Abstract
Breast cancer is the most common malignancy in Singapore women. Ductal carcinoma in situ (DCIS) is the putative, non-obligate precursor of the majority of invasive breast cancers. The efficacy of the Singapore breast-screening pilot project in detecting early stage breast cancer led to the launch of a national breast-screening programme, BreastScreen Singapore (BSS), in January 2002. In this study, we compared clinicopathological and immunohistochemical characteristics, as well as clinical outcomes, between screen-detected and symptomatic DCIS. The study cohort comprised 1202 cases of DCIS diagnosed at Singapore General Hospital from 1994 to 2010. Comparison of clinicopathological parameters, immunohistochemical results of ER, PR, HER2, CK14, EGFR, and 34βE12, and clinical outcomes was carried out between the 2 groups. Amongst 1202 cases, 610 (50.7 %) were screen-detected and 592 (49.3 %) were symptomatic DCIS. Screen-detected cases were smaller in size (P < 0.001), of lower nuclear grade (P = 0.004), and more frequently expressed ER (P < 0.001). Luminal A phenotype was more frequently observed in screen-detected DCIS, while triple-negative and HER2 phenotypes were more common in symptomatic DCIS (P < 0.001). The basal-like phenotype was also more frequent in symptomatic DCIS (P = 0.041). Mean and median follow-up was 99.7 and 97.8 months, respectively, with a maximum follow-up of 246.0 months. More symptomatic patients developed invasive recurrences compared to screen-detected patients (P = 0.001). A trend for better disease-free survival was observed in screen-detected patients (P = 0.076). Patients who were screen-detected experienced better overall survival than those with symptomatic DCIS (P = 0.007). Our data indicate a more favourable outcome of screen-detected DCIS patients confirming the role of BSS in early identification of this curable disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ductal carcinoma in situ (DCIS) is a heterogeneous entity composed of an intraductal malignant proliferation of ductal epithelial cells in the breast [1]. It represents the early, noninvasive stage of breast cancer and is reported to be the putative precursor to many invasive breast carcinomas. Previously a relatively uncommon disease with a pre-mammography incidence of less than 5 % [2], the incidence of DCIS today has risen markedly due to mammographic breast screening. The United States National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) cancer statistics review reported 63,846 (17.2 %) cases of DCIS out of a total of 370,343 cases of breast cancers from 2008 to 2012 [3]. In Singapore, statistics from 2002 to 2007 showed that DCIS comprised 26 % of all breast cancers detected during BreastScreen Singapore (BSS), a population-based mammographic breast-screening programme launched in January 2002 [4].
The marked increase in the incidence of DCIS after the introduction of mammographic screening has led to discussions regarding overdiagnosis [5, 6]. A study by Coldman and Phillips [7] estimated overdiagnosis of DCIS in a screening programme to be at 11.9 %. Overdiagnosis refers to the detection of lesions that will not become symptomatic or result in death during a patient’s lifetime [6]. As there are currently no established guidelines to determine which cases of DCIS will remain indolent and which will progress to invasive carcinoma, it is possible that many cases of DCIS are potentially overtreated with surgery, radiation, and/or adjuvant hormonal therapy [8].
Although these reports are concerned with overdiagnosis of DCIS, several studies have maintained the view that it is a minor phenomenon [9–11] and that the benefits of breast screening outweigh the risks of DCIS overdiagnosis [12]. Yen et al. [13] estimated that 37 % of DCIS detected at a prevalence screen and only 4 % at an incidence screen would not progress, suggesting that the majority of DCIS lesions are significant.
As symptomatic lesions have been known to carry a poorer prognosis than screen-detected ones [14, 15], it is worthwhile to ascertain the clinicopathological and immunohistochemical differences as well as clinical outcomes of screen-detected and symptomatic DCIS. A meta-analysis of 6 studies that examined the association between modes of DCIS detection and clinical outcomes described an increased risk of local invasive recurrence in patients whose DCIS was picked up symptomatically, compared to those detected via mammography (HR = 1.38, 95 % CI 1.12–1.63) [16]
In this study, we endeavoured to establish the clinicopathological and immunohistochemical characteristics, as well as clinical outcomes, of a large series of women diagnosed with both screen-detected and symptomatic DCIS in our institution.
Materials and methods
Patients and tumours
The study cohort comprised cases of DCIS diagnosed at the Department of Pathology, Singapore General Hospital (SGH) from 1994 to 2010. Institutional review board approval was obtained for this study (CIRB Ref: 2010/610/F). All screen-detected DCIS cases were derived from the database of the Health Promotion Board. Mammographic findings were recorded. As comparison, all cases of DCIS presenting symptomatically over the same time period were similarly reviewed and investigated. Clinical, radiological, and pathological information was obtained from hospital records.
Histology slides for each case were retrieved and reviewed. Histopathological parameters assessed included tumour size, nuclear grade, necrosis, calcifications, microinvasion, and the morphological subtype of DCIS. Nuclear grade was categorised as low, intermediate, or high based on the degree of nuclear pleomorphism [17]. Low-nuclear grade lesions were identified by their relatively uniform nuclei; intermediate-nuclear grade lesions had moderately enlarged vesicular and variably sized nuclei; high-nuclear grade DCIS contained markedly enlarged and pleomorphic nuclei, where mitoses could be readily discerned [18]. Microinvasion was defined as the presence of invasion not exceeding 1 mm in extent [17]. Morphological subtyping was classified into comedo, cribriform, micropapillary, papillary, solid, and mixed groups.
Immunohistochemistry
Archival formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved. Sections (4 µm thick) were cut from the FFPE blocks and fished onto positively charged Bond Plus glass slides (Leica Biosystems, Inc., Richmond, IL, USA). This was followed by incubation in an oven overnight at 80 °C to increase adhesion of the sections to the slides. Immunohistochemistry (IHC) was performed using antibodies to oestrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), epidermal growth factor receptor (EGFR), and cytokeratins (CK14 and 34βE12) according to previously published protocol [19]. Details of the antibodies, dilution factors, and antigen retrieval methods are provided in Table 1. An appropriate positive control was run with each batch of slides.
Nuclear reactivity for ER and PR, cytoplasmic membrane decoration for HER2 and EGFR, and cytoplasmic staining for CK14 and 34βE12 were assessed. The staining intensity and percentage of positively stained tumour cells were recorded. The staining intensities were scored as 0, 1+, 2+, and 3+, denoting no staining, weak, moderate, and strong staining, respectively [20]. For ER, PR, CK14, EGFR, and 34βE12, a positive result was defined by the presence of at least 1 % of tumour cells displaying positive staining [21]. For HER2, positive expression was defined as >10 % of tumour cells exhibiting 3+ membrane staining [22].
Molecular subtypes of DCIS were categorised based on immunohistochemical surrogates as Luminal A (ER+, and/or PR+, HER2−), Luminal B (ER+, and/or PR+, HER2+), triple negative (ER−, PR−, HER2− and HER2 type (ER−, PR−, HER2+) [23]. Basal-like DCIS was defined by positive staining of any of a tri-panel of CK14, EGFR, and 34βE12 [20]. It is acknowledged that Ki-67 is also used as a discriminant for Luminal A and Luminal B molecular subtypes, but for the purposes of this study on DCIS lesions, only ER, PR, and HER2 were applied.
Follow-up
Follow-up data were obtained from case notes. Recurrences included in situ and invasive local relapses and distant metastases. Local recurrence was defined as the re-occurrence of a tumour in the ipsilateral breast or on the chest wall. Contralateral breast cancers (CBCs) were taken into account in this study for a complete reflection of all events that occurred. Distant metastasis referred to tumours that occurred away from locoregional locations, in distant sites. Disease-free survival (DFS) and overall survival (OS) were defined as time from the date of diagnosis to the date of recurrence or death, respectively, or to the date of last follow-up [24].
Statistical analysis
Findings were analysed using SPSS for Windows, Version 18 (SPSS, Inc., Chicago, IL, USA). χ 2 and Fisher’s exact tests were used to evaluate the relationship of clinicopathological parameters between screen-detected and symptomatic DCIS. Survival outcomes were estimated using the Kaplan–Meier estimator and compared between screen-detected and symptomatic DCIS groups using the log-rank test. Cox proportional hazards models were used to determine the effect of the two categories of DCIS on survival outcomes. A P value of < 0.05 defined statistical significance.
Results
The study cohort comprised 1254 DCIS cases which were diagnosed in the Department of Pathology, Singapore General Hospital, from 1994 to 2010. Upon further review, 52 cases were excluded as they had a previous history of invasive breast carcinoma, or had contained an invasive component that was present in a prior specimen. Of the final 1202 cases, 610 (50.7 %) were screen-detected and 592 (49.3 %) were symptomatic DCIS. Amongst the symptomatic group, 540 (91.2 %) patients presented with a breast lump, 44 (7.4 %) with nipple discharge, 3 (0.5 %) with lump and nipple discharge, and 5 (0.9 %) with mastitis. Radiological findings showed calcifications in 515 (84.4 %) of the screen-detected cases, mass in 74 (12.1 %), and both calcifications and mass in 21 (3.5 %) cases (Fig. 1). Patients were treated with either breast-conserving therapy or mastectomy. Adjuvant radiation therapy consisting of whole-breast radiation delivered to 50 Gy followed by a 10-Gy boost to the tumour bed was administered for those who underwent breast conservation. Of the screen-detected patients, 380 (62.3 %) underwent breast-conserving therapy, compared to 310 (52.4 %) of symptomatic patients. Two hundred and thirty (37.7 %) screen-detected patients had mastectomy compared to 282 (47.6 %) symptomatic patients.
A comparison of the clinicopathological parameters between screen-detected and symptomatic DCIS is shown in Table 2 with morphological patterns of DCIS demonstrated in Fig. 2. While immunohistochemical staining results (Fig. 3) and different molecular subtypes observed in our cohort of DCIS lesions are presented in Table 3.
Follow-up ranged from 0.3 to 246.0 months (mean 99.7 months, median 97.8 months). There were 140 (11.6 %) recurrences. Of these, 69 (49.3 %) were in situ, 63 (45.0 %) invasive, and 8 (5.7 %) direct distant metastases without a prior record of invasive locoregional recurrence. A comparison of recurrence patterns between the screen-detected and symptomatic groups is shown in Table 4. We observed more cases of recurrence amongst the symptomatic (14.2 %) than screen-detected (9.2 %) patients (P = 0.001). The most common pattern of recurrence in the screen-detected population was ipsilateral DCIS (3.3 %) and in the symptomatic population was ipsilateral invasive ductal carcinoma (IDC) (4.3 %). Patients who underwent mastectomy had a lower rate of recurrence (1.9 %) than those who had breast-conserving therapy (4.8 %). There were 15 (1.2 %) patients who developed distant metastases; 10 were patients who presented symptomatically, and 5 were from the screen-detected group. Of these 15 patients, 7 presented with invasive locoregional recurrences prior to the metastatic event, while 8 had no documented invasive locoregional recurrences (direct metastasis). Details of the 8 patients with direct metastasis are summarised in Table 5. There was no significant association of clinicopathological parameters and biomarkers with recurrence risk.
A total of 28 (2.3 %) patients died: 8 (0.7 %) were breast cancer-specific deaths and the other 20 were due to other causes, such as cardiac arrest, cervical cancer, chronic renal failure, colon cancer, diabetes, gastric cancer, laryngeal cancer, lung cancer, peritoneal malignancy, sarcoma, septicaemia, and vaginal cancer. All 8 breast cancer-specific deaths were from the symptomatic cohort. Of these, 6 patients developed recurrences of IDC and in 5 of them, metastasised and subsequently caused death. One patient died from IDC with no documented metastasis, while the remaining 2 patients developed distant metastasis without a prior invasive locoregional recurrence. A summary of the breast cancer-specific deaths is presented in Table 6.
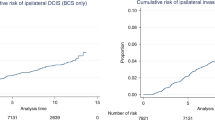
A trend for better DFS (P = 0.076) was observed in screen-detected patients. Patients who presented with DCIS symptomatically disclosed poorer OS (P = 0.007) (Fig. 4). DFS and OS rates at 5 and 10 years with the inclusion and exclusion of contralateral events are summarised in Table 7.
DFS and OS were also separately assessed with the exclusion of microinvasive cases. There was no significant change in both DFS and OS when these cases were excluded.
Discussion
In this study, we observed a larger proportion of women aged 50 years and above in the screen-detected group compared to the symptomatic group, which was to be expected as our national breast-screening programme is targeted at women aged 50 years and above [25]. Younger age at presentation has been associated with increased risk of local recurrence [26, 27], although our study did not reveal age as a factor for recurrence risk.
We noted that screen-detected DCIS cases were of smaller size than those from the symptomatic group, underscoring the efficacy of mammographic screening in identifying smaller tumours, concordant with previous studies [28, 29] where the median tumour size of screen-detected DCIS was also smaller than that of symptomatic lesions.
A higher proportion of low-nuclear grade DCIS was noted in screen-detected than symptomatic groups, a finding which differs from previous studies [28–31] that observed no significant difference in tumour grades between screen-detected and symptomatic cases. Low-nuclear grade DCIS is biologically less aggressive than high-nuclear grade DCIS, but it is also not entirely innocuous. Page et al. [32, 33] and Sanders et al. [34] documented that 39.0 % of patients with low-grade noncomedo DCIS who underwent only a diagnostic biopsy procedure, developed invasive recurrences over a follow-up period of 46 years. Of these patients, 45.0 % subsequently died from metastatic disease. Progression of DCIS to invasive disease reported in other follow-up studies [35, 36] of untreated low-grade DCIS (previously misdiagnosed as benign breast disease) ranged from 14.0 to 75.0 % of patients, reinforcing the fact that low-grade DCIS left undetected and untreated can develop into invasive carcinoma. A prospective study by Wong et al. [37] concluded that the rate of local recurrence was substantially high at an estimated 5-year rate of 12.0 % when cases of small-, low-, or intermediate-grade DCIS were treated only with wide excision, even though margins were ≥1 cm. These findings suggest that untreated low-grade DCIS has a substantial risk of developing into invasive breast carcinoma, and this risk is enhanced in high-grade DCIS.
Even though screen-detected DCIS has a significantly higher proportion of low-nuclear grade tumours than symptomatic ones, it is noteworthy that intermediate- and high-nuclear grade DCIS constituted a significant proportion of this screen-detected group, with 234 (38.4 %) and 235 (38.5 %) cases, respectively. High-grade DCIS is known to be a biologically aggressive subgroup of DCIS, associated with progression to high-grade invasive carcinoma [30] and higher rates of recurrence [38, 39]. Approximately half of these recurrent lesions have invasive carcinoma, and the grade of the invasive carcinoma is strongly associated with the grade of the original DCIS [30]. Even though no statistically significant difference was observed in the comparison of tumour grade between screen-detected and symptomatic DCIS in previous studies [28–31], a similar trend of high-grade DCIS predominance was observed in both the groups. In our study, however, tumour grade as a whole was not significantly associated with risk of recurrence.
Calcifications were largely observed in our cohort of screen-detected DCIS compared to symptomatic DCIS. This is not surprising because calcifications are readily picked up on screening mammography and is the most common mammographic feature of DCIS [40]. Our results are in concordance with findings by Ma et al. [29], where 63.2 % of their screen-detected DCIS showed microcalcifications, compared to only 12.5 % of the symptomatic DCIS. The same study also showed that high-grade DCIS is more likely to display abnormal microcalcifications compared to low- and intermediate-grade DCIS.
We report a higher rate of ER positivity in the screen-detected group compared to the symptomatic group. This is in agreement with findings from Barnes et al. [28] where the screen-detected group had 86.0 % ER positivity compared to 72.0 % in the symptomatic group. Studies [41–43] have shown ER negativity to be associated with a higher risk of local recurrence. However, none of the biomarkers in our study were significantly associated with recurrence risk. In line with the higher proportion of ER positive screen-detected cases, the luminal A phenotype was more frequent in the same group as well. The luminal A phenotype has also been associated with a lower risk of recurrence [44].
Triple-negative and HER2 subtypes were more common in the symptomatic group. The rate of triple-negative DCIS in our study was noted to be at 7.1 %, which does not differ greatly from the findings of Clark et al. [45] and Zhou et al. [46], whose documented rates of triple-negative DCIS were 7.5 and 7.8 %, respectively. We observed a higher proportion of triple-negative DCIS subtype in the symptomatic group compared to the screen-detected group. According to Zhou et al., the risk of recurrence for triple-negative DCIS was significantly higher amongst all the molecular subtypes after a follow-up period of 10 years [47]. Triple-negative DCIS has been found to be associated with triple-negative invasive breast cancer, and is likely to be the precursor lesion [19]. The same study found that 97.9 % of DCIS associated with triple-negative invasive breast cancer were also triple negative, suggesting that its higher occurrence amongst our cases of symptomatic DCIS may implicate aggressive behaviour of invasive recurrences.
HER2 status has been known to be associated with a higher risk of recurrence [47]. Although HER2 status showed no significant difference between our screen-detected and symptomatic groups, we observed the HER2 phenotype to be slightly more frequent in symptomatic DCIS than in screen-detected DCIS. Amongst the 4 molecular subtypes, the HER2+ ER− phenotype has been associated with the highest risk of local recurrence during the first 10 years, although the difference was not statistically significant [47].
Of our cohort of DCIS, 33.7 % were identified to be basal-like. This differs from the reported rates of basal-like DCIS from studies by Livasy et al. [48] at 8.0 %, Zhou et al. [46] at 8.2 %, and Clark et al. [45] at 4.2 %. This difference may be due to the higher sensitivity of 34βE12, which targets 4 cytokeratins (CK1, 5, 10, and 14). If we were to look at CK14 and EGFR individually, the rates of basal-like DCIS would be 6.5 % and 3.1 %, respectively. Similar to triple-negative DCIS, basal-like DCIS is believed to be the precursor lesion to basal-like invasive breast carcinoma. Dabbs et al. [49] reported that DCIS present along with basal-like invasive breast carcinoma displayed the same immunophenotype as their invasive counterpart. There were slightly more cases of symptomatic DCIS with the basal-like phenotype than screen-detected DCIS in our study, thereby potentially portending a poorer prognosis for the symptomatic cohort.
Including contralateral events and direct metastases, the overall rate of recurrence for our cohort of patients is 11.6 %, with a median follow-up period of 97.8 months and a mean of 99.7 months. This rate of recurrence is comparable to a study by Sprague et al. [50], who disclosed an 8.5 % rate of recurrence (including contralateral events) with a slightly shorter mean follow-up period of 85.2 months. If contralateral events were excluded, the rate of recurrence in our series would be 6.7 %. This is relatively low compared to other studies such as Kong et al. [27], who reported a 13.0 % rate of local recurrence after breast-conserving surgery and radiotherapy with a median follow-up of 120.0 months, and Bijker et al. [26], who recorded a 16.0 % rate of local recurrence after breast-conserving surgery with or without radiotherapy with a median follow-up of 64.8 months. This difference in rates of recurrence is likely due to the inclusion of patients who underwent mastectomy in our study.
Excluding contralateral events, the rates of recurrence at 5 and 10 years were 3.5 % and 7.1 % (screen-detected patients), and 4.5 % and 7.2 % (symptomatic patients), respectively. This is slightly higher compared to the 5 and 10 year recurrence rates (2.3 % and 4.2 %) of a previous study by Wong et al. [51] because their study focused on low-risk DCIS comprising low- to intermediate-grade lesions (size range 3–25 mm) and high-grade lesions (size range 3–10 mm) with margins ≥3 mm.
Distant metastasis after a diagnosis of DCIS is a phenomenon that has been encountered. DCIS is, by definition, not invasive and therefore theoretically unable to metastasise to regional lymph nodes [52]. Roses et al. [53] documented a distant metastases rate of 1.0 % after an initial diagnosis of pure DCIS, where some of the patients also had no prior invasive locoregional recurrences. Donker et al. [15] also disclosed 3.2 % of direct distant metastasis in their study cohort. A likely explanation for this occurrence could be due to unsampled invasive foci. In our study, of the 8 patients with distant metastasis but no prior invasive locoregional recurrences, 3 had DCIS with microinvasion. Tumour size of DCIS has been suggested to be a vital predictive factor for occult invasion [18]. Of the 8 cases of direct metastasis, 6 had tumour sizes ≥20 mm. Progression from DCIS to metastasis is an indication of aggressive biological behaviour [53]. In our patients with direct distant metastasis, only 2 were from the screen-detected group, while the rest were symptomatic cases, suggesting that symptomatic DCIS is more likely to recur and even potentially metastasise.
Breast cancer-specific mortality in this study was 0.7 % and all were from the symptomatic group of patients. This is relatively low compared to other studies by Donker et al. [15] who disclosed a breast cancer-related death rate of 4.6 %, and Ernster et al. [54] with a documented breast cancer-related death rate of 2.1 %. Of the breast cancer-related deaths in this study by Ernster et al., 4.2 % were diagnosed in 1978–1983, where breast screening was uncommon, while 1.5 % was diagnosed during 1984–1989 after mammography became increasingly commonplace. This concurs with our study where screen-detected patients displayed better OS compared to symptomatic patients.
The exclusion of microinvasive DCIS in this study had no significant impact on DFS and OS. This is in agreement with studies showing that DCIS with microinvasion generally carries a good prognosis [55], and that there is no significant difference between DCIS and DCIS with microinvasion with regard to clinical outcomes, thereby supporting a similar therapeutic approach [39, 56].
In conclusion, we demonstrated that screen-detected DCIS lesions were smaller, of lower nuclear grade, and more frequently ER positive compared to symptomatic DCIS. Nevertheless, these favourable features cannot be equated to indolent insignificant disease, since a significant proportion of screen-detected lesions are intermediate- and high-nuclear grade. Triple-negative, HER2, and basal-like phenotypes were more common in symptomatic DCIS than screen detected. Patients who were screen-detected showed better OS and a trend of better DFS over the duration of follow-up achieved in this study, affirming the role of breast screening in the early identification of this disease.
References
Silverstein MJ (1998) Ductal carcinoma in situ of the breast. BMJ 317(7160):734–739
Frykberg ER, Bland KI (1994) Overview of the biology and management of ductal carcinoma in situ of the breast. Cancer 74(1 Suppl):350–361
Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2014). SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. http://seer.cancer.gov/csr/1975_2012/. Accessed 5 April 2014
Jara-Lazaro AR, Thilagaratnam S, Tan PH (2010) Breast cancer in Singapore: some perspectives. Breast Cancer 17(1):23–28. doi:10.1007/s12282-009-0155-3
Jorgensen KJ, Keen JD, Gotzsche PC (2011) Is mammographic screening justifiable considering its substantial overdiagnosis rate and minor effect on mortality? Radiology 260(3):621–627. doi:10.1148/radiol.11110210
Welch HG, Woloshin S, Schwartz LM (2008) The sea of uncertainty surrounding ductal carcinoma in situ–the price of screening mammography. J Natl Cancer Inst 100(4):228–229. doi:10.1093/jnci/djn013
Coldman A, Phillips N (2013) Incidence of breast cancer and estimates of overdiagnosis after the initiation of a population-based mammography screening program. CMAJ 185(10):E492–E498. doi:10.1503/cmaj.121791
Kerlikowske K (2010) Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010(41):139–141. doi:10.1093/jncimonographs/lgq027
Duffy SW, Agbaje O, Tabar L, Vitak B, Bjurstam N, Bjorneld L, Myles JP, Warwick J (2005) Overdiagnosis and overtreatment of breast cancer: estimates of overdiagnosis from two trials of mammographic screening for breast cancer. Breast Cancer Res 7(6):258–265. doi:10.1186/bcr1354
Moss S (2005) Overdiagnosis and overtreatment of breast cancer: overdiagnosis in randomised controlled trials of breast cancer screening. Breast Cancer Res 7(5):230–234. doi:10.1186/bcr1314
Paci E, Duffy S (2005) Overdiagnosis and overtreatment of breast cancer: overdiagnosis and overtreatment in service screening. Breast Cancer Res 7(6):266–270. doi:10.1186/bcr1339
Kopans DB, Smith RA, Duffy SW (2011) Mammographic screening and “overdiagnosis”. Radiology 260(3):616–620. doi:10.1148/radiol.11110716
Yen MF, Tabar L, Vitak B, Smith RA, Chen HH, Duffy SW (2003) Quantifying the potential problem of overdiagnosis of ductal carcinoma in situ in breast cancer screening. Eur J Cancer 39(12):1746–1754
Collins LC, Achacoso N, Haque R, Nekhlyudov L, Fletcher SW, Quesenberry CP Jr, Schnitt SJ, Habel LA (2013) Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast Cancer Res Treat 139(2):453–460. doi:10.1007/s10549-013-2539-5
Donker M, Litiere S, Werutsky G, Julien JP, Fentiman IS, Agresti R, Rouanet P, de Lara CT, Bartelink H, Duez N, Rutgers EJ, Bijker N (2013) Breast-conserving treatment with or without radiotherapy in ductal carcinoma In Situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 31(32):4054–4059. doi:10.1200/JCO.2013.49.5077
Zhang X, Dai H, Liu B, Song F, Chen K (2015) Predictors for local invasive recurrence of ductal carcinoma in situ of the breast: a meta-analysis. Eur J Cancer Prev. doi:10.1097/CEJ.0000000000000131
Lakhani SR, Ellias IO, Schnitt SJ, Tan PH, van de Vijver MJ (2012) WHO classification of tumours of the breast, 4th edn. IARC, Lyon
Tan PH (2001) Pathology of ductal carcinoma in situ of the breast: a heterogeneous entity in need of greater understanding. Ann Acad Med Singap 30(6):671–676 quiz 677
Thike AA, Iqbal J, Cheok PY, Tse GM, Tan PH (2013) Ductal carcinoma in situ associated with triple negative invasive breast cancer: evidence for a precursor-product relationship. J Clin Pathol 66(8):665–670. doi:10.1136/jclinpath-2012-201428
Thike AA, Cheok PY, Jara-Lazaro AR, Tan B, Tan P, Tan PH (2010) Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol 23(1):123–133. doi:10.1038/modpathol.2009.145
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11(2):155–168
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013. doi:10.1200/JCO.2013.50.9984
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 295(21):2492–2502. doi:10.1001/jama.295.21.2492
Thike AA, Iqbal J, Cheok PY, Chong AP, Tse GM, Tan B, Tan P, Wong NS, Tan PH (2010) Triple negative breast cancer: outcome correlation with immunohistochemical detection of basal markers. Am J Surg Pathol 34(7):956–964. doi:10.1097/PAS.0b013e3181e02f45
Health Promotion Board (2015) BreastScreen Singapore. http://www.hpb.gov.sg/HOPPortal/programmes-article/3324. Accessed 26 Jan 2015
Bijker N, Peterse JL, Duchateau L, Julien JP, Fentiman IS, Duval C, Di Palma S, Simony-Lafontaine J, de Mascarel I, van de Vijver MJ (2001) Risk factors for recurrence and metastasis after breast-conserving therapy for ductal carcinoma-in situ: analysis of European Organization for Research and Treatment of Cancer Trial 10853. J Clin Oncol 19(8):2263–2271
Kong I, Narod SA, Taylor C, Paszat L, Saskin R, Nofech-Moses S, Thiruchelvam D, Hanna W, Pignol JP, Sengupta S, Elavathil L, Jani PA, Done SJ, Metcalfe S, Rakovitch E (2014) Age at diagnosis predicts local recurrence in women treated with breast-conserving surgery and postoperative radiation therapy for ductal carcinoma in situ: a population-based outcomes analysis. Curr Oncol 21(1):e96–e104. doi:10.3747/co.21.1604
Barnes NL, Dimopoulos N, Williams KE, Howe M, Bundred NJ (2014) The frequency of presentation and clinico-pathological characteristics of symptomatic versus screen detected ductal carcinoma in situ of the breast. Eur J Surg Oncol 40(3):249–254. doi:10.1016/j.ejso.2013.12.013
Ma KK, Lau SS, Cheung PS (2013) Ductal carcinoma in situ in Chinese women undergoing opportunistic breast cancer screening. Surg Pract 18(1):8–14. doi:10.1111/1744-1633.12042
Evans AJ, Pinder SE, Ellis IO, Wilson AR (2001) Screen detected ductal carcinoma in situ (DCIS): overdiagnosis or an obligate precursor of invasive disease? J Med Screen 8(3):149–151
Kessar P, Perry N, Vinnicombe SJ, Hussain HK, Carpenter R, Wells CA (2002) How significant is detection of ductal carcinoma in situ in a breast screening programme? Clin Radiol 57(9):807–814
Page DL, Dupont WD, Rogers LW, Jensen RA, Schuyler PA (1995) Continued local recurrence of carcinoma 15–25 years after a diagnosis of low grade ductal carcinoma in situ of the breast treated only by biopsy. Cancer 76(7):1197–1200
Page DL, Dupont WD, Rogers LW, Landenberger M (1982) Intraductal carcinoma of the breast: follow-up after biopsy only. Cancer 49(4):751–758
Sanders ME, Schuyler PA, Dupont WD, Page DL (2005) The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 103(12):2481–2484. doi:10.1002/cncr.21069
Betsill WL Jr, Rosen PP, Lieberman PH, Robbins GF (1978) Intraductal carcinoma. Long-term follow-up after treatment by biopsy alone. JAMA 239(18):1863–1867
Leonard GD, Swain SM (2004) Ductal carcinoma in situ, complexities and challenges. J Natl Cancer Inst 96(12):906–920
Wong JS, Kaelin CM, Troyan SL, Gadd MA, Gelman R, Lester SC, Schnitt SJ, Sgroi DC, Silver BJ, Harris JR, Smith BL (2006) Prospective study of wide excision alone for ductal carcinoma in situ of the breast. J Clin Oncol 24(7):1031–1036. doi:10.1200/JCO.2005.02.9975
Silverstein MJ (2003) The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg 186(4):337–343
Sue GR, Chagpar AB (2015) Predictors of recurrence in patients diagnosed with ductal carcinoma in situ. Am Surg 81(1):48–51
Evans A (2003) The diagnosis and management of pre-invasive breast disease: radiological diagnosis. Breast Cancer Res 5(5):250–253. doi:10.1186/bcr621
Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, Sanchez H, Jimenez C, Stewart K, Chew K, Ljung BM, Tlsty TD (2010) Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst 102(9):627–637. doi:10.1093/jnci/djq101
Provenzano E, Hopper JL, Giles GG, Marr G, Venter DJ, Armes JE (2003) Biological markers that predict clinical recurrence in ductal carcinoma in situ of the breast. Eur J Cancer 39(5):622–630
Roka S, Rudas M, Taucher S, Dubsky P, Bachleitner-Hofmann T, Kandioler D, Gnant M, Jakesz R (2004) High nuclear grade and negative estrogen receptor are significant risk factors for recurrence in DCIS. Eur J Surg Oncol 30(3):243–247. doi:10.1016/j.ejso.2003.11.004
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H (2010) Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 28(10):1684–1691. doi:10.1200/JCO.2009.24.9284
Clark SE, Warwick J, Carpenter R, Bowen RL, Duffy SW, Jones JL (2011) Molecular subtyping of DCIS: heterogeneity of breast cancer reflected in pre-invasive disease. Br J Cancer 104(1):120–127. doi:10.1038/sj.bjc.6606021
Zhou W, Jirstrom K, Johansson C, Amini RM, Blomqvist C, Agbaje O, Warnberg F (2010) Long-term survival of women with basal-like ductal carcinoma in situ of the breast: a population-based cohort study. BMC Cancer 10:653. doi:10.1186/1471-2407-10-653
Zhou W, Jirstrom K, Amini RM, Fjallskog ML, Sollie T, Lindman H, Sorlie T, Blomqvist C, Warnberg F (2013) Molecular subtypes in ductal carcinoma in situ of the breast and their relation to prognosis: a population-based cohort study. BMC Cancer 13:512. doi:10.1186/1471-2407-13-512
Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, Tse CK, Nyante S, Millikan RC (2007) Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol 38(2):197–204. doi:10.1016/j.humpath.2006.08.017
Dabbs DJ, Chivukula M, Carter G, Bhargava R (2006) Basal phenotype of ductal carcinoma in situ: recognition and immunohistologic profile. Mod Pathol 19(11):1506–1511. doi:10.1038/modpathol.3800678
Sprague BL, McLaughlin V, Hampton JM, Newcomb PA, Trentham-Dietz A (2013) Disease-free survival by treatment after a DCIS diagnosis in a population-based cohort study. Breast Cancer Res Treat 141(1):145–154. doi:10.1007/s10549-013-2670-3
Wong FY, Wang F, Chen JJ, Tan CH, Tan PH (2014) Outcomes of low-risk ductal carcinoma in situ in Southeast Asian women treated with breast conservation therapy. Int J Radiat Oncol Biol Phys 88(5):998–1003. doi:10.1016/j.ijrobp.2014.01.018
Yen TW, Hunt KK, Ross MI, Mirza NQ, Babiera GV, Meric-Bernstam F, Singletary SE, Symmans WF, Giordano SH, Feig BW, Ames FC, Kuerer HM (2005) Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: a guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg 200(4):516–526. doi:10.1016/j.jamcollsurg.2004.11.012
Roses RE, Arun BK, Lari SA, Mittendorf EA, Lucci A, Hunt KK, Kuerer HM (2011) Ductal carcinoma-in situ of the breast with subsequent distant metastasis and death. Ann Surg Oncol 18(10):2873–2878. doi:10.1245/s10434-011-1707-2
Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R (2000) Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med 160(7):953–958
Shatat L, Gloyeske N, Madan R, O’Neil M, Tawfik O, Fan F (2013) Microinvasive breast carcinoma carries an excellent prognosis regardless of the tumor characteristics. Hum Pathol 44(12):2684–2689. doi:10.1016/j.humpath.2013.07.010
Parikh RR, Haffty BG, Lannin D, Moran MS (2012) Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys 82(1):7–13. doi:10.1016/j.ijrobp.2010.08.027
Acknowledgments
This study was supported by the Health Services Research Competitive Research Grant, HSRG/0009/2010, from the Ministry of Health, Singapore.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koh, V.C.Y., Lim, J.C.T., Thike, A.A. et al. Characteristics and behaviour of screen-detected ductal carcinoma in situ of the breast: comparison with symptomatic patients. Breast Cancer Res Treat 152, 293–304 (2015). https://doi.org/10.1007/s10549-015-3472-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3472-6