Abstract
The epidermal growth factor receptor (EGFR) and the estrogen receptor (ER) modulator Amplified In Breast cancer-1 (AIB1) have been reported to be of importance for the prognosis of breast cancer patients. We have analyzed AIB1 and EGFR by immunohistochemistry in primary breast cancers (n = 297) arranged in a tissue microarray in order to predict outcome after adjuvant endocrine therapy with tamoxifen for two years. High expression of AIB1 was associated with DNA-nondiploidy, high S-phase fraction, HER2 amplification, and short term (≤2 years) distant disease-free survival (DDFS), independent of ER status. High expression of EGFR was strongly associated to ER negativity and also correlated with progesterone receptor negativity, high S-phase fraction, and inversely correlated with nodal metastases. In univariate analysis, high EGFR was associated with shorter DDFS (hazard ratio 2.1; P = 0.017), and reached borderline significance in a multivariate analysis, adjusting for ER, menopausal and lymph node status, tumor size, and HER2 (P = 0.057).
In conclusion, both AIB1 and EGFR were associated to DDFS for breast cancer patients treated with two years of adjuvant tamoxifen; AIB1 with the development of early distant recurrences, indicating association between high AIB1 and resistance to tamoxifen during treatment, and EGFR with distant recurrences up to a follow up of five years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estrogen receptor (ER) and progesterone receptor (PgR) status at the time of breast cancer surgery are well-established markers of hormone dependency and predictors of response to endocrine therapy. Overview of randomized trials has shown that tamoxifen, given adjuvantly for about five years after primary operation, reduced breast cancer mortality by 9.2% for ER positive disease after 15 years of follow up [1]. Despite the presence of ER in their tumors, some patients will present with either primary (de novo) resistance to endocrine therapy, or acquire a resistance with relapse and progression of the disease after endocrine therapy [2, 3]. It is therefore important to identify the subgroups of patients recurring and not recurring after tamoxifen therapy, respectively, the former subgroup being candidates for other treatment strategies, either alone or in combination with tamoxifen. The underlying mechanisms for endocrine resistance in ER expressing tumours are still not fully elucidated and several mechanisms have been studied. It has been suggested that crosstalk between ER and various growth factors could explain the endocrine resistance [3–5]. The downstream events initiated by epidermal growth factor receptor (EGFR) and HER2 include stimulation of protein kinases such as mitogen-activated kinase (MAPK). The kinases activate ER, independent of estrogens, through phosphorylation and contribute to the tamoxifen resistance. Growth factor-induced ER activation could lead to a further increase in the expression of several EGFR ligands and establishment of a new self-propagating autocrine growth regulatory loop that efficiently drives the resistant cell growth [2–6]. Overexpression/amplification of HER2, in the ER positive subgroup, is generally accepted to imply a poor effect of tamoxifen, and that alternative treatments strategies are preferable, e.g., aromatase inhibitors in postmenopausal breast cancer [7]. EGFR is a well established marker of endocrine resistance in experimental models of breast cancer cells, but robust results from clinical studies and validated techniques demonstrating the importance of analysing EGFR are lacking [8, 9]. Recently, attention has also been focused on ER-interacting proteins. These co-activators and co-repressors may have significant roles in mediating transcriptional activation of target genes by ER. Amplified In Breast cancer-1 (AIB1) is a co-activator of ER and the estrogen agonist activity of tamoxifen can be enhanced by high levels of AIB1 [10, 11]. However, quite opposite results have been obtained in another study, showing that patients with high AIB1 nuclear expression tended to be successfully treated by hormonal therapy [12].
The aim of this study was to further elucidate the importance of the protein expression of EGFR and AIB1 as markers for the prognosis after two years of adjuvant tamoxifen treatment of breast cancer patients during five years of follow-up.
Material and methods
Patients
The patients (median age = 63 years; range 26–81) had stage II (pT2pN0pM0, pT1-2pN1pM0) breast carcinoma, and were diagnosed in the South Sweden Health Care Region (1985–1994). All patients were treated with tamoxifen for two years, irrespective of ER status, and has previously been selected from two randomized clinical trials [13, 14] in order to investigate the compatibility of different laboratory methods for evaluation of hormonal receptor status [14]. All patients received radical surgery in the form of a modified radical mastectomy or breast-conserving surgery with axillary lymph node dissection (levels I and II). After breast-conserving surgery, radiotherapy (50 Gy) was given to the breast, and in patients with axillary lymph node metastases, locoregional radiotherapy was delivered. The patients were followed until five years with annual mammogram and clinical investigation. None of the patients received any systemic adjuvant therapy besides tamoxifen.
Effects on distant disease-free survival (DDFS), during a maximum follow up time of five years, were studied. Information on clinical outcome, patient and tumor related factors were already available. These factors include information on menopausal status, tumor size, lymph node status, ER and PgR status, HER2 amplification, DNA ploidy status, and flow cytometric S-phase fraction. In the present study tissue microarrays from paraffin embedded tumor samples were used, with approval from the Ethics Committee at Lund University.
Tissue microarray
Tissue microarrays from the primary tumors have been constructed with specimens from all 445 patients included in the previous study [15]. Representative areas of invasive breast cancer, embedded in paraffin block, were marked, and 3 mm x 0.6 mm diameter tissue core biopsies from the tumor blocks were punched out using a precision device, a manual arrayer (Beecher Instruments, Sun Prairie, WI) and positioned into a recipient paraffin array block. Tissue microarray slides were immunohistochemically stained using antibodies for AIB1 and EGFR. Staining with hematoxylin and cytokeratin (AE1/AE3) was carried out for morphological overview and localization of invasive breast cancer cells. This series of primary breast cancer specimens has previously been used to study various other potential prognostic factors and markers in breast cancer.
Immunohistochemical staining
Sections of 4 μm were cut, mounted onto capillary microscope slides (DAKO, Glostrup, Denmark) and dried overnight at room temperature followed by 1–2 h at 60°C. The sections were deparaffinized in xylene and rehydrated in a graded series of ethanol. Antigen retrieval was performed by microwaving the slides (AIB1 and cytokeratin AE1/AE3) in Tris-EDTA, pH 9, at 800 W for 7 min followed by 15 min at 350 W. The slides were cooled down to room temperature for 20 min. For EGFR, antigen retrieval was achieved by incubating the slides in a pepsin solution [0.4% (w/v) in 10 mM HCl] for 1 h at 37°C. The specimens were then washed in distilled water for 10 min followed by washing in Tris-buffer with Tween 20, pH 7.4 for 5 min.
For AIB1 detection, a mouse monoclonal IgG antibody, recognising the amino acids 376–389, was used in a 1:100 dilution, as the primary antibody (BD BioScience, San José, CA). Monoclonal mouse IgG, EGFR pharm DX (clone 2–18C9, 1:50, DAKO, Glostrup, Denmark) and cytokeratin (clone AE1/AE3, 1:500, DAKO, Glostrup, Denmark) were used as primary antibodies for EGFR and cytokeratin, respectively. The immunostaining was performed by an automatic immunostainer (TechMateTM 500 Plus, DAKO, Glostrup, Denmark). DAKO EnvisionTM was used for AIB1 and cytokerain visualization, whereas DAKO ChemMate Kit was used for visualization of EGFR. The slides were then counterstained with hematoxylin. Finally, the sections were dehydrated in ethanol, cleared in xylene, and coverslipped. All series included positive controls.
AIB1 and EGFR staining evaluation
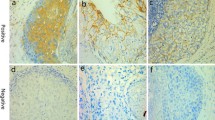
The immunohistochemical staining for AIB1 and EGFR were examined by light microscopy blinded to clinical and tumor characteristic data. Results for AIB1 was estimated semi-quantitatively as the percentage of stained nuclei (proportion score) and intensity of the positive tumor cells (intensity score). The cases were scored 0–3 according to the amount of staining. Score 0 represented negative staining, score 1 = 1–10%, score 2 = 11–50%, and score 3 = 51–100% positive AIB1 staining of tumor cells. The extent of positive AIB1 nuclei stained intensity was scored 0–3, which represented negative (0) to intense (3) staining. The proportion and intensity scores were then added to obtain a total score, which ranged from 0 to 6. In the literature, no consensus for the choice of cut-off value could be found. Therefore, a cut-off close to the median value was used.
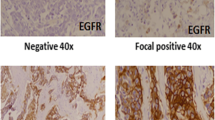
Expression of EGFR was defined as positive membrane staining. The estimated proportion of positively stained tumor cells for each case was assigned a proportion score (score 0 = negative, score 1 = 1–10%, score 2 = 11–50%, score 3 = 51–99%, and score 4 = 100%). An intensity score (0–3) was assigned that represented the average intensity of the positive tumor cells, in which score 0 represented a negative intensity and score 3 represented a strong EGFR membrane staining intensity. The proportion and intensity scores were then added to obtain a total score, which ranged from 0 to 7 for EGFR. A cut-off resulting in a similar distribution of EGFR over-expressing tumors as has previously been reported in the literature, around 10–15%, was chosen [6–9].
In the following, only the total scores for both AIB1 and EGFR were considered. In case of discrepant staining result of AIB1 and EGFR between the three cores from the same patients, the core with the highest score was used in the further analyses.
Analysis of other tumor characteristics
ER and PgR were analyzed by using immunohistochemistry on formalin-fixed, paraffin-embedded breast carcinoma [15]. Tumors with more than 10% positive nuclei staining were considered ER and PgR positive. S-phase fraction was analyzed in frozen tumor samples by flow cytometric analyses, where ≥ 12% was classified as high S-phase fraction [16]. Flow cytometric analysis was also used to investigate DNA ploidy status; diploid = one cell population and non-diploid = more than one cell population [16]. Chromogenic in situ hybridization analysis with HER2/neu DNA probe was used to evaluate HER2 amplification as previously described [16]. More than six copies per cell were defined as HER2 amplified.
Statistical analysis
The Kaplan–Meier method was used to estimate survival and the log-rank test to evaluate null hypotheses of equal survival in two patient strata. Uni- and multivariate Cox-regression was used to calculate hazard ratios (HRs) for the factors in relation to DDFS. A Cox model with an interaction term was used to test if the AIB1 effect was the same in the two ER subgroups. The prognostic effect of the two factors studied (AIB1 and EGFR) was found to decline with time leading to non proportional hazards. Hence, the survival times were censored at five years when Cox-models were fitted. The same rule was used when Kaplan-Meier curves were drawn. Comparisons of AIB1 and EGFR with other tumor characteristics were made by Fisher’s exact test. P-values of less than 0.05 derived from two-sided tests were considered significant. The statistical software package Stata 9.2 (StataCorp. 2006. College Station, TX, USA) was used for all the statistical calculations.
Results
Immunohistochemical expression of AIB1 and EGFR
Tissue microarrays, either torn or displayed mostly with stroma, were excluded for further analysis leaving 303 cases evaluable for AIB1 and 305 for EGFR. In the following, only cases with information of both AIB1 and EGFR were included (n = 297).
AIB1 antibody binding in the nucleus of breast cancer cells was revealed in 247 (83%) of the 297 cases. Positive reactions in most cases were restricted to the nuclei. Four percent were assigned a total score of 2, score 3 = 3%, score 4 = 24%, score 5 = 29%, and 23% were assigned score 6. In order to get a cut-off value close to the median, samples with a total score of 5 or higher were classified as high AIB1 (52% of the samples) and those with a total score below 5 as low AIB1 (48% of the samples).
As far as EGFR is concerned, 193 (65%) of the 297 tumors exhibited no membrane staining and were assigned score 0. Positive EGFR membrane immunoreactivity was observed in 104 (35%) of the 297 tumors investigated. Twenty-one (7%) of the breast carcinomas received the total score 2, score 3 = 5%, score 4 = 4%, score 5 = 2%, and 5% received score 6. Eleven per cent of the cases showed intense membrane staining in every one of the cancer cells, and therefore were assigned the highest total score 7. This group was considered to have high EGFR (11%), and those below 7 as low EGFR (89%).
Correlation between AIB1, EGFR immunoreactivity and other clinicopathologic variables
In comparison to the low AIB1 group, breast cancer classified as high AIB1 were more likely to express markers for cell proliferation, e.g. high S-phase fraction (P = 0.049), HER2 amplification (P = 0.005), and DNA-nondiploidy (P = 0.029; Table 1; Fisher´s exact test). No significant association was found between nuclear AIB1 expression and the other clinicopathological variables such as: ER and PgR status, menopausal status, tumor size, and lymph node status.
Compared with the low EGFR group, tumors classified as high EGFR were more likely to have negative ER and PgR status (P < 0.001 for both variables). Only three out of 203 (1.5%) ER positive samples had high EGFR, compared to 29 out of 91 (32%) ER negative. The corresponding figures for PgR were 4/141 (2.8%) and 28/153 (18%), respectively. Tumors with high EGFR were also more likely to express high S-phase fraction (P = 0.004), compared to those with low EGFR. A significant inverse correlation was found between high EGFR and number of lymph node metastases (P = 0.010). No significant association was found between EGFR status and the other clinicopathological features, and AIB1.
Association of AIB1 and EGFR with DDFS–univariate analyses
When considering the whole follow up period of five years, AIB1 expression did not yield predictive information for the prognosis after tamoxifen, and was not associated with DDFS [HR = 1.3; P = 0.28 (Table 2, Fig.1a)]. The lack of association with DDFS was independent of ER status; HR = 1.4 (P = 0.31) in the ER negative subgroup, and HR = 1.0 (P = 1.0) in the ER positive subgroup. However, when only early recurrences, within two years of follow up, were considered, high AIB1 was significantly associated to DDFS (HR = 3.6; 95% CI = 1.5–8.8; P = 0.006). This association persisted when the analysis was adjusted for ER status (HR = 3.0; 95% CI = 1.2–7.5; P = 0.017). AIB1 was significantly associated to DDFS in the ER positive subgroup, but not in the ER negative subgroup (HR = 9.0; 95% CI = 1.1–72; P = 0.039 vs. HR = 1.9; 95% CI = 0.70–5.4; P = 0.21). This difference is, however, not statistically significant (P interaction = 0.19).
As far as EGFR is concerned, patients with tumors expressing high EGFR had a 2.1 fold higher hazard of developing distant recurrence in comparison with patients with low EGFR during the whole follow-up period of five years (P = 0.015; Table 2, Fig. 1b). To study the importance of EGFR in relation to ER status was not meaningful, since only three tumors had high EGFR in the ER positive subgroup.
Association of basic clinicopathologic variables with DDFS–univariate analyses
ER positivity, postmenopausal status, and no HER2 amplification were factors associated with a prolonged DDFS (Table 2). Although high S-phase fraction, positive nodal status, larger tumor size, and PgR negativity showed a trend toward a worse outcome, these correlations were not statistically significant.
Multivariate analysis
Factors being significant in the univariate analyses during the whole follow up of five years, i.e., EGFR, ER, menopausal status, and HER2, were included in the multivariate analysis, as well as the well established clinicopathological prognostic factors, lymph node status and tumor size. HER2 was the strongest independent predictor for DDFS, followed by lymph node status, tumor size, and menopausal status (Table 3). Patients with tumors expressing high levels of EGFR were 2.0 times more likely to relapse. However, this correlation only reached a borderline of significance (P = 0.057). Finally, the analysis showed also a trend towards a lower hazard ratio for patients with ER positive carcinomas, compared to those with ER negative tumors. AIB1 was not included in the multivariate analysis, as it was not a significant factor in the univariate analysis when considering the whole follow-up period (Table 2).
Discussion
Tamoxifen is to date the most extensively used adjuvant endocrine therapy for all stages of breast cancer, for both pre- and postmenopausal patients. The current study primarily addresses the association of the estrogen receptor modulator AIB1 and EGFR expression and clinical outcome after two years of adjuvant tamoxifen therapy in a cohort of 297 breast cancer patients not receiving any chemotherapy. Fifty-two percents of the cases were considered positive for AIB1 (total score ≥ 5). There were significant correlations between high AIB1 and HER2 amplification, DNA non-diploidy, and high SPF. Thus, tumors with high levels of AIB1 were associated with a more aggressive phenotype. AIB1 was also significantly associated to early recurrences (P = 0.006), within a short duration (<= 2 years) of follow-up, but not to DDFS when taking the whole follow-up period of five years into consideration. The association between AIB1 and early recurrences was furthermore independent of ER status. As the benefit of adjuvant tamoxifen is only found among patients with ER positive tumors, it is noteworthy that high AIB1 identifies eight out of nine early distant metastases in the ER positive subgroup. It may be speculated that the importance of AIB1 in the ER positive subgroup is restricted to the time period when adjuvant tamoxifen is given (the patients in our study have been treated for two years) indicating an agonistic function for tamoxifen in patients with high nuclear expression of AIB1. Although the importance of AIB1 seems to be strongest in the ER positive subgroup, we cannot exclude that AIB1 maybe of prognostic value also in the ER negative subgroup, since interaction analysis did not reveal any significant difference between the two subgroups. It should therefore, in the future, be interesting to examine if AIB1 is a prognostic factor after a longer follow up time when tamoxifen is given for five years, which may further support the hypothesis for an agonistic function of tamoxifen in this subgroup of patients. In agreement with our results, Osborne and co-workers, using western blot analysis, showed that in a subgroup of 187 patients treated with adjuvant tamoxifen, elevated expression of AIB1 was associated to poorer clinical outcome and to indicators of poor prognosis, such as high S-phase fraction and HER2 overexpression [10]. They emphasized that patients with tumors expressing high levels of both AIB1 and HER2 had a significantly worse prognosis than all other patients combined (P = 0.002; log-rank test). We obtained similar results when patients with high levels of both AIB1 and HER2 (n = 31) were compared with the remaining 257 patients after five years of follow-up (P = 0.002), further supporting the hypothesis that these markers are of importance for predicting outcome in tamoxifen treated patients. Also an in vitro experiment has demonstrated that AIB1 expression is increased in anti-estrogen resistant cells [18]. The recently presented data from Kirkegaard and co-workers using immunohistochemistry based evaluation of AIB1 demonstrated that the AIB1 subgroup did not have an increased risk of relapse during tamoxifen treatment in hormone receptor positive disease, although the combination of HER1–3 and AIB1 defined a subset of patients with an increased risk of relapse during tamoxifen treatment in the ER positive cohort [11]. However, the fraction of patients defined as AIB1 high was not presented and furthermore chemo-endocrine treatment was given to 25% of the included patients which make direct comparisons with our study difficult. In another study, using clinical breast cancer material and the same technology as in our study (immunohistochemistry), only 16% of the cases showed nuclear staining of AIB1 protein (compared to 52% in ours) and AIB1 expression was correlated with ER positivity, but not with other clinicopathological markers [12]. Based on a small subset of 21 patients with metastatic breast cancer treated with endocrine therapy alone (anti-estrogens, aromatase inhibitors, or ovarian ablation), they also suggested that patients with tumors containing high levels of AIB1 tended to be more successfully treated by endocrine therapy [12]. Reasons for discrepancies between their results and those obtained in the study by Osborne and Kirkegaard, and in our study may be differences between the metastatic and adjuvant settings, small number of patients in the work by Iwase et al., differences in methodology, and patient selection bias.
As far as EGFR is concerned, the cut-off value was chosen to get a similar proportion of high EGFR samples (10–15%) as in previous studies [6–9]. We found an association between high EGFR, high S-phase fraction and ER and PgR negativity as previously described [8, 9]. The very strong association between EGFR and ER negativity (only 3 out of 204 ER positive cases had high EGFR) in our study is noteworthy. Interestingly, a significant inverse correlation was found between high EGFR protein over-expression and the number of lymph node metastases. This correlation is maybe a chance finding, since it has not previously been reported. EGFR expression was significantly associated with DDFS in univariate analyses and multivariate analysis showed that EGFR protein expression was of borderline prognostic significance (P = 0.057).
EGFR is a strong candidate as a marker of tamoxifen resistance in experimental models of breast cancer cells. Yarden and colleagues showed that the presence of estrogens, alone, was sufficient to suppress the expression of EGFR in ER positive cultured breast carcinoma, while treatment with tamoxifen resulted in growth arrest and an increase of EGFR expression [19]. An increase in EGFR levels and sensitization to low concentrations of growth factors could provide a means for estrogen-dependent breast cancer cells to avoid cell death in the face of estrogen-depletion. Through a membrane-bound and/or cytoplasmic ER, tamoxifen, like estrogens, can directly activate EGFR and induce tumor growth [20]. The importance of EGFR as a predictor of prognosis for ER positive patients treated with adjuvant tamoxifen has been explored in non-randomized trials showing a correlation between EGFR and outcome in this setting, supporting the hypothesis that high expression of EGFR could reduce the estrogen antagonist activity of tamoxifen-bound ER in breast cancer patients by cross-talk [3, 4, 6]. However, in three independent randomized trials of adjuvant tamoxifen none of the studies were able to confirm the preclinical hypothesis that EGFR is a predictor of tamoxifen resistance [8, 9, 21]. The inverse relationship between ER and EGFR contributing to a very small fraction of ER positive and EGFR positive tumours is speculated to be one explanation to the findings [8, 9] as well as non-standardized methods of analysis using different antibodies and protocols. It should also be mentioned that we have analyzed EGFR and AIB1 on tissue microarray, which in a study by Henriksen and co-workers were comparable to the analyses on whole sections [22]. EGFR has been found to be associated with an aggressive tumour type having prognostic value in clinical studies, including cohorts of patients treated with adjuvant chemotherapy [23–25]. Tsutsui and co-workers showed that patients with high EGFR and negative ER had the worst prognosis, at least in univariate analysis [24].
In conclusion, in this study using immunohistochemical analyses on tissue microarrays, we have found that both AIB1 and EGFR were associated to DDFS; AIB1 with the development of early distant recurrences and EGFR with recurrences up to a follow up of five years. High EGFR expression was also strongly associated to ER negativity.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15–year survival: an overview of the randomised trials. Lancet 365:1687–1717
Johnston S (2005) Molecular insights into endocrine resistance. Eur J Cancer Suppl 3:225–236
Osborne K, Shou J, Massarweh S, Schiff R (2005) Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res Suppl 11:865–870
Arpino G, Weiss H, Lee A, Schiff R, De Placido S, Osborne K, Elledge R (2005) Estrogen receptor–positive, progesterone receptor–negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst 97:1254–1261
Kurebayashi J (2005) Resistence to endocrine therapy in breast cancer. Cancer Chemother Pharmacol Suppl 1 56:39–46
Arpino G, Green S, Allred C, Lew D, Martino S, Osborne K, Elledge R (2004) HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A Southwest Oncology Group Study. Clin Cancer Res 10:5670–5676
Ellis M, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Jänicke F, Miller W, Evans D, Dugan M, Brady C, Quebe-Fehling E, Borgs M (2001) Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for erbB-1– and/or erbB-2–positive, estrogen receptor–positive primary breast cancer: evidence from a phase III randomized trial. J Clin Oncol 19:3808–3816
Knoop AS, Bentzen SM, Nielsen MM, Rasmussen BB, Rose C (2001) Value of epidermal growth factor receptor, HER2, p53, and steroid receptors in predicting the efficacy of tamoxifen in high-risk postmenopausal breast cancer patients. J Clin Oncol 19:3376–3384
Dowsett M, Houghton J, Iden C, Salter J, Farndon J, Hern RA, Sainsbury R, Baum M (2006) Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol 17:818–826
Osborne K, Bardou V, Hopp T, Chamness G, Hilsenbeck S, Fuqua S, Wong J, Allred C, Clark G, Schiff R (2003) Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361
Kirkegaard T, McGlynn L, Campbell F, Müller S, Tovey S, Dunne B, Nielsen K, Cooke T, Bartlett J (2007) Amplified in breast cancer 1 in human epidermal growth factor receptor-positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13:1405–1411
Iwase H, Omoto Y, Toyama T, Yamashita H, Hara Y, Sugiura H, Zhang Z (2003) Clinical significance of AIB1 expression in human breast cancer. Breast Cancer Res Treat 80:339–345
Swedish Breast Cancer Cooperative Group (1996) Randomized trial of two versus five years of adjuvant tamoxifen for postmenopausal early stage breast cancer. J Natl Cancer Inst 88:1543–1549
Rydén L, Jönsson P-E, Chebil G, Dufmats M, Fernö M, Jirström K, Källström A-C, Landberg G, Stål O, Thorstenson S, Nordenskjöld B (2005) Two years of adjuvant tamoxifen in premenopausal patients with breast cancer: a randomised, controlled trial with long-term follow-up. Eur J Cancer 41:256–264
Chebil G, Bendahl PO, Idvall I, Ferno M (2003) Comparison of immunohistochemical and biological assay of steroid receptors in primary breast cancer–clinical associations and reasons for discrepancies. Acta Oncol 42:719–725
Sigurdsson H, Baldetorp B, Borg Å, Dalberg M, Fernö M, Killander D, Olsson H (1990) Indicators of prognosis in node-negative breast caner. N Engl J Med 322:1045–1053
Isola J, Tanner M, Forsyth A, Cooke TG, Watters AD, Bartlett JM (2004) Interlaboratory comparison of HER-2 oncogene aplification as detected by chromogenic and fluoroscence in situ hybridization. Clin Cancer Res 10:4793–4798
Sarvilinna N, Eronen H, Miettinen S, Vienonen A, Ylikomi T (2006) Steroid hormone receptors and coregulators in endocrine-resistant and estrogen-independent breast cancer cells. Int J Cancer 118:832–840
Yarden RI, Wilson MA, Chrysogelos SA (2001) Estrogen suppression of EGFR expression in breast cancer cells: A possible mechanism to modulate growth. J Cell Biochem 81(Suppl. 36):232–246
Schiff R, Massarweh SA, Shou J, Bharwani L, Moshin S, Osborne K (2004) Cross-talk between estrogen receptor and growth factor pathways as a molecular target for overcoming endocrine resistance. Clin Cancer Res 10(Suppl):331–336
De Placido S, De Laurentiis M, Carlomagno C, Gallo C, Perrone F, Pepe S, Ruggiero A, Marinelli A, Pagliarulo C, Panico L, Pettinato G, Petrella G, Bianco A (2003) Twenty-year results of the Naples GUN randomized trial: predictive factors of adjuvant tamoxifen efficacy in early breast cancer. Clin Cancer Res 9:1039–1046
Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT (2006) Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: A comparison between whole sections and tissue microarrays J Clin Pathol 60:397–404
Ferrero JM, Ramaioli A, Largillier R, Formento JL, Francoual M, Ettore F, Namer M, Milano G (2001) Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol 12:841–846
DiGiovanna MP, Stern DF, Edgerton SM, Whalen SG, More II D, Thor AD (2005) Relationship of epidermal growth factor receptor expression to ErbB-2 signaling activity and prognosis in breast cancer patients. J Clin Oncol 23:1152–1160
Niu Y, Fu X, Lv A, Fan Y, Wang Y (2002) Potential markers predicting distant metastasis in axillary node-negative breast carcinoma. Int J Cancer 98:754–760
Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S (2002) Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat 71:67–75
Acknowledgments
We are indebted to participating departments of the South Sweden Breast Cancer Group for providing us with breast cancer samples and clinical follow-up data. The study was supported by funds from the Swedish Cancer Society, the Swedish Research Council, the Gunnar, Arvid, and Elisabeth Nilsson Foundation, the Mrs Berta Kamprad Foundation, and the University Hospital of Lund Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dihge, L., Bendahl, PO., Grabau, D. et al. Epidermal growth factor receptor (EGFR) and the estrogen receptor modulator amplified in breast cancer (AIB1) for predicting clinical outcome after adjuvant tamoxifen in breast cancer. Breast Cancer Res Treat 109, 255–262 (2008). https://doi.org/10.1007/s10549-007-9645-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9645-1