Abstract
Since the rate of persistence to adjuvant endocrine therapy such as 5-year aromatase inhibitors (AI) would decrease over time in patients with hormone-sensitive breast cancer, it is necessary to investigate if a patient support program could modify patients’ beliefs and improve their persistence to AI treatment. This was a prospective, multicenter, controlled, observational study to evaluate the efficacy of a patient support program in improving postmenopausal patients’ persistence to adjuvant AI medication for early stage breast cancer (NCT00769080). The primary objective was to compare the rates of 1-year persistence to upfront adjuvant AI for patients in the two observational arms (standard treatment group and standard treatment plus patient support program group). In this study, 262 patients were enrolled in the standard treatment group and 241 patients in the standard treatment plus patient support program group. The mean 1-year persistence rates were 95.9 and 95.8 % for the standard treatment group and the standard treatment plus patient support program group, respectively (P = 0.95). The mean times to treatment discontinuation were 231.2 days in the standard treatment group and 227.8 days in the standard treatment plus patient support program group, with no statistically significant difference between the two groups (P = 0.96). There was also no statistically significant difference in the reason for treatment discontinuation (P = 0.32). There was a significant relationship between the patient centered care questionnaire and poor persistence (odds ratio = 3.9; 95 % CI, 1.1–13.7; P = 0.035), suggesting that the persistence rate of patients with whom the doctor always or usually spends time is greater than that of patients with whom the doctor sometimes or never spends time. Patients’ persistence to adjuvant AI medication for postmenopausal, early stage breast cancer is relatively high in the first year and is not significantly increased by adding a patient support program to standard treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The long-term use of medication raises persistence issues [1], and the available evidence indicates patients who are prescribed oral anti-neoplastic agents have trouble adhering to treatment regimens [2]. The rate of persistence to adjuvant tamoxifen for early stage breast cancer ranges from 25 to 96 % [3–5] and decreases over time [4, 6]. A similar trend has been observed for the initial adjuvant anastrozole, which is prescribed to women with early stage breast cancer [7]. Across diseases, persistence has been cited as one of the most important modifiable factors that influences treatment outcomes. Non-persistence may result in unnecessary costs, increased morbidity, and increased mortality [8]. Therefore, efforts should be made to improve patient persistence to adjuvant hormonal therapy for early stage breast cancer. The World Health Organization acknowledges the necessity of supporting patients’ self-management efforts because the major barrier to persistence is a lack of information. A recent European patient survey demonstrated that patients need more information about all the treatment options available to them, need to understand how different endocrine therapies work, and need to understand the predicted side-effects [9].
Currently, there are two major compliance evaluation programs using information or educational material aimed at improving the persistence to upfront adjuvant aromatase inhibitors (AI) for postmenopausal women with hormonally sensitive, early stage breast cancer. In the European observational study compliance of aromatase inhibitors in assessment daily practice through educational approach (CARIATIDE, NCT00681122), the impact of educational material on women’s compliance and persistence rates was evaluated [10]. Another randomized compliance evaluation program named patient’s anastrozole compliance therapy program (PACT, NCT00555867) addressed the influence of informational material on compliance and the persistence rates of adjuvant anastrozole treatment in clinical practice [11].
The upfront use of AI as a 5-year adjuvant has been established as a standard of care for postmenopausal, hormone-sensitive, early stage breast cancer; however, the issue of persistence to upfront adjuvant AI is also emerging. Currently, the patient support program adapted to Chinese patients’ needs is being established with one major objective: to improve persistence to upfront adjuvant AI. It is necessary to investigate if the patient support program could modify patients’ beliefs and change their behavior, thereby improving their treatment persistence. Thus far, there are few Chinese findings available on this topic. Given the differences in social, cultural, and economic situations, as well as differences in the health-care systems, caution should be paid when directly applying research results from developed countries to China. Here, we report on a prospective, multicenter, controlled, observational study to evaluate the efficacy of a patient support program in improving patients’ persistence to adjuvant AI medication prescribed to postmenopausal patients with early stage breast cancer (NCT00769080). Because the results from the studies with tamoxifen [4] and anastrozole [7] suggest that the persistence issue appeared to be the most critical during the first year following adjuvant hormonal therapy initiation, we believe the first year would provide the ideal time frame to assess the efficacy of our patient support program in improving patients’ persistence.
Patients and methods
Study design and objects
This is a prospective, multicenter, controlled, observational study to evaluate the efficacy of the patient support program in improving patients’ persistence to upfront adjuvant AI for postmenopausal, early stage breast cancer. Eligible patients from ten sites covered by the patient support program constituted the standard treatment plus patient support program arm. Patients from the other ten sites with a matched location and comparable medical care quality but without the systematic patient support program were enrolled as the standard treatment arm. Because patients in the same site may have had communication with each other and a patient’s persistence to treatment might be affected by her surrounding patients, all the patients in one site were enrolled on the same arm to minimize the influence of inter-patient communication. The investigators collected the following information at recruitment: socio-demographic and economic profile, disease and treatment profile, and the date of the initiation of upfront AI. One year after the initiation of the upfront AI prescription, records of AI prescription refills were collected, information on non-persistence was recorded, and the number of tablets remaining with the patient was noted. The patients were also asked to complete the patient centered care questionnaire (PCCQ). The follow-up period was approximately 1 year, unless there was evidence of recurrence or death or withdrawal of informed consent. The subjects who stopped hormonal therapy or were lost to follow-up were categorized as premature discontinuation, and the time and reason for premature discontinuation were documented. In cases where the patients recalled interruptions in medication use, the reasons for interruption were collected. If the patients switched to other endocrine agents, the time and reason for switching were recorded.
The primary objective was to compare the rates of 1-year persistence to upfront adjuvant AI for postmenopausal, early stage breast cancer patients in two observational arms, the standard treatment group and the standard treatment plus patient support program group. The secondary objectives were to explore potential predictors for poor persistence to upfront adjuvant AI medication. The persistence rate of the upfront adjuvant AI medication after 1 year was defined as the proportion of days covered by prescription refills for AI over the 364 days following the initiation of AI prescription. Poor persistence was defined as a persistence rate after 1 year below 80 %. The protocol was reviewed and approved by the independent Ethical Committee and Institutional Review Board of Shanghai Cancer Center, Fudan University. All the patients provided written informed consent before inclusion in this study.
Eligibility
Postmenopausal women who have been prescribed upfront adjuvant AI medication (anastrozole or letrozole) for hormone-sensitive, early stage breast cancers were candidates for this study. The upfront AI medication taken before enrollment in this study must not have exceeded 8 weeks. For inclusion into this observational study, the subjects must have fulfilled all the following criteria: provided signed and dated written informed consent, taking upfront AI adjuvant therapy, capable of completing drug intake by herself, and capable of understanding a Chinese questionnaire. The exclusion criteria included using upfront adjuvant hormonal therapy with an AI to which an upfront adjuvant indication has not been granted by the state food and drug administration (SFDA) of China, taking upfront adjuvant AI medication for over 8 weeks, previous adjuvant hormonal therapy other than an AI for breast cancer lasting over 8 weeks, involvement in the planning and conduct of the study (e.g., staff at the study site), participation in any other clinical study addressing hormonal treatments, and patients who, for whatever reason, were unlikely to comply with the study requirements.
The subjects could withdraw from the study at any time. The specific reasons for terminating a subject’s participation in this study were as follows: (1) voluntary withdrawal by the subject who was free to discontinue her participation in the study at any time without prejudice to her further treatment; (2) severe non-compliance to the study protocol as judged by the investigator; (3) incorrect enrollment (i.e., the subject did not meet the required inclusion/exclusion criteria); (4) lost to follow-up; and (5) patient no longer treated with an adjuvant upfront AI.
Patient support program
The patient support program in the present study consisted mainly of educational support material and a follow-up reminder service. The educational material included a welcome package and quarterly newsletters, which provided information about early stage breast cancer and treatments, with a focus on the persistence issue of upfront adjuvant hormonal therapy. The welcome package consisted mainly of a booklet that contains disease information, an introduction to the program, and a 10-year follow-up reminder card. The follow-up service consisted of reminder calls to the patients and a programmed automatic reminder installed on the sites’ computers for physicians. The patient support program was stopped if breast cancer recurrence, death, withdrawal of informed consent, or hormonal therapy discontinuation were documented. The welcome package was distributed by investigators to the subjects after having obtained written informed consent.
Statistics
The sample size was calculated according to the anticipated persistence difference (6 %) between the standard treatment group (89 %) and the standard treatment plus patient support program group (95 %) at 1 year. A minimum of 250 assessable patients in each group were required for a one-sided significance level of 0.05 with 80 % power in a superiority design (i.e., the patient support program group might be better). Considering a 5 % withdrawal rate, we needed 262 patients for each arm. Thus, 524 postmenopausal women diagnosed with hormone-sensitive early breast cancer and prescribed upfront AI medication were enrolled across 20 sites (ten sites in each arm). Continuous variables were presented as the mean/median and analyzed by Student’s t test; categorical variables were presented as the frequency and percentage and analyzed by Pearson’s χ2 test. Two-sided tests were used with a significance level of 0.05, and all confidence intervals (CI) are 95 % unless otherwise specified.
Results
Patients’ characteristics
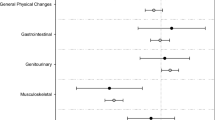
The first study subject was enrolled on September 26, 2008, and the last scheduled patient visit was completed on October 27, 2010. In this study, 516 patients were initially screened, 264 were enrolled in the standard treatment group, and 252 were enrolled in the standard treatment plus patient support program group. Finally, 262 of 264 patients in the standard treatment group and 241 of 252 patients in the standard treatment plus patient support program group were included into intent-to-treat (ITT) analysis set (see the CONSORT diagram in Fig. 1). The demographic and baseline characteristics are shown in Table 1. The mean ages of the standard treatment group and standard treatment plus patient support program group were 59.5 and 59.3 years, respectively (P > 0.05). There was a statistically significant difference in education level and occupation between the two groups (P < 0.0001), but not for risk of recurrence and the treatment modalities (all P > 0.05).
One-year persistence rate to upfront adjuvant AI
ITT analysis showed that the mean persistence rates were 95.9 and 95.8 % for the standard treatment group and the standard treatment plus patient support program group, respectively (P = 0.95), indicating no statistically significant difference in 1-year persistence rates between the two groups (Table 2).
Time to and reason for treatment discontinuation
In the ITT population, the mean times to treatment discontinuation were 231.2 days in the standard treatment group and 227.8 days in the standard treatment plus patient support program group, with no statistically significant difference between the two groups (P = 0.96) (Table 3). There was also no statistically significant difference in the reason for treatment discontinuation (P = 0.32).
Association of patients’ persistence at 1-year with PCCQ scores
The question in part-9 of the PCCQ is “In the last 12 months, how often did this doctor spend enough time with you?” The optimal care responses were “always” and “usually,” and the sub-optimal care responses were “sometimes” and “never.” There was a statistically significant relationship between this PCCQ question and poor persistence (odds ratio = 3.878; 95 % CI, 1.10–13.69, P = 0.035), suggesting that the persistence rate of patients with whom the doctor always or usually spends time is greater than that of the patients with whom the doctor sometimes or never spends time.
Discussion
The results of this observational trial showed that there was no statistically significant difference in 1-year persistence rates between the two compared groups, with a persistence rate of 95.9 % in the standard treatment group and 95.8 % in the standard treatment plus patient support program group. These findings suggest that patients’ persistence to adjuvant AI medication for postmenopausal, early stage breast cancer in the first year is relatively high and is not significantly affected by adding patient support program to the standard treatments.
The mean times to treatment discontinuation of upfront adjuvant AI medication in the standard treatment group and the standard treatment plus patient support program group were similar. Furthermore, there was no statistically significant difference in the reason for treatment discontinuation between the two groups. The most common reason for treatment discontinuation was disease recurrence for the standard treatment group and informed consent withdrawal for the standard treatment plus patient support program group. No patient withdrew from study because of adverse events. Our study in a Chinese population had results similar to outcomes from two other larger prospective studies. One study is the PACT [11], which evaluated the influence of a standardized information service on compliance in postmenopausal women with early stage breast cancer. PACT is a prospective, randomized, two-arm parallel-group trial in Germany. Women on anastrozole for hormone receptor-positive disease were randomized, and the primary endpoints were compliance and persistence rates after 12 months. In this study, 4,924 women were enrolled, with an average age of 64.7 years. After 12 months, there was no difference between the standard and educational arm in compliance; however, compliance was higher in patients who had no adverse events (P < 0.001) and who regularly attended follow-up visits (P < 0.001). The other global observational study CARIATIDE [10] randomized 2,758 patients to the standard therapy group and the standard therapy plus educational materials group. The 2-year follow-up results, which reconfirmed their findings at the 1-year follow-up, showed no statistically significant difference in compliance with AI therapy between the two groups (82 and 82 %, respectively, P = 0.99). Over the 2-year follow-up, AI treatment discontinuation rates of 8 and 9 % were observed in the standard therapy group and the standard therapy plus educational materials group, respectively, with discontinuation more frequently attributed to AI-related side effects. The investigator concluded that at the 2-year follow-up, the educational materials did not appear to improve compliance with the initial AI. In addition, in our study, an analysis of the relationship between scores on the patient centered care questionnaire (PCCQ) and patient persistence after 1-year indicated that the persistence rates of the patients with whom doctors always or usually spend time was greater than that of the patients with whom doctors sometimes or never spend time.
China is different from western countries in its social, cultural, and economic environments, as well as in its health-care systems. Studies evaluating persistence in oncology patients are very rare in China, and relevant information is limited. Although the results of the present study help us to better understand the persistence behaviors of hormone receptor-positive early breast cancer patients in China, the limitations of this study, such as a short-term follow-up time, limited sample size, and the influence of China’s unique medical insurance, should also be considered. Larger and longer-term studies are needed to further validate our present findings in the Chinese population.
References
Claxton AJ, Cramer J, Pierce C (2001) A systematic review of the associations between dose regimens and medication compliance. Clin Ther 23:1296–1310
Partridge AH, Avorn J, Wang PS, Winer EP (2002) Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst 94:652–661
Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA (2006) Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 99:215–220
Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ (2007) Early discontinuation of tamoxifen: a lesson for oncologists. Cancer 109:832–839
Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA (2004) Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor-positive breast cancer. J Clin Oncol 22:3309–3315
Partridge AH, Wang PS, Winer EP, Avorn J (2003) Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol 21:602–606
Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A (2008) Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 26:556–562
DiMatteo MR (1994) Enhancing patient adherence to medical recommendations. J Am Med Assoc 271(79):83
Wengstrom Y, Aapro M, di Priolo SL, Cannon H, Georgiou V (2007) Patients’ knowledge and experience of adjuvant endocrine therapy for early breast cancer: a European study. Breast 16:462–468
Arican A, Ozaslan C, Benekli M, Gulcelik M, Sevinc A, Altundag M, Bese N (2011) The impact of educational material (EM) on compliance and persistence rates with adjuvant aromatase inhibitor (AI) treatment: first year data from Turkish arm of the CARIATIDE study. San Antonio breast cancer symposium P5-08-02. University of Texas Health Science Center, San Antonio
Lück H, Hadji P, Harbeck N, Jackisch C, Blettner M, Glados M, Terhaag J, Hackenberg R, Goehler T, Zaun S, von Fircks AR, Kreienberg R (2011) 24-month follow-up from the patient’s anastrozole compliance to therapy (PACT) program evaluating the influence of a standardized information service on compliance in postmenopausal women with early breast cancer. J Clin Oncol 29:s526
Acknowledgments
This research is supported by grants from the National Natural Science Foundation of China (30971143, 30972936, 81001169, 81102003), the Shanghai United Developing Technology Project of Municipal Hospitals (SHDC12010116), the Key Clinical Program of the Ministry of Health (2010–2012), the Zhuo-Xue Project of Fudan University, and the Shanghai Committee of Science and Technology Fund for 2011 Qimingxing Project (11QA1401400). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors have declared that no competing interests exist.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ke-Da Yu, Ying Zhou, Guang-Yu Liu and Bin Li are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, KD., Zhou, Y., Liu, GY. et al. A prospective, multicenter, controlled, observational study to evaluate the efficacy of a patient support program in improving patients’ persistence to adjuvant aromatase inhibitor medication for postmenopausal, early stage breast cancer. Breast Cancer Res Treat 134, 307–313 (2012). https://doi.org/10.1007/s10549-012-2059-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2059-8