Abstract
Toxicity due to treatment causes a negative impact on quality of life in breast cancer survivors. Hot flash symptoms, described as intense sensations of heat, sweating and flushing occur in more than 50 % of breast cancer patients taking tamoxifen. We hypothesized that venlafaxine, a selective-norepinephrine reuptake inhibitor drug, was effective for reducing patient-reported hot flash scores among women treated for breast cancer compared to other non-hormonal treatments. We searched Medline, Scopus, and Cochrane Central Register of Controlled Trials from inception till May 2015 for venlafaxine (75 mg once daily or greater) with non-hormonal comparators for the treatment of hot flashes in female breast cancer patients. The primary outcome was hot flash score (derived from patient-reported hot flash severity and frequency) in randomized controlled trials. Standardized mean differences (SMD) were calculated for each study due to variation in the outcome measures. Heterogeneity was determined using I 2 statistics, and publication bias was assessed using a contour funnel plot and Egger’s tests. Pooled analyses demonstrated that venlafaxine significantly reduced hot flash scores compared to the trial comparators (overall SMD 2.06; 95 % confidence interval (CI) [0.40, 3.72]). There was significant heterogeneity among these studies (I 2 = 98.7 %, P < 0.001). Asymmetry in the contour funnel plot suggests the presence of publication bias and a trend towards small study effects (Egger’s test, P = 0.096). Venlafaxine is efficacious in managing hot flashes among women with breast cancer. This review highlights methodological issues that arise from eligible trials and recommends a collaborative approach in survivorship studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxicity due to breast cancer treatment affects quality of life. In one study, more than half of the women treated with endocrine therapy for breast cancer reported hot flash symptoms [1]. Hot flashes, classified as vasomotor symptoms, are described as intense sensations of heat associated with sweating and flushing that affect the face and chest [2]. Patients may also experience discomfort due to palpitations and anxiety [3]. The constellation of these symptoms can last between 3 and 10 min, and women can experience several episodes daily. The etiology of these symptoms is complex but may be associated with estrogen withdrawal effects on the hypothalamic thermoregulatory center and other neurotransmitters [4, 5].
In women without breast cancer who suffer from hot flashes, hormone replacement therapy (HRT) is considered effective and remains first-line treatment [6]. Hormonal agents, such as megestrol acetate and a progestogen, demonstrated some benefit in reducing hot flashes and menopausal symptoms among women with breast cancer [7, 8]. However, concerns about the possibility of breast cancer recurrence led to early closure of a randomized clinical trial investigating the use of HRT for hot flash symptoms [9]. Several non-hormonal agents within the anti-depressant and anticonvulsant class have also been investigated for the management of hot flashes [10–12]. Paroxetine is the only FDA approved non-hormonal therapy for hot flashes. [13] However, paroxetine should be used with caution in women taking tamoxifen because of cytochrome P450 2D6 (CYP2D6) inhibition caused by drug–drug interaction, leading to reduced efficacy of tamoxifen [14, 15].
Venlafaxine, a selective-norepinephrine reuptake inhibitor (SNRI), is commonly prescribed in the management of hot flashes for breast cancer patients. Loprinzi et al., had shown reduction of hot flash symptoms in ninety-two participants administered venlafaxine at dose greater than 37.5 mg compared to those administered placebo [12]. Therefore 75 mg of venlafaxine has been used commonly for management of hot flashes. Since this seminal study, there have been several randomized controlled trials designed to investigate hot flash symptoms, comparing venlafaxine to therapeutic agents such as clonidine and acupuncture. Some studies have shown that the comparators are safe and more effective at reducing hot flash symptoms, therefore the effect of venlafaxine is unclear [16, 17]. Furthermore a systematic review demonstrated favorable results of both venlafaxine and gabapentin in the management of hot flashes [18]. The efficacy of venlafaxine alone in management of hot flashes among treated breast cancer patients from the available evidence has not been determined. Therefore, we pooled the available evidence to determine the reduction in symptoms of hot flashes between venlafaxine and comparators amongst patients treated for breast cancer.
Methods
Search strategy and selection criteria
We searched Medline, Scopus, the Cochrane Central Register of Controlled Trials (CENTRAL) (Supplementary Appendix 1) from the inception of each database till January 2014, and this was repeated again in May 2015. We also reviewed conference proceedings, relevant reviews, editorials, meta-analyses, and reference lists of identified reports for randomized or quasi-randomized trials in any language that compared venlafaxine with non-hormonal comparators such as anti-depressants (gabapentin, Selective Serotonin Reuptake Inhibitor (SSRI), amitriptyline), clonidine, vitamin E, and acupuncture. Two investigators screened 47 abstracts independently for potential inclusion followed by review of full text and determined their eligibility in duplicate (Supplementary Appendix 1). To verify our search was current at the time of manuscript submission, we repeated the Medline, Scopus, and CENTRAL searches December 7, 2014 and found no additional relevant trials to include in this report.
Data collection and quality assessment
We extracted characteristics of trials, patients, and interventions, including study design, length of follow-up, components of methodological quality, and source of funding, median age, World Health Organization performance status, treatment with surgery, chemotherapy or hormonal therapy, hot flash scores at baseline, and at intervals stipulated by the studies. As components of methodological quality, we assessed concealment of allocation, blinding of investigators adjudicating clinical events, and the inclusion of all randomized individuals in the analysis according to the intention-to-treat principle. Concealment of allocation was considered adequate if the investigators responsible for the selection of patients did not know before allocation which treatment was next in line (central randomization, sealed, opaque, sequentially numbered assignment envelopes, etc.). Any procedures based on predictable generation of allocation sequences, and potentially transparent attempts to conceal allocation, such as assignment envelopes, which were not opaque or not sealed, were considered inadequate [19]. The analysis was considered to be according to the intention-to-treat principle if all randomized patients were analyzed in the group they were originally allocated to, regardless of the treatment actually received [20]. All data were extracted by one reviewer (RR) and subsequently independently checked by a second reviewer (MV/DMP).
Outcomes
We pre-specified any effects on hot flash score at a dose of 75 mg of venlafaxine or higher as the primary efficacy outcome against a non-hormonal comparator. The once daily dose of 75 mg of venlafaxine was used after the study by Loprinzi et al., which had demonstrated resolution of hot flashes at doses above 37.5 mg [12]. Hot flash scores were derived from prospective studies and consist of frequency multiplied by severity of hot flashes among trial participants who completed a symptom diary [21]. These outcomes were extracted by one of the authors (RR) and independently verified by another author (MV). Data on the outcomes from baseline to end of treatment were extracted from tables or by measurement of graphs in published trials. In addition, factors such as the quality of allocation and concealment of allocation were assessed for each study by two authors using criteria from the Cochrane Handbook (Supplementary Appendix 3) [22].
In the absence of hot flash score, the frequency and severity measurements were multiplied by each other and divided by the number of participants analyzed at the specified time point. Confidence intervals, when reported, were used to derive a standard error and used for deriving standard deviations. Data on hot flash score and frequency from Carpenter et al. and Buijs et al. were requested from the authors directly for analysis. Carpenter et al. provided data that were incorporated into the primary analysis [23, 24]. Data from Buijs et al. were analyzed using data extracted from studies that used the same comparator in a sensitivity analysis (Supplementary Fig. 1) [24]. Studies that were not randomized control trials or did not meet these primary efficacy outcomes, and frequencies were excluded.
Statistical analyses
To establish a standard measure between studies, the percentage change in hot flash score from baseline to the end of venlafaxine or control treatment was obtained. For crossover study designs, study authors randomized between groups that received venlafaxine first or control first. In the analysis of crossover studies, authors averaged the effects of venlafaxine or comparator over time [25]. Therefore the average effect after the venlafaxine phase and control phase of treatment were obtained at the end of the treatment period. The mean change for the participants in the venlafaxine and control groups was used to calculate the standardized mean differences (SMD) [26]. SMD were calculated due to variation in the outcome measures [26]. SMD was derived as follows for comparator (con) and venlafaxine (exp) arms:
Data on 95 % confidence intervals or standard error for the estimates of percentage change in the hot flash score from baseline were used to derive the pooled standard deviation sdpooled.
SMD and pooled SD were analyzed using random effects meta-analysis to obtain a pooled estimate and 95 % confidence interval (95 % CI). Heterogeneity was assessed using I 2 statistics, and publication bias was determined using a contour funnel plot and Egger’s tests [27, 28]. Due to missing hot flash severity data, hot flash scores could not be derived for the Buijs et al. study comparing venlafaxine and clonidine, therefore a sensitivity analysis was performed with participants, extrapolating the standard error and hot flash severity data from Boekhout et al., which had the same comparators (Supplementary Fig. 1) [16, 24]. All data were analyzed using STATA 13.1 (STATA Corp, College Station, TX).
Results
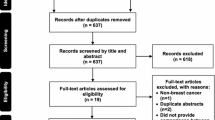
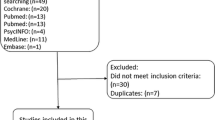
There were 47 references identified in the literature search and there were 15 reports that were eligible as per the inclusion criteria (Supplementary Appendix 1). Nine articles were excluded when full texts were reviewed, as they were ineligible due to study designs, comparators or the publications were editorials on the venlafaxine use. Two studies involved European centers, one study was from Canada and the others were from the United States. The Buijs et al. study did not have the required information to calculate hot flash scores, and the authors did not provide the original data for the pooled analysis in this study. Therefore the final analysis involved five articles describing five trials of venlafaxine versus a non-hormonal comparator with 525 participants randomized to either venlafaxine or comparators [12, 16, 17, 23, 29].
Table 1 describes the characteristics of all eligible trials used for the analysis. The comparators that were used in these trials include clonidine, acupuncture, gabapentin, and placebo agents. Participants in the Boekhout et al. study were randomized to either clonidine, venlafaxine, or placebo and therefore hot flash scores were analyzed between these three arms separately [16]. Four out of five trials had tapered dosing where participants were started on a lower dose of venlafaxine (37.5 mg), and this was increased to 75 mg to improve tolerability, reduce adverse effects, and improve compliance [12]. The median age of participants ranged from 50 to 56 years and duration of follow-up ranged from 4 to 52 weeks. Three studies were crossover trials [23, 24, 29]. Participants within all studies were women who were treated with radiotherapy, chemotherapy, and oral endocrine therapy. Loprinzi et al., and Carpenter et al., conducted trials with varying doses of venlafaxine [12, 23]. Only study arms with doses at 75 mg or greater once a day were analyzed for this study. Data from Loibl et al. was excluded, as the dose of venlafaxine was 37.5 mg twice daily rather than 75 mg once daily (Supplementary Appendix 1) [30].
Figure 1 presents the results of the random-effects meta-analysis assessing the efficacy of venlafaxine to reduce hot flash scores compared to each non-hormonal treatment/placebo (overall SMD 2.06; 95 % confidence interval (CI) [0.40, 3.72]). The results of the meta-analysis were driven by Loprinzi et al. study, where the largest benefit of Venlafaxine was recorded. Here doses of 75 mg (SMD = 4.85, 95 % CI = 4.45, 5.26) and 150 mg (SMD = 4.57, 95 % CI = 4.18, 4.97) were compared to placebo. Clonidine was favorable compared to venlafaxine in reduction of hot flash score compared to baseline (SMD −0.49; 95 % CI [−0.98, −0.01]). This was also observed in the sensitivity analysis where we used estimates from Buijs et al. study where the comparator was clonidine (SMD −0.11; 95 % confidence interval (CI) [−0.52, 0.31], Supplementary Fig. 1). Acupuncture was inferior to venlafaxine (SMD 1.83; 95 % CI [1.07, 2.60]), and this was also seen with gabapentin as a comparator (SMD 0.89; 95 % CI [0.38, 1.39]). The forest plot presents the sample sizes of all studies in this meta-analysis were comparable, and there were no large population studies. There was significant heterogeneity between these studies (I 2 = 98.7 %, P < 0.001).
Effect of venlafaxine and comparator on standardized mean difference in hot flash scores from baseline to completion of treatment in all eligible studies. Forest plot includes in all eligible studies with participants administered venlafaxine (n) and venlafaxine dose in milligrams (mg) against type of comparator. Standardized mean difference is represented as solid square (effect size (ES) with 95 % confidence intervals (CI) as side bars
The contour funnel plot demonstrates asymmetry when reviewing all the studies together (Fig. 2). Three points are within a boundary where the effect size and standard error are significant (P < 1 %). These three points represent data from Boekhout et al. and Loprinzi et al. studies [12, 16]. There are three studies that are within P > 10 % contour, therefore Walker et al., Carpenter et al. and Bordeleau et al., not significant in the funnel plot [17, 23, 29]. There are no studies within significance levels between 1 and 10 % and highlights asymmetry in the contour funnel plot. The asymmetry may be due to publication bias against negative trials. All studies in this meta-analysis have a standard error >0.1. Furthermore, there are few studies that show a negative effect size at a higher significance level. Egger’s test for the absence of small study effects was not significant (P = 0.096) indicating that the effects seen in small studies vary from those estimated in larger studies, which may be due to the instability of the effect sizes in small sizes or reporting bias [31].
Contour funnel plot to highlight the effect estimate and standard error of the studies included in this meta-analysis. A large trial would mean a smaller standard error as a measure of statistical precision, therefore trials scattered in the shape of an inverted funnel indicate the absence of bias with large trials scattering little at the top and small trials scattering at the bottom. The contours distinguish between gray areas of significance where P < 1 %, 1 % < P < 5 %, 5 % < P < 10 % and white areas are P > 10 %
Figure 3 demonstrates the methodological trial characteristics. None of the studies were analyzed as intention-to-treat analysis. Concealment of allocation was unclear among 3 out of 6 studies. Blinding was not feasible in Walker et al., due to the nature of the study design and acupuncture as the comparator, therefore in 5 out of 6 studies, blinding of outcome assessment was unclear or absent. In all 6 studies, incomplete outcome data were not addressed fully as missing data were not equal in all groups, and authors did not comment on the impact of missing data on effect size (Supplementary Appendix 3). The primary outcomes and power calculations were specified and reported in 5 out of 6 studies. Walker et al., did report on primary outcomes stipulated; however, power calculation was not clearly reported. As follow-up and assessment was over 1 year in this trial, the study does not account for the drop out rate in the power calculations.
Figure to demonstrate the bias within eligible studies as noted by authors. Adapted from Stradling et al., and Cochrane Handbook version 5.0 derived criteria (Appendix 3). For each study, the presence (+) and absence (−) of a characteristic are recorded. If the characteristic was not clear in the trial, then it was marked as uncertain (?)
Discussion
This meta-analysis of six randomized trials in 525 breast cancer patients suggests that venlafaxine is superior compared to placebo or other non-hormonal therapies in the management of hot flashes. We also found that clonidine, a selective alpha-agonist licensed in management of blood pressure, demonstrated marginal benefit compared to venlafaxine in the management of hot flashes. This is the first meta-analysis to review venlafaxine alone in treatment of hot flashes in breast cancer and the first to evaluate the methodological quality of existing randomized controlled trials on this crucial issue.
Venlafaxine at a dose of 75 mg is efficacious in treating hot flashes among women treated for breast cancer and should be considered as a first-line treatment. This effect was pronounced in studies comparing venlafaxine and placebo. Acupuncture, a non-therapeutic method of treatment, has shown benefit in reducing hot flash severity and frequency in breast cancer patients [17]. However, in comparison with venlafaxine, a non-blinded study showed that the change of hot flash scores were similar in the two groups over a follow-up period. Administration of acupuncture may not be a service that is readily available to all populations and is an operator-dependent procedure therefore results may vary [32]. This review did not compare the side effect profiles and the impact of these non-hormonal therapies on other symptoms such as insomnia or sexual dysfunction. However, in the study investigating venlafaxine and gabapentin, participants preferred venlafaxine compared to gabapentin, due to fewer adverse events and side effects [29].
Within this meta-analysis, a secondary analysis using imputed data from Buijs et al. also confirmed that clonidine reduced hot flash scores compared to venlafaxine. One trial comparing clonidine to placebo shown a reduction in hot flash symptoms in breast cancer patients [33]. A randomized control trial using transdermal administration of clonidine showed benefit in 55 participants compared to placebo [34]. The mechanism of action is unclear; however, it may reduce the vascular reactivity associated with hot flashes [34]. A meta-analysis reviewing the effect of venlafaxine and gabapentin also demonstrated the potential benefit of clonidine in managing hot flashes in breast cancer patients [18]. Despite the potential benefit of clonidine, venlafaxine does have an immediate impact on reducing hot flash scores compared to clonidine, which may have led to higher discontinuation rates among participants randomized to clonidine in the Boekhout et al. study [16].
The safety of supportive medicines is paramount and it is imperative that these medicines are not associated with increased breast cancer mortality. Paroxetine is a drug within the selective serotonin reuptake inhibitors (SSRI) and is licensed for hot flashes in breast cancer. However, this class inhibits CYP2D6 and among a cohort of women administered SSRI drugs in combination with tamoxifen there was an increase in breast cancer related morality due to reduced efficacy of tamoxifen [35, 36]. Venlafaxine is least likely to interfere with CYP2D6 activity and therefore provides a safe and effective alternative in breast cancer patients undergoing tamoxifen treatment [37].
Our meta-analysis demonstrates high heterogeneity, indicating substantial differences among trials. These differences can be explained in conjunction with varying study methodological quality of individual trials and lack of standardized measurements of hot flash symptom scores in all trials. The effect of crossover study design in the interpretation of hot flash scores is also uncertain as the effect of multiple therapies even in the presence of the washout period may have an effect on subjective measurement tools [25]. Hot flash measurement tools, which are used in the majority of studies included in this meta-analysis, are subjective and have been validated in the North American population; there may be differences in reporting symptoms in breast cancer patients in Boekhout et al. study where participants were from Europe [16, 21, 24]. None of the studies use the intention-to-treat analysis, thereby leading to increased confounding and reducing the validity of results. Due to uneven attrition rates, per protocol analysis would likely result in underpowered analyses in these studies.
This report also highlights study design issues in trials on cancer survivorship issues, due to the heterogeneity in trial methodology and outcome assessment used between different trials. This is an important issue to consider with the rising prevalence of cancer survivors and the absence of clear guidelines on management of symptoms after cancer treatment. With a high incidence of these hot flash symptoms in this population, it is important that investigators consider a multi-center collaborative model that is commonly used in conventional cancer therapy trials or as was used in the MsFLASH research network trials investigating hot flash interventions in midlife women without cancer [38]. This would allow adequately powered studies and improve the internal validity of trials within this setting.
In conclusion, venlafaxine should be considered an appropriate first-line treatment in the management of hot flash symptoms in women with breast cancer.
References
Couzi RJ, Helzisouer KJ, Fetting JH (1995) Prevalence of menopausal symptoms among women with a history of breast cancer and attitudes toward estrogen replacement therapy. J Clin Oncol 13(11):2737–2744
Stan D, Loprinzi CL, Ruddy KJ (2013) Breast cancer survivorship. Hematol Oncol Clin North Am 27(4):805–827
Kronenberg F (1994) Hot flashes: phenomenology, quality of life, and search for treatment options. Exp Gerontol 29(3–4):319–336
Hickey M, Saunders CM, Stuckey BG (2005) Management of menopausal symptoms in patients with breast cancer: an evidence-based approach. Lancet oncol 6(9):687–695
Rance NE, Dacks PA, Mittleman-Smith MA et al (2013) Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot fluhes. Front Neuroendocrinol 34(3):211–227
Krause MS, Nakajima ST (2015) Hormonal and nonhormal treatment of vasomotor symptoms. Obstet Gynecol Clin North Am 42(1):163–179
Quella SK, Loprinzi CL, Sloan JA et al (1998) Long term use of megestrol acetate by cancer survivors for the treatment of hot flashes. Cancer 82(9):1784–1788
Goodwin JW, Green SJ, Moinpour CM et al (2008) Phase III randomized placebo-controlled trial of two doses of megestrol acetate as treatment for menopausal symptoms in women with breast cancer: Southwest Oncology Group Study 9626. J Clin Oncol 26(10):1650–1656
Holmberg L, Anderson H, For the HABITS Steering and Data Monitoring Committees (2004) HABITS (hormonal replacement therapy after breast cancer–is it safe?), a randomised comparision: trial stopped. Lancet 363:453–455
Stearns V, Isaacs C, Rowland J et al (2000) A pilot trial assessing the efficacu of paroxetine hydrocholride (Paxil) in controlling hot flashes in breast cancer survivors. Ann Oncol 11:17–22
Pandya KJ, Thummala AR, Griggs JJ et al (2004) Pilot study using gabapentin for tamoxifen-induced hot flashes in women with breast cancer. Breast Cancer Res Treat 83:87–89
Loprinzi CL, Kugler JW, Sloan JA et al (2000) Venlafaxine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet 356:2059–2063
Weber L, Thacker HL (2014) Paroxetine: a first for selective serotonin reuptake inhibitors—a new use: approved for vasomotor symptoms in postmenopausal women. Womens Health (London Engl) 10(2):147–154
Jin Y, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97(1):30–39
Binkhorst L, Mathijssen RH, van Herk-Sukel MP et al (2013) Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen. Breast Cancer Res Treat 139(3):923–929
Boekhout AH, Vincent AD, Dalesio OB et al (2011) Management of hot flashes in patients who have breast cancer with Venladaxine and Clonidine: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 29:3862–3868
Walker EM, Rodriguez AI, Kohn B et al (2010) Acupuncture versus venlafaxine for the management of vasomotor symptoms in patients with hormone receptor-positive breast cancer: a randomized controlled trial. J Clin Oncol 28:634–640
Yamaguchi N, Okajima Y, Fujii T et al (2013) The efficacy of nonestrogenic therapy to hot flashes in cancer patients under hormone manipulation therapy: a systematic review and meta-analysis. J Cancer Res Clin Oncol 139:1701–1707
Juni P, Altman DG, Egger M (2001) Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 323:42–46
Hollis S, Campbell F (1999) What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 319:670–674
Sloan JA, Loprinzi CL, Novotny PJ et al (2001) Methodologic lessons learned from hot flash studies. J Clin Oncol 19(23):4280–4290
Stradling C, Chen YF, Russell T et al (2012) The effects of dietary intervention on HIV dyslipidaemia: a systematic review and meta-analysis. PLoS One 7(6):e38121
Carpenter JS, Stomiolo AM, Johns S et al (2007) Randomized, double-blind, placebo-controlled crossover trials of venlafaxine for hot flashes after breast cancer. Oncologist 12:124–135
Buijs C, Mom CH, Willemse PHB, Boezen HM et al (2009) Venlafaxine versus clonidine for the treatment of hot flashes in breast cancer patients: a double-blind, randomized cross-over study. Breast Cancer Res Treat 115:573–580
Elbourne DR, Altman DG, Higgins JPT et al (2002) Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 31(1):140–149
Da Costa BR, Rutjes AWS, Johnston BC et al (2012) Methods to convert continuous outcomes into odds ratios of treatment response and numbers needed to treat: meta-epidemiological study. Int J Epidemiol 41(5):1445–1459
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Nüesch E, Trelle S, Reichenbach S et al (2010) Small study effects in meta-analyses of osteoarthritis trials: a meta-epidemiological study. BMJ 341:c3515
Bordeleau L, Pritchard KI, Loprinzi CL et al (2010) Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol 28:5147–5152
Loibl S, Schwedler K, von Minckwitz G et al (2007) Venlafaxine is superior to clonidine as treatment of hot flashes in breast cancer patinets—a double-blind, randomized study. Ann Oncol 18:689–693
Harbord RM, Harris RJ, Sterne JAC (2009) Updated tests for small-study effects in meta-analyses. Stata J 9(2):197–210
Haddad NE, Palesh O (2014) Acupuncture in the treatment of cancer-related psychological symptoms. Integr Cancer Ther 13(5):371–385
Pandya KJ, Raubertas RF, Flynn PJ et al (2000) Oral clonidine in postmenopausal patients with breast cancer experiencing tamoxifen-induced hot flashes: a University of Rochester Cancer Center Community Clinical Oncology Program study. Ann Intern Med 132:788–793
Goldberg RM, Loprinzi CL, O’Fallon JR et al (1994) Transdermal clonidine for ameliorating tamoxifen-induced hot flashes. J Clin Oncol 12:155–158
Kelly CM, Juurlink DN, Gomes T et al (2010) Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ 8(340):c693
Stearns V, Johnson MD, Rae JM et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Henry NL, Stearns V, Flockhart DA et al (2008) Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am J Psychiatry 165:1251–1255
Newton KM, Carpenter JS, Guthrie KA et al (2014) Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause 21(1):45–58
Conflict of interest
Dr. Kalesan is employed by PPD (Contract research organization) after this study was completed. This study was not done at PPD. This does not pose a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramaswami, R., Villarreal, M.D., Pitta, D.M. et al. Venlafaxine in management of hot flashes in women with breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat 152, 231–237 (2015). https://doi.org/10.1007/s10549-015-3465-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3465-5