Abstract
Purpose
HER2 copy number by fluorescence in situ hybridization (FISH) is typically reported relative to the centromere enumeration probe 17 (CEP17). HER2/CEP17 ratio could be impacted by alterations in the number of chromosome 17 copies. Monosomy of chromosome 17 (m17) is found in ~ 1900 cases of early-stage HER2-positive breast cancer annually in the United States; however, the efficacy of HER2-directed trastuzumab therapy in these patients is not well characterized. Here, we retrospectively identified HER2-amplified, stage I–III breast cancers with m17 and characterized the impact of trastuzumab treatment.
Methods
From January 1, 2000 to June 1, 2011, we identified 99 women with HER2-amplified m17 breast cancers, as defined by a CEP17 signal of < 1.5 per nucleus and a HER2/CEP17 ratio of ≥ 2.0.
Results
Most HER2-amplified m17 patients were treated with trastuzumab plus chemotherapy (51%, n = 50), whereas 31% (n = 31) received chemotherapy alone and 18% (n = 18) received no chemotherapy. The 4-year overall survival (OS) was superior with trastuzumab compared to chemotherapy alone or no chemotherapy (100 vs. 93 vs. 81%, respectively; p = 0.005). OS was not influenced by estrogen/progesterone-receptor (ER/PR) status, tumor stage, or degree of FISH positivity. A proportion of patients who would be considered HER2-negative by standard immunohistochemistry staging criteria (0–1+) were HER2 amplified by FISH.
Conclusions
In the largest series reported to date, patients with HER2-amplified m17 cancers treated with trastuzumab have outcomes comparable to patients from the large phase III adjuvant trastuzumab trials who were HER2-positive, supporting the critical role of HER2-directed therapy in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gene for human epidermal growth factor receptor 2 (HER2) is located on the long arm of chromosome 17, and when amplified, is generally associated with overexpression of the oncoprotein [1, 2]. HER2-“positive” tumors have extra copies of the HER2 gene by fluorescence in situ hybridization (FISH) and/or high levels of the HER2 protein by immunohistochemistry (IHC). HER2 copy number by FISH has typically been reported relative to a control for the expected number of chromosomes, namely the centromere enumeration probe 17 (CEP17) nuclear signal, although some tests increasingly report only the total HER2 copy number. The successful development of HER2-directed therapies, such as trastuzumab, has dramatically improved breast cancer-specific outcomes in HER2-positive breast cancers [3, 4]. However, HER2/CEP17 ratio as a diagnostic tool is influenced by the number of chromosome 17 copies and the impact of this on treatment decisions is unknown.
A relatively small proportion of HER2-positive early-stage breast cancers exhibit a gain of HER2 copy number and loss of chromosome 17 copy number, suggestive of HER2-amplified monosomy 17 (m17). While the overall incidence is unknown, HER2-amplified m17 can be identified in the clinic using standard-of-care HER2-testing with dual-probe FISH, which uses enumeration probes to quantify copy numbers of both the HER2 gene and CEP17. By this method, m17 is detected in an estimated 4.2% of HER2-positive cases, which translates to an incidence of approximately 1900 cases annually in the United States [5, 6].
The clinical implications of HER2-amplified m17 in early-stage breast cancer are unknown. In the N9831 adjuvant trastuzumab trial, a small number of patients with m17 (n = 79) were treated with or without trastuzumab, and the hazard ratio for benefit with trastuzumab was not statistically significant (HR 1.21, CI 0.43–4.62, p = 0.78), raising the possibility that patients may not benefit from trastuzumab in this subgroup [5]. Notably, a retrospective series of patients with metastatic, HER2-positive breast cancer identified inferior response rates to trastuzumab-containing chemotherapy regimens in subjects with m17, compared to those with normal or increased chromosome 17 copy number (response rate: m17 = 53%, non-m17 = 92%) [7].
There are at least two possible reasons that patients with HER2-amplified m17 may exhibit unique prognostic or therapeutic response patterns when compared to other HER2-“positive” tumors. First, chromosome 17 contains additional genes that are central to breast cancer pathogenesis and DNA repair, including topoisomerase II-α (TOP2A), breast cancer 1 (BRCA1), tumor protein P53 (TP53), and others, and therefore loss of chromosome 17 may have biologic implications that may influence prognosis or therapeutic response [8]. For example, TOP2A expression may be associated with anthracycline sensitivity and therefore loss of TOP2A may mediate chemoresistance [9, 10].
In addition, tumors that test positive for m17 by FISH may in fact not actually have increased copy numbers of HER2. Specifically, genetic alterations that impair CEP17 binding may result in falsely elevated HER2/CEP17 ratios without true HER2-amplification, and therefore these tumors may not respond to anti-HER2 therapy. Peri-centromeric genomic alterations, for example, mutations/amplifications or translocation events, may perturb binding of the CEP17 probe and lead to modest elevations in HER2/CEP17 ratio without copy number increases in HER2. For example, an isochromosome [i.e., i(q17)] translocation event by which the long (q) arm is duplicated with a tandem loss of the short (p) arm would yield a HER2/CEP17 ratio of 3, whereas a heterozygous mutational event that disturbs CEP17 binding would yield a HER2/CEP17 ratio of 2.
A better understanding of the clinical characteristics of HER2-amplified m17 could help to inform clinical management. The current standard-of-care is to treat patients with these cancers the same as patients with HER2-positive early-stage disease. Most commonly, this means using trastuzumab plus chemotherapy, either in the neoadjuvant or adjuvant setting [3]. However, in light of the US Food and Drug Administration (FDA) approval of next-generation HER2-directed therapies such as lapatinib, trastuzumab-emtansine (T-DM1), and pertuzumab, the standard may soon evolve to include these agents and, for lower risk cohorts, we may reduce or fully eliminate conventional chemotherapy in early-stage disease in the future. Therefore, overtreatment of m17 could lead to added cost and toxicity while undertreatment could compromise breast cancer-specific outcomes. Thus, understanding the impact of m17 is important across the spectrum of HER2-positive disease.
Here, we undertook a retrospective single-institution study to explore the impact of m17 on outcomes for women with HER2-“positive” stage I–III breast cancer treated at Memorial Sloan Kettering Cancer Center (MSKCC) between 2000 and 2011. We evaluated the treatment course and clinical outcomes of all patients with elevated HER2/CEP17 ratio (≥ 2.0) and low CEP17 (< 1.5) on routine, dual-probe FISH. We further compared HER2/CEP17 ratio levels to HER2 protein levels using IHC.
Materials and methods
Study population and definitions
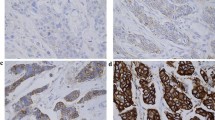
Under an investigational review board-approved waiver of consent, the MSKCC breast cancer database was interrogated. All women diagnosed between 1/1/2000 and 6/1/2011 with HER2-amplified m17 early-stage breast cancer by dual-probe FISH (defined as HER2/CEP17 ratio ≥ 2.0 and CEP17 < 1.5 per nucleus) for whom clinical follow-up was available for review were identified. Because no standard definition for monosomy 17 is established, the definition derived from the largest reported series was used, which defines monosomy 17 as < 1.5 CEP17 signals per nucleus (Table 1; Fig. 1) [11, 12]. These criteria highly enrich for, but do not exclusively identify HER2-amplified m17, because alternative genomic alterations (for example, peri-centromeric translocations or mutations) may disturb binding of CEP17 to the chromosome and may generate a falsely elevated HER2/CEP17 ratio with low CEP17. However, for clarity unless otherwise specified, HER2-amplified m17 will refer to all cases meeting the above criteria.
A clinical chart review was conducted on subjects with HER2-amplified m17 tumors to ascertain whether trastuzumab and or chemotherapy was employed in the neoadjuvant or adjuvant setting. The inclusion of anthracycline was documented.
FISH and IHC
As per the MSKCC institutional standard, IHC was performed routinely on all early-stage breast cancer cases according to standard methods as previously published, using ASCO guidelines as negative (0, 1+), equivocal (2+), or positive (3+) [13, 14]. FISH was conducted reflexively to define HER2 status in the setting of equivocal IHC expression since 2009, and was performed in all invasive breast cancer cases before 2009. FISH was also performed on selected IHC HER2-negative cases, for example, for women who are enrolled on investigational protocols requiring FISH or when requested by the treating clinician. In all cases, deparaffinized tissue sections were evaluated using the US FDA-approved Vysis PathVysion probe set, which consists of a SpectumGreen-conjugated probe (CEP17) to the alpha satellite DNA located on 17p11.1-q11.1 of chromosome 17, and a SpectrumOrange-conjugated probe to the HER2 gene (Abbott Diagnostics, Chicago, IL) [15].
Statistical methods
This was a retrospective study designed to explore potential associations of various (neo)adjuvant treatment strategies (chemotherapy plus trastuzumab, chemotherapy alone, versus no chemotherapy) with long-term outcome in patients harboring monosomy 17. The χ 2 test was used to identify clinicopathologic imbalances across treatment groups, including estrogen/progesterone-receptor (ER/PR) status, age, chemotherapy agent (anthracycline versus no anthracycline), and American Joint Committee on Cancer Staging (AJCC) stage. The principle outcome measure was overall survival, although other endpoints as defined by Standardized Definitions for Efficacy End Points (STEEP) were also evaluated [16]. Kaplan–Meier survival curves were generated for graphical analysis, and when feasible based on sample size, differences in outcome were compared across treatment groups by the log-rank test. Analyses were conducted using the R statistical software package and Graphpad Prism.
Results
Summary of study population
From 1/1/2000 to 6/1/2011, 99 subjects with HER2-amplified cancers and m17 had clinical follow-up data available. The clinicopathologic features of these patients are summarized in Table 2 and are stratified by the type of chemotherapy regimen received in the curative setting (chemotherapy + trastuzumab, chemotherapy alone, or no chemotherapy). About half of the subjects received chemotherapy + trastuzumab (51%, n = 50), whereas a smaller subset of patients received chemotherapy without trastuzumab (31%, n = 31) or no chemotherapy (18%, n = 18). With a median follow-up of 7.1 years (0.16–13.4 years), the median overall survival (OS) was not reached, and the landmark 4-year OS was 95% (CI 88–98%, Fig. 2).
We conducted χ 2 analyses to evaluate potential confounders in baseline characteristics between patients in the three treatment groups (Table 2). Subject age and tumor histology were balanced across treatment groups, whereas hormone receptor status and AJCC stage were not. The chemotherapy + trastuzumab group contained more ER/PR-positive tumors relative to the chemotherapy only and no-chemotherapy groups (p = 0.04), whereas the no-chemotherapy group had lower AJCC stage tumors compared to the chemotherapy + trastuzumab and chemotherapy groups (p = 0.01). There was also a trend (p = 0.1) towards imbalance in anthracycline use, with a smaller proportion of trastuzumab-treated subjects receiving an anthracycline (82%) compared to chemotherapy only subjects (97%, Table 2).
Improved outcomes for patients treated with trastuzumab-based chemotherapy
We performed Kaplan–Meier analyses to evaluate the potential influence of therapy on outcomes. Despite receiving less intensive chemotherapy, as indicated by a trend towards less anthracycline use (Table 2), subjects receiving chemotherapy + trastuzumab demonstrated a statistically superior OS compared to subjects receiving either chemotherapy alone or no chemotherapy (p = 0.001; Fig. 3). Furthermore, subjects receiving chemotherapy + trastuzumab had improved STEEP outcomes, with recurrence-free survival (RFS, p = 0.04; Fig. 3) and distant recurrence-free survival (DRFS, p = 0.005; data not shown) reaching statistical significance.
Kaplan–Meier curves according to treatment group (chemo + tras, chemo alone, and no chemo). a Overall survival. b Recurrence-free survival by STEEP criteria [16]. c Overall survival, AJCC Stage I/II only. d Overall survival, ER/PR-positive only. tras trastuzumab, chemo chemotherapy, STEEP standardized definitions for efficacy end points, AJCC American Joint Commission on Cancer Staging, ER estrogen receptor, PR progesterone receptor
To assess the potential influence of imbalance in AJCC staging and hormone receptor status, we repeated the above analysis independently for each AJCC subgroup (stage I/II versus stage III) and each ER/PR subgroup (ER/PR-positive versus ER/PR-negative). OS was superior in the chemotherapy + trastuzumab group relative to chemotherapy alone or no-chemotherapy group in AJCC Stage I/II subjects (p = 0.003; (Fig. 3) and in ER/PR-positive subjects (p = 0.0006; Fig. 3). The ER/PR-negative (n = 12) and AJCC Stage III (n = 15) subgroups were too small for further statistical analyses; however, chemotherapy + trastuzumab appeared to be superior in each of these subgroups.
HER2-positive m17 patients with potential alterations in CEP17 binding still benefit from trastuzumab
We next examined patients with potential m17-related genetic alterations that could impair CEP17 binding, which would result in artificially elevated HER2/CEP17 ratios due to decreased CEP17 rather than increased HER2 copy number. We separately evaluated outcomes for patients with HER2/CEP17 ratios between 2 and 3.5, which are more likely to include non-m17 causes of low CEP17 binding, including i(q17) translocation and heterozygous mutational events. Such cases would still be classified as HER2-positive by 2015 NCCN guidelines [17]. In our series, tumors with FISH ratios within this range constituted only 23% of HER2-amplified m17 cases (n = 23/99), and were much less likely to test positive by IHC (only 13% IHC 3+, vs. 84% IHC 3+ for tumors with FISH ratio ≥ 3.5). These patients had Kaplan–Meier survival curves which overlapped with those of FISH-high patients (p = 0.321; Fig. 4). Despite the small sample size, subjects in this group who received chemotherapy + trastuzumab had improved OS compared to subjects who did not receive trastuzumab (p = 0.018; Fig. 4).
Kaplan–Meier curves according to magnitude of HER2/CEP17 FISH ratio: a Overall survival, FISH ratio 2–3.5 versus ≥ 3.5. b Overall survival among HER2-amplified m17 subjects with FISH ratio 2–3.5, by treatment group. tras trastuzumab; chemo chemotherapy, HER2 human epidermal growth factor receptor 2, IHC immunohistochemistry, FISH fluorescence in situ hybridization, m17 monosomy 17
Some patients with CEP17 copy number loss had discordant HER2-status by FISH versus IHC
All but two subjects (2/99, 2%) in this series were tested by both FISH and IHC, thereby providing the opportunity to evaluate concordance of these two testing modalities in determining HER2-positivity in subjects with loss of CEP17 binding. The majority (61%, n = 59/97) of cases were concordantly HER2-positive, with HER2/CEP17 ratios ≥ 2.0 by FISH and 3+ expression by IHC (Fig. 5). An additional 26% (n = 25/97) had HER2/CEP17 ratios ≥ 2.0 and IHC in the equivocal range (2+); these cases are still considered concordant because NCCN guidelines recommend reflexive FISH testing in the setting of equivocal IHC [14, 17]. However, 13% of cases (n = 13/97) were IHC-negative (0–1+) and FISH-positive (HER2/CEP17 ratio ≥ 2.0), which represents a subset of putatively HER2-positive patients (by FISH) who may not be identified as HER2-positive by standard-of-care IHC testing alone.
Correlation of HER2 expression by IHC versus FISH ratio in HER2-amplified m17 early-stage breast cancers. Solid lines represent group medians and the dotted line crosses at FISH ratio = 2.0, the threshold for HER2-positivity by NCCN guidelines [17]. HER2 human epidermal growth factor receptor 2, IHC immunohistochemistry, FISH fluorescence in situ hybridization, m17 monosomy 17, NCCN National Comprehensive Care Network
Figure 5 illustrates the distribution of IHC scores and the corresponding HER2/CEP17 ratios for each patient. Overall, the intensity of HER2 staining by IHC correlated with FISH ratios. However, FISH ratios were widely distributed across each IHC score, with a proportion of IHC-negative patients (n = 4/13) exhibiting FISH ratios greater than 4.0. While sample size precluded statistical assessment of outcomes in the IHC/FISH-discordant subjects, OS appeared similar to the entire cohort, with 4-year OS of 93%. Three of the 13 IHC/FISH-discordant subjects received chemotherapy + trastuzumab, and all of these subjects remain alive with a minimum follow-up of 5.2 years. However, one of these subjects experienced an in-breast recurrence at 6 years which was resected with no subsequent recurrence.
Conclusion
The large absolute annual incidence of breast cancer makes even uncommon events, such as HER2-amplified m17, potentially important. Based on national registries and the largest prospective analysis of chromosome 17 copy number in trastuzumab-treated early-stage breast cancers, the best estimate for incidence of HER2-amplified m17 early-stage breast cancer is 1900 patients annually in the United States [5]. This is a comparable population to male breast cancer or synovial sarcoma [18]. Despite this, HER2-amplified m17 is under-investigated, and trastuzumab-based chemotherapy remains an unproven and largely empiric standard-of-care for these patients.
Here, we report the largest case series describing outcomes for women with HER2-amplified m17 early-stage breast cancers. We found that the survival of these subjects (4 year OS 95%) was comparable to all-comer HER2-positive early-stage breast cancers as reported in large phase III clinical trials such as the N9831, which reported a 4-year OS of ~ 89% following trastuzumab-containing chemotherapy [5]. Second, we found that HER2-amplified m17 subjects appeared to benefit from trastuzumab, as indicated by improved survival and STEEP outcomes, despite less anthracycline-based chemotherapy in these subjects compared to similar subjects who were not treated with trastuzumab. These findings were consistent across all ER/PR and AJCC stage subgroups, addressing the potential concern of confounders based upon imbalances in baseline characteristics across the treatment groups. These data support the current standard treatment of HER2-amplified m17 early-stage breast cancers with trastuzumab-based chemotherapy and suggest that prognosis is similar to that of HER2-positive breast cancers.
These findings may become more clinically relevant with the advent of newer HER2-targeted regimens. For example, the anti-HER2 antibody drug conjugate, T-DM1, is now FDA approved as monotherapy for use in metastatic HER2-positive breast cancer, based on a phase III trial demonstrating improved OS compared to capecitabine plus lapatinib [19]. One potential concern is that if subjects with m17 by FISH do not benefit from trastuzumab, then monotherapy with T-DM1—which uses a trastuzumab moiety to deliver cytotoxic therapy to HER2-positive cells—may not be clinically active. Our retrospective analysis showed that m17 patients appear to benefit from trastuzumab, providing indirect reassurance that T-DM1 should also be effective in this patient population. T-DM1 is being evaluated for expanded clinical indications, potentially as a first-line regimen in metastatic breast cancer based on phase II and phase III data comparing T-DM1 to taxane/trastuzumab, and in the neoadjuvant/adjuvant setting with several ongoing trials such as the ATEMPT trial comparing T-DM1 to paclitaxel/trastuzumab in stage I breast cancer [20,21,22]. In light of these possible expanding indications, we would urge investigators of all T-DM1 trials to conduct a subset analysis of m17-treated patients to confirm that these patients benefit from the therapy. Meta-analyses of similar trials may be necessary to obtain sufficient sample size to accomplish this goal.
Our data highlight another potential clinical implication of HER2-amplified m17, namely that a proportion of patients with HER2-amplified tumors and m17 may potentially be misdiagnosed as HER2-negative. In our series, we identified a subset of patients with IHC 0–1+ and concomitant FISH HER2/CEP17 ratios above 2.0. Since FISH testing is not reflexively requested if IHC protein expression is in the 0–1+ range, per ASCO CAP or NCCN guidelines, these patients would not be identified as HER2-positive in the absence of FISH testing [17]. Our series is too small to provide information on the prognosis or impact of trastuzumab in these patients. However, these findings may justify a more extensive assessment of the prevalence and clinical characteristics of discordant HER2-positive m17 breast cancers across recent phase III clinical trials.
A critical limitation of our analysis is that, by standard-of-care dual-probe FISH testing, it would be impossible to discern cases of true HER2-amplified m17 from false-positive cases which may be the result of alternative genomic events. For example, heterozygous somatic mutations in the CEP17 probe binding region may result in a HER2 copy number of 2, a CEP17 copy number 1, and therefore a HER2/CEP17 ratio of 2. Another possible mechanism of eusomic CEP17 copy number loss is isochromosome 17 [i(17q)], a described translocation by which the long (q) arm is duplicated with a tandem loss of the short (p) arm. i(17q) has been previously identified in breast cancer cell lines, is described in hematologic and CNS malignancies, and is thought to arise from a translocation at a locus of genomic instability in the 17p11.2 band related to large, palindromic repeats [23, 24]. The effect of an i(17q) event on CEP17 centromeric probe binding has not been described, but this may theoretically disturb binding of the probe to the centromere. More sophisticated genomic testing, such as the use of additional chromosome 17 reference probes, could be conducted to differentiate true m17 versus other causes of CEP17 probe binding loss, such as i(17q) [25]. In our data, subjects with FISH between 2.0 and 3.5 appeared to benefit from trastuzumab and had similar outcomes when compared to subjects with more robust elevations in HER2/CEP17 ratio. These findings suggest that false-positive HER2-amplified m17 cases may either constitute a small proportion of HER2-amplified m17, or have similar prognosis/response patterns compared to true-positive HER2-amplified cases. Therefore, we believe that additional genomic testing would be of limited value for this rare subset of patients.
Despite the limitations of a small, retrospective single-institution report, our study provides the largest single series of HER2-amplified m17 early-stage breast cancer to date and supports the continued use of trastuzumab in this setting. These data also serve to highlight the potential utility of studying rare subsets in breast cancer, which despite constituting small relative proportions of patients, may represent distinct biological entities with large absolute incidences both across the US and worldwide.
References
Bartlett JM, Going JJ, Mallon EA et al (2001) Evaluating HER2 amplification and overexpression in breast cancer. J Pathol 195:422–428
Bartlett J, Mallon E, Cooke T (2003) The clinical evaluation of HER-2 status: which test to use? J Pathol 199:411–417
Hudis CA (2007) Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 357:39–51
Slamon D, Eiermann W, Robert N et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365:1273–1283
Perez EA, Reinholz MM, Hillman DW et al (2010) HER2 and chromosome 17 effect on patient outcome in the N9831 adjuvant trastuzumab trial. J Clin Oncol 28:4307–4315
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30
Risio M, Casorzo L, Redana S, Montemurro F (2005) HER2 gene-amplified breast cancers with monosomy of chromosome 17 are poorly responsive to trastuzumab-based treatment. Oncol Rep 13:305–309
Bieche I, Tomasetto C, Regnier CH, Moog-Lutz C, Rio MC, Lidereau R (1996) Two distinct amplified regions at 17q11-q21 involved in human primary breast cancer. Can Res 56:3886–3890
Engstrom MJ, Ytterhus B, Vatten LJ, Opdahl S, Bofin AM (2014) TOP2A gene copy number change in breast cancer. J Clin Pathol 67:420–425
Press MF, Sauter G, Buyse M et al (2011) Alteration of topoisomerase II-alpha gene in human breast cancer: association with responsiveness to anthracycline-based chemotherapy. J Clin Oncol 29:859–867
Reinholz MM, Bruzek AK, Visscher DW et al (2009) Breast cancer and aneusomy 17: implications for carcinogenesis and therapeutic response. Lancet Oncol 10:267–277
Ma Y, Lespagnard L, Durbecq V et al (2005) Polysomy 17 in HER-2/neu status elaboration in breast cancer: effect on daily practice. Clin Cancer Res 11:4393–4399
Perez EA, Suman VJ, Davidson NE et al (2006) HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol 24:3032–3038
Wolff AC, Hammond ME, Hicks DG et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013
Perez EA, Press MF, Dueck AC et al (2013) Immunohistochemistry and fluorescence in situ hybridization assessment of HER2 in clinical trials of adjuvant therapy for breast cancer (NCCTG N9831, BCIRG 006, and BCIRG 005). Breast Cancer Res Treat 138:99–108
Hudis CA, Barlow WE, Costantino JP et al (2007) Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol 25:2127–2132
NCCN Clinical Practice Guidelines in Oncology (2015) Breast Cancer, Version 3.2015. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 24 July 2015
Greenlee RT, Goodman MT, Lynch CF, Platz CE, Havener LA, Howe HL (2010) The occurrence of rare cancers in U.S. adults, 1995–2004. Public Health Rep 125:28–43
Verma S, Miles D, Gianni L et al (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–1791
Hurvitz SA, Dirix L, Kocsis J et al (2013) Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 31:1157–1163
Frontline T-DM1 Results Disappointing in Phase III MARIANNE Trial (2014) http://www.onclive.com/web-exclusives/Frontline-T-DM1-Results-Disappointing-in-Phase-III-MARIANNE-Trial. Accessed 27 July 27 2015
Trial identifier NCT01853748 https://clinicaltrials.gov/ct2/show/NCT01853748 Accessed 27 July 2015
Mertens F, Johansson B, Mitelman F (1994) Isochromosomes in neoplasia. Genes Chromosom Cancer 10:221–230
Pandrangi SL, Raju Bagadi SA, Sinha NK et al (2014) Establishment and characterization of two primary breast cancer cell lines from young Indian breast cancer patients: mutation analysis. Cancer Cell Int 14:14
Tse CH, Hwang HC, Goldstein LC et al (2011) Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J Clin Oncol 29:4168–4174
Watters AD, Going JJ, Cooke TG et al (2003) Chromosome 17 aneusomy is associated with poor prognostic factors in invasive breast carcinoma. Breast Cancer Res Treat 77:109–114
Acknowledgements
This study was supported in part by a National Institute of Health/National Cancer Institute P30CA008748 Institutional grant awarded to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
David B. Page and Hannah Wen are co-first authors.
Rights and permissions
About this article
Cite this article
Page, D.B., Wen, H., Brogi, E. et al. Monosomy 17 in potentially curable HER2-amplified breast cancer: prognostic and predictive impact. Breast Cancer Res Treat 167, 547–554 (2018). https://doi.org/10.1007/s10549-017-4520-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4520-1