Abstract
Thymidylate synthase (TYMS), which catalyzes the conversion of deoxyuridine monophosphate to deoxythymidine monophosphate, is a central enzyme in the folate metabolic pathway. Epidemiological studies have evaluated the association between TYMS gene polymorphisms and breast cancer susceptibility; however, the published data are still inconclusive. To derive a more precise assessment of this relationship, we performed a meta-analysis based on currently available data by searching PubMed, EMBASE databases, and the Cochrane Library. A total of 10 eligible studies were identified for the TYMS TSER polymorphism (six studies with 2,718 cases and 3,423 controls) and for the TYMS TS3′-UTR polymorphism (five studies with 1,969 cases and 2,290 controls). The overall odds ratio (OR) and the corresponding 95% confidence interval (CI) showed a statistical association between the TSER polymorphism and breast cancer risk under homozygote comparison (2R/2R vs. non-2R/non-2R; OR 1.25; 95% CI 1.04–1.50), allele contrast (2R vs. non-2R; OR 1.09; 95% CI 1.01–1.19) and the recessive model (OR 1.19; 95% CI 1.01–1.39). In the subgroup analysis by ethnicity, a statistically significant increase in cancer risk was found among Caucasians for homozygote comparison (OR 1.31; 95% CI 1.10–1.57), the allele contrast model (OR 1.12; 95% CI 1.02–1.23) and the dominant model (OR 1.40; 95% CI 1.00–1.95). For the TS3′-UTR polymorphism, significant effects were shown using the allele contrast model (OR 1.33; 95% CI 1.03–1.73). However, the TS3′-UTR polymorphism increased breast cancer risk among Asian women (del6 vs. ins6; OR 1.41; 95% CI 1.01–1.98) but not Caucasian women using the homozygote comparison. In conclusion, our meta-analysis suggests that the TSER polymorphism may increase susceptibility to breast cancer in the Caucasian population and the TS3′-UTR polymorphism may be a genetic determinant for developing breast cancer in the Asian population; therefore, ethnic background should be carefully considered in further studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the second leading cause of death by cancer in women and is the most common cancer among women across the world [1]. Some epidemiological studies indicate that various types of genetic damage induced by endogenous metabolites and exogenous hazards may play an important role in the development of breast cancer [2]. Folate is an important nutrient required for DNA synthesis, and it is also involved in the methionine metabolic pathway, which is crucial for DNA methylation, DNA synthesis, and DNA repair [3]. Several large, prospective epidemiological studies have implicated folate deficiency in the development of several cancers [4–8].
Many key enzymatic regulators are involved in folate metabolism. Among them, thymidylate synthase (TYMS) catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP) in DNA synthesis, using 5, 10-methylenetetrahydrofolate as a methyl donor. This process is essential for the synthesis of thymidine, a nucleotide needed for DNA synthesis and repair [9]. TYMS is also the target for the widely used chemotherapeutic agent 5-fluorouracil (5-FU) [10]. Recently, it has been shown that functional polymorphisms in the TYMS gene may contribute to breast cancer risk via effects on nucleotide synthesis. This is particularly true if the genetic variation is combined with low levels of folate or coenzymes of folate metabolism (vitamins B12 and B6) [8]. Therefore, genetic alterations in TYMS enzyme efficiency and/or expression level may result in individual susceptibility to breast and other cancers.
A tandem-repeat polymorphism has been identified in the TYMS promoter enhancer region (TSER), which contains triple (3R) or double (2R) repeats of a 28-bp sequence and several rare alleles containing 4, 5, or 9 repeats [11]. In vitro and in vivo studies show that TYMS expression is TSER genotype-dependent and that the 3R allele is associated with an increase in TYMS expression [12]. Recently, a novel, potentially functional polymorphism within the 3′-UTR of the TYMS gene has also been identified, which consists of a 6-bp deletion/insertion (del6/ins6) at nucleotide 1494 of the TYMS mRNA (TS3′-UTR). This polymorphism may affect RNA stability and reduce TYMS protein expression [13].
Considering the potential influence of altering TYMS activation on folate metabolism, many epidemiological studies have explored the association between these two TYMS variants and increased breast cancer risk development [14–23]. However, the results remain inconclusive, which may be due to limitations in individual studies. Therefore, we performed a meta-analysis of 10 published case–control studies covering 4,443 cases and 5,447 controls to obtain a more precise estimation of the relationship between TYMS polymorphisms and breast cancer risk.
Methods
Search strategy
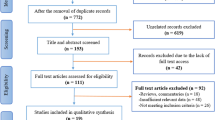
We searched the literature from PubMed, EMBASE, and the Cochrane Library to identify relevant and available published articles. The keywords and subject terms “thymidylate synthase,” “TYMS,” “TS,” “polymorphism,” and “breast cancer’’ and combinations of these phrases were used for the search (last search: July 31, 2010). The language that the papers were written in was not restricted. All eligible studies were retrieved, and their bibliographies were checked for other relevant publications. Review articles and bibliographies of other identified, relevant studies were searched by hand for additional eligible studies. Only published studies with full text articles were included. The following criteria were used to select the eligible studies: (a) a case–control study on the association between TYMS polymorphisms and breast cancer risk, (b) an available genotype or allele frequency, (c) number of different genotypes for estimating an odds ratio (OR) with a 95% confidence interval (CI), and (d) a genotype distribution among the control populations consistent with Hardy–Weinberg Equilibrium (HWE). When authors reported two or more publications on possibly the same patient populations, only the most recent or complete study was included in the review to avoid overlap between the cohorts.
Data extraction
Two reviewers (J. Wang and J. Bi) independently evaluated the final articles selected for further meta-analysis. Disagreements were resolved by discussions between the two authors. If they could not reach a consensus, a third investigator (B. Wang) resolved the disagreements. Reviews, non-original articles, non-case–control studies, and studies involving breast cancer cells and animal models were excluded from this meta-analysis. Data retrieved from the reports included the following: first author’s name, publication year, country of origin, source of controls, racial decent of the study population (categorized as Caucasian and Asian descents), genotyping method, eligible and genotyped cases and controls, the number of cases and controls for each TYMS single-nucleotide polymorphism (SNP) genotype, and the minor allele frequency for the controls. In each study, we did not define a minimum number of patients for inclusion in this meta-analysis.
Statistical methods
Crude ORs and their corresponding 95% CIs were used to assess the strength of association between the studied TSER polymorphism and breast cancer susceptibility. Five different ORs were calculated using the following models: the dominant genetic model (2R/2R + non-2R/2R vs. non-2R/non-2R), the recessive genetic model (2R/2R vs. non-2R/non-2R + non-2R/2R), the homozygote comparison (2R/2R vs. non-2R/non-2R), the allele contrast model (2R vs. non-2R), and the non-2R/2R vs. non-2R/non-2R comparison. The same methods were applied to the analysis of the TS3′-UTR polymorphism.
The χ2-based Q statistic was used to investigate the degree of heterogeneity between the studies, and a P value <0.1 was interpreted as significant heterogeneity among the studies [24]. The I 2 index expresses the percentage of the total variation across studies due to heterogeneity. I 2 values of 25, 50, and 75% were used as evidence of low, moderate, and high heterogeneity, respectively. When there was no statistical heterogeneity, we used a fixed-effects model (Mantel–Haenszel method). If heterogeneity was present, we used a random-effects model (DerSimonian–Laird method) to account for inter-study heterogeneity. HWE was tested by the χ2 test. All statistical analyses were performed using Review Manage (Version 5.0; Oxford, England).
Results
Study characteristics
A total of 10 studies (4,443 cases and 5,447 controls) met the inclusion criteria (Table 1). In these studies, a total of four TYMS polymorphisms were reported, and the most studied genetic variants were TSER and TS3′-UTR; however, one study did report the association between rs16948322 or rs2298581 and breast cancer risk [20]. Therefore, our meta-analysis focused on the two genetic variants TSER and TS3′-UTR. A total of nine studies were included in the meta-analysis for the TSER (2,718 cases and 3,423 controls) [15, 16, 18, 21–23] and TS3′-UTR polymorphisms (1,969 cases and 2,290 controls) [14, 17–19, 23]. These studies were published between 2004 and 2010. All studies were carried out in various ethnic populations, including four studies in Asian populations [17, 20, 21, 23] and six studies in Caucasian populations [14–16, 18, 19, 22]. The main characteristics of these studies are summarized in Tables 2 and 3 for the TSER and TS3′-UTR polymorphisms, respectively. Several genotyping methods were used, and they included the polymerase chain reaction-restriction fragment-length polymorphism (PCR-RFLP), the TaqMan assay, and the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). Except for two studies, the genotype distributions in the study controls were in agreement with HWE [14, 18].
Meta-analysis
The results of this meta-analysis are shown in Table 4. As there was no statistical heterogeneity for the TSER polymorphism (P = 0.67, I 2 = 0), we used a fixed-effects model. The 2R allele was significantly associated with breast cancer risk compared to the non-2R allele (OR 1.09; 95% CI 1.01–1.19). Breast cancer risks were also significantly increased using the homozygote comparison (OR 1.25; 95% CI 1.04–1.50) and the recessive model (OR 1.19; 95% CI 1.01–1.39). No significant association was found between the TSER polymorphism and breast cancer risk in the overall population using the dominant model (OR 1.05; 95% CI 0.92–1.19) or the 2R/non-2R vs. non-2R/non-2R comparison (OR 1.23; 95% CI 0.91–1.65). Whether genotype frequencies in the control population were consistent with HWE or not, when all subjects were included, we also found significant associations between the TSER polymorphism and breast cancer risk under allele contrast (OR 1.13; 95% CI 1.05–1.23) and homozygote comparison models (OR 1.30; 95% CI 1.10–1.53).
In the subgroup analysis by ethnicity, the differences between the allele, homozygote, recessive, and dominant models were insignificant in the Asian population. However, significantly increased risks were observed among Caucasian women (allele contrast: OR 1.13; 95% CI 1.02–1.25; 2R/2R vs. non-2R/non-2R comparison: OR 1.26; 95% CI 1.03–1.53; and dominant model: OR 1.17; 95% CI 1.00–1.36).
For the TS3′-UTR polymorphism, the association between this genetic variant and breast cancer risk was significant under homozygote comparison (OR 1.33; 95% CI 1.03–1.73). We found no significant associations between this genetic variant and breast cancer risk in the other comparison models tested (allele contrast: OR 1.07; 95% CI 0.96–1.19; for ins6/del6 vs. ins6/ins6: OR 1.26; 95% CI 0.85–1.86; recessive model del6/del6 vs. (ins6/ins6 + ins6/del6): OR 0.93; 95% CI 0.71–1.22; and dominant model del6/del6 + ins6/del6 vs. ins6/ins6: OR 1.03; 95% CI 0.80–1.33). However, when stratified by ethnicity, a significantly elevated risk among Asian women was found using the homozygote comparison (OR 1.41; 95% CI 1.01–1.98). When studies with genotype frequencies in the control population were not consistent with HWE and were also included [14], the results were in agreement with the findings from the foregoing analysis.
Discussion
In the present study, we collected all available, published studies and performed a meta-analysis to examine the association between the TYMS polymorphisms TSER and TS3′-UTR and susceptibility to breast cancer. We performed this study to clarify controversial results from previous reports. Six and five studies on the TSER and TS3′-UTR genetic genotypes, respectively, were critically reviewed. Our meta-analysis showed that breast cancer risk was significantly increased in TSER 2R genotype carriers. Our data also indicated that the TS3′-UTR del6/del6 genotype might be a risk factor for breast cancer susceptibility in Asian individuals, but it did not show a significant role in all subjects or in the Caucasian population.
Folate has a central role in one-carbon metabolism and is a critical coenzyme for both nucleotide synthesis and methylation reactions [3]. Folate depletion alone is sufficient to diminish the methyl pool, and a high level of dietary folate intake is associated with a decreased risk for several common cancers, including breast cancer [4–8]. Enzymes such as methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTH), and TYMS play key roles in one-carbon and folate metabolisms. Previously, functional polymorphisms in genes of these folate-related enzymes were found to contribute to the alteration in folate metabolism and were independently associated with breast cancer risk [5, 20, 21, 25, 26]. TYMS is a key enzyme in the nucleotide biosynthetic pathway that converts the methylation of dUMP to dTMP using 5, 10-methylene tetrahydrofolate as a cofactor. This reaction is a key source of de novo cellular thymidylate production [9]. A SNP in the 5′ tandem repeats of the TYMS gene (TSER) has been discovered. Individuals with the wild-type form of 3R show higher transcription of TYMS than those with the variant form that includes 2R and 4, 5, or 9 repeats [27]. In addition, another novel polymorphism of TS3′-UTR, consisting of a 6-bp deletion at nucleotide 1494 of the TYMS mRNA, has been associated with decreased mRNA stability, an enhanced rate of mRNA decay and lower tumor TYMS expression. Thus, functional genetic variants of the TYMS gene may represent risk factors for breast cancer because of their central role in cellular folate metabolism.
Previous studies showed that the TS3′-UTR variant is associated with several types of cancer risk [28, 29]; however, the results remain mixed. In contrast, the TSER genotype appears to be a protective factor against bladder cancer development [30]. Only Henríquez-Hernández et al. reported a significant and increased risk for breast cancer in women who were homozygous for TSER 2R (OR 1.63; 95% CI 1.05–2.52) [16]. In a Chinese population, Zhai et al. found that a significantly reduced risk for breast cancer was associated with the ins6/ins6 homozygous variant (OR 0.58; 95% CI 0.35–0.97) [23]. Our meta-analysis confirmed a significant association between the TSER polymorphism and breast cancer risk in Caucasian women. However, based on currently available data, there is a lack of evidence supporting that the TSER variant genotype is associated with an individual’s susceptibility to breast cancer in the Asian population. In addition, this meta-analysis revealed a modest association between the TS3′-UTR polymorphism and breast cancer risk in Asian women. Thus, the same polymorphisms may play varying roles in different ethnicities due to differences in genetic backgrounds and environmental exposure. Sangrajrang et al. examined the TYMS rs16948322 and rs2298581 polymorphisms and found that they were not risk factors for developing breast cancer (rs16948322: OR 1.14, 95% CI 0.87–1.50; rs2298581: OR 0.95, 95% CI 0.72–1.26) [20]. Although results of the study conducted by Xu et al. found that the rs2298581 SNP was associated with a marginally significant decrease in endometrial cancer risk [31], our collected data were not sufficient to determine the effects of the two TYMS SNPs on breast cancer risk because few studies exist on these SNPs together. Therefore, the roles of rs16948322 and rs2298581 polymorphisms in breast cancer risk need further investigation.
Presumably, genetic factors alone have weak effects on an individual’s phenotype. These effects may be measurable, except in the context of some environmental factors, such as smoking, aging, and alcohol, green tea, and folate intake. Inoue et al. suggested that the MTHFR/TYMS genotypes influence breast cancer risk in women, and their effects may be modified by weekly/daily green tea intake [17]. In bladder cancer, Rouissi et al. reported the correlation between TYMS gene variations and bladder cancer risk among smokers [30]. The lung cancer risk associated with the TS3′-UTR genotype was more pronounced in older people, current and heavy smokers and current drinkers [28]. However, green tea intake, tobacco smoking and drinking have different effects on different cancer types. No association study has been restricted to populations exposed to only one environmental factor; therefore, it is difficult to examine the interaction between the TYMS polymorphisms and dietary and environment factors in breast cancer development.
The interaction of different polymorphisms in the same gene or between different genes might contribute to breast cancer risk. The combined effects of TYMS polymorphisms and other genetic polymorphisms, including those in MTHFR and MTR, which are involved in folate metabolism or one-carbon metabolism, were evaluated in breast cancer [16, 17] and other types of cancer risk [30]. A case–control analysis in a Chinese population revealed no statistical evidence for interactions between the combined variant genotypes in the TYMS gene and risks for breast cancer [23]. We evaluated the combined effects of these polymorphisms on susceptibility to breast cancer, and unfortunately, the data were not sufficient for simply putting them together in a meta-analysis.
Heterogeneity for the TSER and TS3′-UTR polymorphisms was observed among these studies. The heterogeneity may be due to various factors, such as diversity in the population characteristics, differences in the number of cases and controls, genotyping methods and study design. To eliminate heterogeneity, we placed the studies in subgroups. When significant heterogeneity was present, we used a random-effects model to pool the results. There was one study each for TSER [18] and TS3′-UTR [14] that was derived from the HWE, which may contribute to selection bias. In addition, some unpublished, eligible publications were not available in the present meta-analysis, which could have affected the results.
In conclusion, we found several significant associations between the TYMS polymorphisms and breast cancer risk. For the TSER polymorphism, risk effects were found under some genetic models. In addition, the TSER polymorphism might increase breast cancer risk in Caucasian women, which indicates a difference between populations. We also found the TS3′-UTR del6/del6 genotype might be a risk factor in the susceptibility of breast cancer in Asian individuals under the homozygote comparison. However, studies based on larger, stratified case–control populations are still needed to clarify the different effects of the two key TYMS polymorphisms in Asian and Caucasian women. Also, studies examining the combined effects of the TSER and TS3′-UTR polymorphisms or different polymorphisms in enzymes involved in folate metabolism should be investigated.
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43–66
Mavaddat N, Antoniou AC, Easton DF, Garcia-Closas M (2010) Genetic susceptibility to breast cancer. Mol Oncol 4:174–191
Kim YI (1999) Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem 10:66–88
Larsson SC, Giovannucci E, Wolk A (2007) Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst 99:64–76
Ma E, Iwasaki M, Kobayashi M, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Tsugane S (2009) Dietary intake of folate, vitamin B2, vitamin B6, vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: a case–control study in Japan. Nutr Cancer 61:447–456
Rohan TE, Jain MG, Howe GR, Miller AB (2000) Dietary folate consumption and breast cancer risk. J Natl Cancer Inst 92:266–269
Shrubsole MJ, Jin F, Dai Q, Shu XO, Potter JD, Hebert JR, Gao YT, Zheng W (2001) Dietary folate intake and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Res 61:7136–7141
Zhang SM, Willett WC, Selhub J, Hunter DJ, Giovannucci EL, Holmes MD, Colditz GA, Hankinson SE (2003) Plasma folate, vitamin B6, vitamin B12, homocysteine, and risk of breast cancer. J Natl Cancer Inst 95:373–380
Stover PJ (2009) One-carbon metabolism-genome interactions in folate-associated pathologies. J Nutr 139:2402–2405
Peters GJ, Backus HH, Freemantle S, van Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, van Groeningen CJ, Pinedo HM (2002) Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta 1587:194–205
Marsh S, McKay JA, Cassidy J, McLeod HL (2001) Polymorphism in the thymidylate synthase promoter enhancer region in colorectal cancer. Int J Oncol 19:383–386
Kawakami K, Omura K, Kanehira E, Watanabe Y (1999) Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res 19:3249–3252
Gao CM, Takezaki T, Wu JZ, Liu YT, Ding JH, Li SP, Su P, Hu X, Kai HT, Li ZY, Matsuo K, Hamajima N, Sugimura H, Tajima K (2004) Polymorphisms in thymidylate synthase and methylenetetrahydrofolate reductase genes and the susceptibility to esophageal and stomach cancer with smoking. Asian Pac J Cancer Prev 5:133–138
Akisik E, Dalay N (2007) Functional polymorphism of thymidylate synthase, but not of the COMT and IL-1B genes, is associated with breast cancer. J Clin Lab Anal 21:97–102
Grieu F, Powell B, Beilby J, Iacopetta B (2004) Methylenetetrahydrofolate reductase and thymidylate synthase polymorphisms are not associated with breast cancer risk or phenotype. Anticancer Res 24:3215–3219
Henriquez-Hernandez LA, Murias-Rosales A, Hernandez Gonzalez A, Cabrera De Leon A, Diaz-Chico BN, Mori De Santiago M, Fernandez Perez L (2009) Gene polymorphisms in TYMS, MTHFR, p53 and MDR1 as risk factors for breast cancer: a case–control study. Oncol Rep 22:1425–1433
Inoue M, Robien K, Wang R, Van Den Berg DJ, Koh WP, Yu MC (2008) Green tea intake, MTHFR/TYMS genotype and breast cancer risk: the Singapore Chinese Health Study. Carcinogenesis 29:1967–1972
Jakubowska A, Gronwald J, Menkiszak J, Gorski B, Huzarski T, Byrski T, Toloczko-Grabarek A, Gilbert M, Edler L, Zapatka M, Eils R, Lubinski J, Scott RJ, Hamann U (2010) BRCA1-associated breast and ovarian cancer risks in Poland: no association with commonly studied polymorphisms. Breast Cancer Res Treat 119:201–211
Justenhoven C, Hamann U, Pierl CB, Rabstein S, Pesch B, Harth V, Baisch C, Vollmert C, Illig T, Bruning T, Ko Y, Brauch H (2005) One-carbon metabolism and breast cancer risk: no association of MTHFR, MTR, and TYMS polymorphisms in the GENICA study from Germany. Cancer Epidemiol Biomarkers Prev 14:3015–3018
Sangrajrang S, Sato Y, Sakamoto H, Ohnami S, Khuhaprema T, Yoshida T (2010) Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: a case–control study in Thai women. Breast Cancer Res Treat 123(3):885–893
Suzuki T, Matsuo K, Hirose K, Hiraki A, Kawase T, Watanabe M, Yamashita T, Iwata H, Tajima K (2008) One-carbon metabolism-related gene polymorphisms and risk of breast cancer. Carcinogenesis 29:356–362
Xu X, Gammon MD, Zhang H, Wetmur JG, Rao M, Teitelbaum SL, Britton JA, Neugut AI, Santella RM, Chen J (2007) Polymorphisms of one-carbon-metabolizing genes and risk of breast cancer in a population-based study. Carcinogenesis 28:1504–1509
Zhai X, Gao J, Hu Z, Tang J, Qin J, Wang S, Wang X, Jin G, Liu J, Chen W, Chen F, Wei Q, Shen H (2006) Polymorphisms in thymidylate synthase gene and susceptibility to breast cancer in a Chinese population: a case–control analysis. BMC Cancer 6:138
Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127:820–826
Maruti SS, Ulrich CM, Jupe ER, White E (2009) MTHFR C677T and postmenopausal breast cancer risk by intakes of one-carbon metabolism nutrients: a nested case–control study. Breast Cancer Res 11:R91
Tao MH, Shields PG, Nie J, Marian C, Ambrosone CB, McCann SE, Platek M, Krishnan SS, Xie B, Edge SB, Winston J, Vito D, Trevisan M, Freudenheim JL (2009) DNA promoter methylation in breast tumors: no association with genetic polymorphisms in MTHFR and MTR. Cancer Epidemiol Biomarkers Prev 18:998–1002
Lincz LF, Scorgie FE, Garg MB, Ackland SP (2007) Identification of a novel single nucleotide polymorphism in the first tandem repeat sequence of the thymidylate synthase 2R allele. Int J Cancer 120:1930–1934
Shi Q, Zhang Z, Neumann AS, Li G, Spitz MR, Wei Q (2005) Case–control analysis of thymidylate synthase polymorphisms and risk of lung cancer. Carcinogenesis 26:649–656
Suzuki T, Matsuo K, Hiraki A, Saito T, Sato S, Yatabe Y, Mitsudomi T, Hida T, Ueda R, Tajima K (2007) Impact of one-carbon metabolism-related gene polymorphisms on risk of lung cancer in Japan: a case control study. Carcinogenesis 28:1718–1725
Rouissi K, Ouerhani S, Oliveira E, Marrakchi R, Cherni L, Ben Othman F, Ben Slama MR, Sfaxi M, Ayed M, Chebil M, Amorim A, Prata MJ, Benammar Elgaaied A (2009) Polymorphisms in one-carbon metabolism pathway genes and risk for bladder cancer in a Tunisian population. Cancer Genet Cytogenet 195:43–53
Xu WH, Long JR, Zheng W, Ruan ZX, Cai Q, Cheng JR, Zhao GM, Xiang YB, Shu XO (2009) Association of thymidylate synthase gene with endometrial cancer risk in a Chinese population. Cancer Epidemiol Biomarkers Prev 18:579–584
Acknowledgments
This study was supported by the National Nature Science Foundation of China (30901788) and Shandong Provincial Nature Science Foundation of China (ZR2010HQ038 and ZR2010HM059).
Conflict of interest
The authors have no financial or personal relationships to disclose that could inappropriately influence or bias this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Wang, B., Bi, J. et al. The association between two polymorphisms in the TYMS gene and breast cancer risk: a meta-analysis. Breast Cancer Res Treat 128, 203–209 (2011). https://doi.org/10.1007/s10549-010-1314-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1314-0