Abstract
Previous regional-based diffusion tensor imaging (DTI) studies focused on impairment of the arcuate fasciculus in aphasia; little is known about the extent to which aphasia severity is affected by damage to both ventral and dorsal language white matter (WM) pathways. To understand whether disconnection of these pathways contributes to clinical symptoms, we assessed the relationship between the alterations of WM integrity and clinical characteristics in acute aphasia after stroke. Eighteen patients with acute aphasia and age-, gender-, and education-matched healthy controls underwent language assessment and DTI scanning. The whole brain unbiased tract-based spatial statistics method was employed to quantitate WM integrity (fractional anisotropy) for both groups. Linear correlation analyses were performed to evaluate the relationship between WM integrity and clinical features. The aphasic patients showed decreased WM integrity in the left inferior fronto-occipital fasciculus/inferior longitudinal fasciculus (IFOF/ILF) and the left uncinate fasciculus, which represents components of ventral language pathway, and the left superior longitudinal fasciculus (SLF), which relates to dorsal language pathway. In addition, WM integrity of the left IFOF and SLF showed a positive correlation with aphasia quotient, performance quotient, and cortical quotient, respectively. These findings suggested that impaired WM integrity in both language pathways not only contributed to language performance, but also to general cognitive status. We suggest that aphasia involves a breakdown of multiple connections of dorsal and ventral streams that directly contributes to language deficits. Damage to these dual-streams may serve as a neuromarker for aphasias after stroke.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aphasia is an umbrella term used to describe a collection of disturbances in the comprehension and formulation of language usually following a stroke (Damasio 1992; Schlaug et al. 2009; Jang 2013). Post-stroke aphasia is usually caused by left hemisphere lesions, and substantially increases deficits in language abilities (Thomas and Lincoln 2008).
Previous grey matter integrity on structural magnetic resonance imaging (MRI) (Geva et al. 2012) and brain activation on functional neuroimaging studies (Warren et al. 2009; van Hees et al. 2014b; Zhu et al. 2014; Crinion and Leff 2015; Yang et al. 2016a, b) have investigated the fine-grain details involved in language architecture. Much less is known however about white matter (WM) microstructure changes and neurobiological mechanisms underlying acute aphasia. Moreover, aphasia symptoms and severity cannot be fully explained by grey matter damage alone as it has been shown that local tissue necrosis can cause local fiber displacement and reorganization of WM (Selnes et al. 2002). Diffusion tensor imaging (DTI) is a promising technique that allows in vivo examination of WM integrity and reconstruction of WM fiber tracts based on the diffusion properties of water in neural tissue (Basser et al. 1994). Fractional anisotropy (FA), the most frequently used DTI-derived index, reflects microstructural properties such as fiber density, axonal diameter, and myelination in WM (Kochunov et al. 2007).
Current models on the WM pathways underlie a dual-stream language architecture (Frey et al. 2008; Saur et al. 2008; Dick and Tremblay 2012). A dorsal language pathway includes the superior longitudinal fasciculus (SLF)/arcuate fasciculus (AF), which are responsible for phonological processing, e.g., speech repetition. A ventral language pathway connects temporal and prefrontal regions via the extreme capsule, as well as the inferior fronto-occipital fasciculus (IFOF)/inferior longitudinal fasciculus (ILF), and the uncinate fasciculus (UF), which are associate with semantic processing, e.g., higher-level language comprehension (Hickok and Poeppel 2004, 2007). Most DTI studies in aphasic patients focused on the impairment of the AF (Selnes et al. 2002; Breier et al. 2008; Galantucci et al. 2011; Kim et al. 2011; Tak and Jang 2014) and prediction of aphasia recovery (Kwon and Jang 2011; Forkel et al. 2014; van Hees et al. 2014a), suggesting that the AF in dorsal WM pathway of the dominant hemisphere was an important factor for a speech repetition (Bernal and Ardila 2009). In addition, recent studies have emphasized that the impairment of ventral WM pathway alone or/and disconnection in dorsal and ventral WM pathways in various type of aphasia, indicating a central role for ventral WM pathway in language comprehension (Galantucci et al. 2011; Turken and Dronkers 2011; Kummerer et al. 2013; Rosso et al. 2015).
Although these region of interest (ROI)-based or voxel based analysis methods could provide a wealth of information, both have drawbacks. ROI-based analysis is time-consuming and difficult to objectively place ROIs around thinner tracts and in addition, this approach can only detect alterations in the brain where the ROIs have been placed. Although the voxel based analysis can address these limitations, the issue of misalignment and the choice of smoothing extent can greatly affect the results (Smith et al. 2006). The tract-based spatial statistics (TBSS) method, which keeps the advantages of voxel based analysis while addressing the alignment and smoothing issues, has recently been developed for whole brain DTI analysis (Smith et al. 2006). In contrast to voxel based analysis, TBSS projects an individual FA image into a common space and thus it is not reliant on perfect nonlinear registration. In addition, no spatial smoothing is needed during the image processing. To date, only a few studies have utilized the TBSS method when analyzing aphasia patients (Geva et al. 2015). For example, using TBSS, Geva et al. (2015) found no significant differences in the right hemisphere between aphasia patients and controls. In another study, standard whole-brain TBSS analysis was successfully applied to compare before and after excitatory repetitive transcranial magnetic stimulation treatment of post-stroke aphasia with results indicating increased left-hemispheric FA (Allendorfer et al. 2012). The conflicting data may relate to the investigation paradigm and the heterogeneity of the patient pool.
To the best of our knowledge, few study analyzed the WM microstructure changes and relationship of clinical characteristic in acute aphasias. To eliminate the influence of heterogeneity of the patient pool and WM integrity methodological bias, we evaluated changes in WM microstructure using standard whole-brain TBSS analysis in a group with acute aphasia. In addition, we assessed the relationship between the alterations of WM microstructure and clinical characteristics in acute aphasia.
Materials and Methods
Subjects
Eighteen aphasia patients (all right-handed, six females; age, 53.67 ± 13.66 years) were recruited from admission at the Fuzhou Hospital. Patients were recruited retrospectively according to the following criteria: (1) first stroke occurred in the left hemisphere; (2) age of >18 and <85 years; (3) Chinese native speaker; (4) aphasia persistent at day 1 post-stroke; and (5) right-handed. Participants were excluded if they had the following: (1) any past or current neurological disorders or family history of hereditary neurological disorders; (2) a history of head injury resulting in loss of consciousness; (3) alcohol or substance abuse; (4) claustrophobia; and (5) incompatible implants. All patients experienced a single left-hemisphere ischemic (n = 16) or hemorrhagic (n = 2) stroke (lesion size: 30.60 ± 42.23 cm3) and underwent MRI for an average of 9.7 ± 5.3 days after stroke (Table 1). All patients were Chinese native speakers and right-handed.
All patients received a comprehensive evaluation, including medical history and neurological examination, neuropsychological testing, and neuroimaging. Aphasia was diagnosed based on the aphasia battery of Chinese (ABC), which is the Chinese standardized adaptation of the Western Aphasia Battery (Gao et al. 1992; Lu et al. 2013). The ABC provides the following information: aphasia quotient (AQ), performance quotient (PQ), and cortical quotient (CQ) (Liu et al. 2015). AQ reflects a global measure of severity and type of aphasia. AQ (range, 0–100) is derived from linguistic subtests including spontaneous speech, auditory comprehension, repetition, and naming. The normative and cut-off scores of AQ is 97.11 ± 2.43 (mean ± SD) and 93.25, respectively. Anomic (n = 9), Broca’s (n = 2), and conduction (n = 7) aphasia patients were included according to AQ. PQ (range, 0–40) combines scores of reading/writing, praxis, and construction of patients. CQ (range, 0–100) provides an overall picture of cognitive status (Yu et al. 2013).
A total of 20 age-, gender-, and education-matched healthy controls (HC) (all right-handed, eight females, 54.05 ± 8.43 years of age) were included in this study. The HC were recruited through an advertisement. These volunteers had no history of neurological disorders or psychiatric illnesses and no gross abnormalities on brain MR images.
This study was approved by the local Ethics Committee of the Hospital of Fuzhou and was performed in accordance with the approved guidelines. All participants gave informed consent to participate in the investigation.
Data Acquisition
Imaging was performed on a 3.0T Siemens Vision Scanner (Erlangen, Germany) equipped with high-speed gradients just at the recruited day. The DTI parameters were as follows: repetition time = 8500 ms; echo time = 93 ms; acquisition matrix = 128 × 128; field of view = 240 × 240 mm2; slice thickness = 3 mm, no gap. The diffusion sensitizing gradients were applied along 30 non-collinear directions (b = 1000 s/mm2) with an acquisition without diffusion weighting (b = 0).
Lesion Mapping
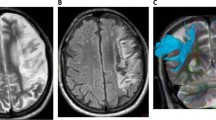
We constructed a lesion overlap image for all aphasic patients. A radiologist (Y.L.) manually traced the outline of the lesion on individual 3D T1 images using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/), thereby creating a lesion mask for each patient. After the spatial normalization process, the union of all individual lesion mask was used to construct a group lesion mask for the patients (Fig. 1).
Data Preprocessing
All diffusion tensor images were processed using FMRIB software library (FSL, v5.0; http://www.fmrib.ox.ac.uk/fsl) software. First, eddy current distortions and head movements were corrected from each dataset. Second, a brain extraction tool was utilized to generate a binary brain mask. Following these steps, a diffusion tensor model was fitted independently for each voxel within the brain mask and a FA image was calculated for each participant. Each subject’s FA image was then non-linearly aligned into 1 × 1 × 1 mm3 MNI standard space using an FMRIB58 FA template, which resulted in a standard space version of the FA image. In addition, we used a cost-function modification to avoid bias during spatial normalization (Brett et al. 2001), as previously studies used (Ji et al. 2015; Liao et al. 2016). Subsequently, the mean image of all aligned FA images was calculated and thinned to create a skeletonized mean FA image with a threshold at 0.2. Finally, each aligned FA image was projected onto this skeleton for statistical analysis.
Statistical Analysis
For the imaging data, analyses were conducted via voxel-wise statistics of the independent two-sample t test using the FSL Randomise tool (version 2.1) via 5000 permutations with the threshold-free cluster enhancement option. Statistical results were corrected for multiple comparison with threshold-free cluster enhancement (TFCE) methods. Clusters with a voxel-wise threshold of P < 0.01 as well as voxels >50 were considered statistically significant. Voxels showing significant differences were assigned to WM tracts using the JHU (Johns Hopkins University) DTI-based white-matter atlases (Hua et al. 2008) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases).
To examine whether clinical features were related to the WM integrity, linear correlation analyses were performed. Correlations between the mean FA values from the significantly different clusters and clinical scores including AQ, PQ and CQ were analyzed. A threshold of P < 0.05 (false discovery rate [FDR]-corrected) was used to indicate statistical significance. Considering AQ highly correlated with PQ (R = 0.8221, P < 0.0001), we examined the interaction effect in how AQ and PQ associated with FA values using a mixed model in general linear model analysis (http://www.math.mcgill.ca/keith/surfstat/).
Results
Demographics and Clinical Characteristics of the Participants
As shown in Table 1, patients with aphasia and healthy controls did not significantly differ in age (two sample t test, P = 0.96), gender (χ2 test, P = 0.67), and years of education (Mann–Whitney U test, P = 0.63). Stroke-related clinical characteristics of patients were tested using the ABC (see details in Table 1).
Differences in FA Values Between Groups
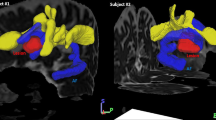
As shown in Table 2 and Fig. 2, voxel-wise statistics revealed four brain WM tracts with lower FA values in patients with aphasia. Compared with healthy controls, patients showed decreased FA in the left IFOF, ILF, UF for ventral language pathway and the SLF for dorsal language pathway, respectively.
WM structures showing significantly lower FA in patients with aphasia (P < 0.01, corrected for multiple comparisons) in (1) the left IFOF, (2) the left ILF, (3) the left SLF, and (4) the left UF. The background image is the standard MNI152 brain template. Green voxels represent the FA WM skeleton. Red-yellow voxels represent regions with lower FA in patients with aphasia compared with healthy controls (Color figure online)
Correlations Between FA Values with Clinical Scores
As shown in Fig. 3, the left IFOF were positively correlated with the AQ (R = 0.5754, P = 0.0125), PQ (R = 0.5774, P = 0.121) and CQ (R = 0.6119, P = 0.0070) scores, respectively. The left SLF were positively correlated with the AQ (R = 0.5630, P = 0.0150), PQ (R = 0.4848, P = 0.0414) and CQ (R = 0.5351, P = 0.0221) scores, respectively. In addition, there is no interact effect between in AQ and PQ that associated with AF value in the left IFOF (F = 2.3771, P = 0.1137), as well as in the SLF (F = 2.5422, P = 0.0983). This auxiliary analysis suggest that AQ and PQ has the same association with FA value in the IFOF (and in the SLF), which would not provide a specific language deficit.
Discussion
We examined WM microstructure changes and associations with clinical characteristics in aphasic patients. We found decreased WM integrity (as assessed by FA) in the both ventral (the SLF) and dorsal (the IFOF/ILF and UF) language pathways in acute aphasic patients after stroke. Although the current findings have already been uncovered in the Dejerines’ studies (Krestel et al. 2013), the whole-brain TBSS technique and an homogenous acute stroke population offer interesting complementary analysis to previous functional neuroimaging works (Price 2012). In addition, WM integrity of the SLF in ventral language pathway and the IFOF in dorsal language pathway were positively correlated with AQ, PQ and CQ, respectively. These findings suggested that impaired WM integrity in both language pathways not only contributed to language performance, but also to general cognitive status.
To eliminate the influences of the methodological bias derived from ROI-based analysis or/and voxel-based analysis, we evaluated changes in WM microstructure using standard whole-brain TBSS analysis. The TBSS method attempts to combine the advantage of voxel-based analysis with the strengths of tractography-based analyses (ideally, being confident that the estimates of FA are truly taken from the relevant voxels) for a more robust and more sensitive way of detecting WM microstructure alterations (Smith et al. 2006). Although a pervious study using TBSS did not find significant differences between patients with chronic aphasia and healthy controls (Geva et al. 2015), the potential influence of patient heterogeneity was not addressed. In the current study, we utilized a homogenous population of patients suffering from acute aphasia following stoke in order to eliminate the possible confounding results associated with the previous study.
Lower FA values in the SLF/AF of the left hemisphere were consistent with previous ROI-based DTI studies in aphasia patients following stroke (Breier et al. 2008; van Hees et al. 2014a; Geva et al. 2015). We found that the anterior segment of the SLF, including all three segments, showed decreased FA in patients with acute aphasia (Fig. 2). The anterior segment of the SLF connects Broca’s territory and Geschwind’s territory (Dick and Tremblay 2012). The SLF/AF is the known proxy for ‘Broca–Wernicke–Geschwind’s language model, and connects then receptive and expressive language areas in the brain (Warren et al. 2005; Hickok and Poeppel 2007). Electrical stimulation of the SLF/AF results in speech arrest/articulation disturbances (Maldonado et al. 2011). DTI measurement (e.g., FA) showed a relationship between damage to the SLF/AF and verbal repetition in patients with left hemisphere stroke (Breier et al. 2008). Furthermore, we found that WM integrity of the SLF was associated with AQ and PQ, suggesting a relationship between WM integrity, aphasia severity and impaired language performance. In agreement with previous studies (Ardila 2010; Kummerer et al. 2013; Rosso et al. 2015), we found that the SLF acts as a core pathway of the dorsal stream of the language network which is responsible for phonological processing in acute aphasia (Saur et al. 2008; Dick and Tremblay 2012).
In addition to impairment in the SLF/AF, we found decreased FA in the left IFOF/ILF (Fig. 2) and confirmed that the WM integrity of the IFOF was associated with AQ and PQ in aphasia. The IFOF/ILF associated with the ventral stream of the language network and it has been postulated to connect occipital cortices to the anterior temporal and inferior frontal cortices (Dick and Tremblay 2012). The ventral pathway may be involved in linking speech to higher-level semantic representations (Hickok and Poeppel 2004; Dick and Tremblay 2012). The IFOF is also part of a temporal lobe fiber network supporting language comprehension (Saur et al. 2008). However, the role of the IFOF/ILF in acute aphasia and severity remains unclear.
The UF has been considered as another potentially major area for a distributed semantic language memory network (Patterson et al. 2007; Tsapkini et al. 2011) and results from the current study found decreased FA in the UF of patients with aphasia compared with healthy controls. As previous studies suggested that the reduction in FA in the UF is related to the semantic variant subtype of primary progressive aphasia (Galantucci et al. 2011) and damage to the UF predicts impaired speech production in aphasia, our data lends supporting evidence to previous studies suggesting an involvement of the UF in the semantic processing of language (Catani et al. 2013; Fridriksson et al. 2013).
Nonetheless, this work has several limitations. First, the sample size was relatively small for studies involving abnormal WM integrity in aphasia. In addition, a longitudinal study is needed to examine whether treatments would improve the integrity of the WM connecting the various cortical language regions (van Hees et al. 2014a). Finally, constructing an anatomical connectome would provide new ways of conceptualizing the disconnections of remote and apparently intact cortical structures in aphasia (Bonilha et al. 2014a, b, 2015).
Conclusions
The current study examined the WM microstructure changes and associations with clinical characteristics in acute aphasias using a TBSS-based DTI method. We found a reduction in FA in both the anterior segment of SLF and IFL/IFOF located in dorsal and ventral language pathways, respectively. These results suggest that aphasias typically involve a breakdown of multiple connections in dorsal and ventral streams of language and that these breakdowns contribute to the core symptoms of this syndrome. In addition, linear correlation analyses suggested that impaired WM integrity in both language pathways not only contributed to language performance, but also to general cognitive status. Taken together, this damaged dual-stream architecture could serve as a neuromarker for acute aphasias after stroke and may aide in the development of treatment targets for this syndrome.
Abbreviations
- ABC:
-
Aphasia battery of Chinese
- AF:
-
Arcuate fasciculus
- AQ:
-
Aphasia quotient
- CQ:
-
Cortical quotient
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- IFOF:
-
Inferior fronto-occipital fasciculus
- ILF:
-
Inferior longitudinal fasciculus
- PQ:
-
Performance quotient
- ROI:
-
Region of interest
- SLF:
-
Superior longitudinal fasciculus
- TBSS:
-
Tract-based spatial statistics
- UF:
-
Uncinate fasciculus
- WM:
-
White matter
References
Allendorfer JB, Storrs JM, Szaflarski JP (2012) Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci 30:103–113
Ardila A (2010) A proposed reinterpretation and reclassification of aphasic syndromes. Aphasiology 24:363–394
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Bernal B, Ardila A (2009) The role of the arcuate fasciculus in conduction aphasia. Brain 132:2309–2316
Bonilha L, Nesland T, Rorden C, Fillmore P, Ratnayake RP, Fridriksson J (2014a) Mapping remote subcortical ramifications of injury after ischemic strokes. Behav Neurol 2014:215380
Bonilha L, Rorden C, Fridriksson J (2014b) Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke 45:988–993
Bonilha L, Gleichgerrcht E, Nesland T, Rorden CFridriksson J (2015) Success of anomia treatment in aphasia is associated with preserved architecture of global and left temporal lobe structural networks. Neurorehabil Neural Repair 30:266–279
Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC (2008) Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol 29:483–487
Brett M, Leff AP, Rorden C, Ashburner J (2001) Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage 14:486–500
Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C et al (2013) A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136:2619–2628
Crinion JT, Leff AP (2015) Using functional imaging to understand therapeutic effects in poststroke aphasia. Curr Opin Neurol 28:330–337
Damasio AR (1992) Aphasia. N Engl J Med 326:531–539
Dick AS, Tremblay P (2012) Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain 135:3529–3550
Forkel SJ, Thiebaut de Schotten M, Dell’Acqua F, Kalra L, Murphy DG, Williams SC et al (2014) Anatomical predictors of aphasia recovery: a tractography study of bilateral perisylvian language networks. Brain 137:2027–2039
Frey S, Campbell JS, Pike GB, Petrides M (2008) Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci 28:11435–11444
Fridriksson J, Guo D, Fillmore P, Holland A, Rorden C (2013) Damage to the anterior arcuate fasciculus predicts non-fluent speech production in aphasia. Brain 136:3451–3460
Galantucci S, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F et al (2011) White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 134:3011–3029
Gao SR, Chu YF, Shi SQ, Peng Y, Dai SD, Wang MH (1992) A standardization research of the aphasia battery of Chinese. Chin Ment Health J (Chinese) 6:125–128
Geva S, Baron JC, Jones PS, Price CJ, Warburton EA (2012) A comparison of VLSM and VBM in a cohort of patients with post-stroke aphasia. Neuroimage Clin 1:37–47
Geva S, Correia MM, Warburton EA (2015) Contributions of bilateral white matter to chronic aphasia symptoms as assessed by diffusion tensor MRI. Brain Lang 150:117–128
Hickok G, Poeppel D (2004) Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition 92:67–99
Hickok G, Poeppel D (2007) The cortical organization of speech processing. Nat Rev Neurosci 8:393–402
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS et al (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347
Jang SH (2013) Diffusion tensor imaging studies on arcuate fasciculus in stroke patients: a review. Front Hum Neurosci 7:749
Ji GJ, Zhang Z, Xu Q, Wei W, Wang J, Wang Z et al (2015) Connectome reorganization associated with surgical outcome in temporal lobe epilepsy. Medicine (Baltimore) 94:e1737
Kim SH, Lee DG, You H, Son SM, Cho YW, Chang MC et al (2011) The clinical application of the arcuate fasciculus for stroke patients with aphasia: a diffusion tensor tractography study. Neurorehabilitation 29:305–310
Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T et al (2007) Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: tract-based spatial statistics study of aging. Neuroimage 35:478–487
Krestel H, Annoni JM, Jagella C (2013) White matter in aphasia: a historical review of the Dejerines’ studies. Brain Lang 127:526–532
Kummerer D, Hartwigsen G, Kellmeyer P, Glauche V, Mader I, Kloppel S et al (2013) Damage to ventral and dorsal language pathways in acute aphasia. Brain 136:619–629
Kwon HG, Jang SH (2011) Excellent recovery of aphasia in a patient with complete injury of the arcuate fasciculus in the dominant hemisphere. Neurorehabilitation 29:401–404
Liao W, Ji GJ, Xu Q, Wei W, Wang J, Wang Z et al (2016) Functional connectome before and following temporal lobectomy in mesial temporal lobe epilepsy. Sci Rep 6:23153
Liu L, Luo XG, Dy CL, Ren Y, Feng Y, Yu HM et al (2015) Characteristics of language impairment in Parkinson’s disease and its influencing factors. Transl Neurodegener 4:2
Lu J, Wu J, Yao C, Zhuang D, Qiu T, Hu X et al (2013) Awake language mapping and 3-Tesla intraoperative MRI-guided volumetric resection for gliomas in language areas. J Clin Neurosci 20:1280–1287
Maldonado IL, Moritz-Gasser S, de Champfleur NM, Bertram L, Moulinie G, Duffau H (2011) Surgery for gliomas involving the left inferior parietal lobule: new insights into the functional anatomy provided by stimulation mapping in awake patients. J Neurosurg 115:770–779
Patterson K, Nestor PJ, Rogers TT (2007) Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci 8:976–987
Price CJ (2012) A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62:816–847
Rosso C, Vargas P, Valabregue R, Arbizu C, Henry-Amar F, Leger A et al (2015) Aphasia severity in chronic stroke patients: a combined disconnection in the dorsal and ventral language pathways. Neurorehabil Neural Repair 29:287–295
Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS et al (2008) Ventral and dorsal pathways for language. Proc Natl Acad Sci USA 105:18035–18040
Schlaug G, Marchina S, Norton A (2009) Evidence for plasticity in white-matter tracts of patients with chronic Broca’s aphasia undergoing intense intonation-based speech therapy. Ann N Y Acad Sci 1169:385–394
Selnes OA, Van Zijl PC, Barker PB, Hillis AE, Mori S (2002) MR diffusion tensor imaging documented arcuate fasciculus lesion in a patient with normal repetition performance. Aphasiology 16:897–902
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Tak HJ, Jang SH (2014) Relation between aphasia and arcuate fasciculus in chronic stroke patients. BMC Neurol 14:46
Thomas SA, Lincoln NB (2008) Predictors of emotional distress after stroke. Stroke 39:1240–1245
Tsapkini K, Frangakis CE, Hillis AE (2011) The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain 134:3094–3105
Turken AU, Dronkers NF (2011) The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front Syst Neurosci 5:1
van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA (2014a) Changes in white matter connectivity following therapy for anomia post stroke. Neurorehabil Neural Repair 28:325–334
van Hees S, McMahon K, Angwin A, de Zubicaray G, Read S, Copland DA (2014b) A functional MRI study of the relationship between naming treatment outcomes and resting state functional connectivity in post-stroke aphasia. Hum Brain Mapp 35:3919–3931
Warren JE, Wise RJ, Warren JD (2005) Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci 28:636–643
Warren JE, Crinion JT, Lambon Ralph MA, Wise RJ (2009) Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain 132:3428–3442
Yang M, Li J, Li Y, Li R, Pang Y, Yao D et al (2016a) Altered intrinsic regional activity and interregional functional connectivity in post-stroke aphasia. Sci Rep 6:24803
Yang M, Li J, Yao D, Chen H (2016b) Disrupted intrinsic local synchronization in poststroke aphasia. Medicine (Baltimore) 95:e3101
Yu ZZ, Jiang SJ, Bi S, Li J, Lei D, Sun LL (2013) Relationship between linguistic functions and cognitive functions in a clinical study of Chinese patients with post-stroke aphasia. Chin Med J (Engl) 126:1252–1256
Zhu D, Chang J, Freeman S, Tan Z, Xiao J, Gao Y et al (2014) Changes of functional connectivity in the left frontoparietal network following aphasic stroke. Front Behav Neurosci 8:167
Acknowledgments
We thank to the radiologist Ying Liu in the Hospital of Fuzhou for manually traced the outline of the lesion. This work was supported by the 973 project (2012CB517901), 863 project (2015AA020505), Natural Science Foundation of China (61533006, and 81471653), China Postdoctoral Science Foundation (No. 2013M532229), and Fundamental Research Funds for the Central Universities (ZYGX2013Z004).
Author Contributions
DY, WL, and HC designed the study. MY, YL, and JL contributed to data collection. MY, YL, JL, WL and HC contributed to data analysis, data interpretation, and manuscript preparation. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Yang, M., Li, Y., Li, J. et al. Beyond the Arcuate Fasciculus: Damage to Ventral and Dorsal Language Pathways in Aphasia. Brain Topogr 30, 249–256 (2017). https://doi.org/10.1007/s10548-016-0503-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-016-0503-5