Abstract
Proton magnetic resonance spectroscopy (1H-MRS) studies have previously reported reduced brain N-acetyl aspartate (NAA) and increased myo-inositol (mI) in people with established Alzheimer’s disease (AD). The earliest structure affected by AD is the hippocampus but relatively few studies have examined its neuronal integrity by MRS in AD and fewer still in people with amnestic mild cognitive impairment (MCI). We measured the hippocampal concentration of NAA, mI, choline (Cho) and creatine + phosphocreatine (Cr + PCr) in 39 patients with AD, 21 subjects with MCI and 38 age matched healthy elderly controls. Patients with AD had a significantly lower hippocampal [NAA] than controls, with subjects with MCI intermediate between the other two groups. [NAA] was positively correlated with memory in the impaired groups. Using mean hippocampal [NAA] and [Cr + PCr] we correctly classified 72% of people with AD, and 75% of controls. Reductions in [NAA] can be detected in the hippocampi of subjects with MCI and hippocampal [NAA] and [Cr + PCr] can distinguish between mild AD and normal elderly controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much research effort has been expended on trying to identify a marker of disease that would firstly help differentiate normal ageing from either MCI or mild AD and secondly help differentiate subjects with MCI due to underlying AD that will progress to dementia from those who will not. This is an important clinical problem representing one of the main reasons for referral to memory clinics. Currently no such biomarker exists although progress has been made in both biochemical (Shaw et al. 2009; Thambisetty et al. 2010) and neuroimaging markers (Fox et al. 1996; Jack et al. 1992; Jack et al. 1997; Juottonen et al. 1999; Xu et al. 2000).
One neuroimaging marker that shows promise is 1H-MRS (Kantarci 2007; Valenzuela and Sachdev 2001). It is clear that there is a pattern of metabolic differences in AD relative to normal controls detectable using 1H-MRS but to date relatively few studies have included subjects with mild AD or MCI and fewer still have included only MCI and control subjects recruited in the community. In this study we have examined hippocampal 1H-MRS in a group of patients with known memory problems (mild AD recruited through memory clinics) and in subjects not previously known to have memory problems (control and MCI subjects recruited in the community). We confirm reduced hippocampal [NAA] in mild AD relative to controls, with MCI subjects recruited in the community demonstrating an intermediate level.
Methods
Subjects
Subjects with mild dementia, diagnosed as probable AD according to NINCDS-ADRDA criteria (McKhann et al. 1984), were recruited from out-patient/memory clinics in South London. Subjects with MCI and unaffected controls were recruited from three general practice lists. All people over the age of 65 years registered with these GPs were contacted and invited to participate in the research project. Subjects were interviewed in the community and classified as normal elderly if they scored between 28 and 30 on the Mini Mental State Exam (MMSE) (Folstein et al. 1975), did not score abnormally on the Consortium to establish a registry for Alzheimer’s disease (CERAD) neuropsychological battery (Morris et al. 1989), were independent and living in the community, did not have a psychiatric or neurological condition and were not depressed using the Geriatric Depression Scale (Yesavage 1988). Subjects were defined as amnestic MCI if they met the criteria of Petersen et al. (2001).
The study was approved by the South London and Maudsley/Institute of Psychiatry Local Research Ethics Committee.
1H-MRS Protocol
Subjects were scanned using a 1.5 Tesla, GE NV/i Signa MR System (General Electric, Milwaukee, WI, USA) at the Maudsley Hospital, London. 3D T1-weighted volume images were acquired in the axial plane with 1.5 mm contiguous sections using acquisition parameters chosen using a contrast simulation tool (Simmons et al. 1996). Repetition time (TR) was 13.8 ms, inversion time (TI) 450 ms, echo time (TE) 2.8 ms, and the flip angle was 20° with one data average and a 256 × 256 × 124 voxel matrix. Acquisition time was 6 min, 27 s.
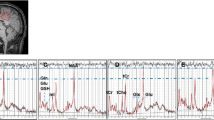
1H-MRS voxels of interest measuring 20 × 20 × 15 mm³ (6 ml) were defined in standard locations in the left and right hippocampi using previously published method (Robertson et al. 2001). We chose hippocampal regions of interest as this is one of the earliest sites of change in Alzheimer’s disease. The anterior extent of the voxel was defined as the coronal slice where the amygdala disappeared, with the posterior extent 20 mm from this (Fig. 1). The hippocampal volume of interest contained both grey and white matter and included the parahippocampal gyrus and the posterior portion of the amygdala.
A point resolved spectroscopy (PRESS) pulse sequence (TE 35 ms, TR 1500 ms, 256 data averages and 2048 points) with automated shimming and water suppression and excellent reproducibility (Simmons et al. 1998) was used to obtain spectra from each voxel after CHESS water suppression with high signal to noise ratio and clearly resolved NAA, Cho, mI and Cr + PCr peaks among other metabolites. Non water-suppressed data were also collected for water referencing, but data was not collected to measure metabolite T1 and T2 relaxation times for individual subjects due to the limited tolerance of Alzheimer patients for MRI scanning. Not all subjects had spectral data from both left and right hippocampus. No significant differences were found in the metabolite content between the right and the left side of hippocampus. Therefore, we averaged the metabolite measures from the left and right hippocampus from the subjects which had data from both hemispheres.
1H-MRS Data Analysis
Differences in proportions of white and grey matter in the 1H MRS voxels may confound group differences in metabolite concentrations. Thus, to ensure that differences in tissue composition did not account for metabolic differences between subject groups, we segmented the SPGR volumes using SPM (Statistical Parametric Mapping) software (http://www.fil.ion.ucl.ac.uk/spm) to determine the percentage of grey matter, white matter and CSF within the MRS voxels. The position of the 1H MRS voxels relative to the segmented 3D dataset was determined automatically using in-house software. T1 and T2 corrections were applied for each metabolite using literature values (Christiansen et al. 1993).
Spectra were processed using LCModel (Provencher 1993) and metabolite concentrations were automatically corrected for CSF contamination of the voxel by dividing by the tissue fraction of the MRS voxel determined using SPM. These corrected concentrations were then calibrated to absolute molar units with respect to a phantom of known concentration, which was scanned in the same scanning session as the subject, using a PRESS acquisition with the same TE and TR.
Statistical Analysis
Comparisons between age, education level and MRS metabolites between the groups were made using univariate general linear models. Differences between gender were tested for using a Chi Squared test. Group differences in metabolite concentrations were tested with one-way analysis of variance (ANOVA), with group as the between subject factor. There were no significant interactions between the side from which 1H-MRS metabolites were measured (left or right hippocampus) or gender and group. Therefore, mean hippocampal metabolite values were considered in the analysis. Differences in metabolite concentrations were considered significant if P < 0.05. Least significant difference (LSD) post hoc tests were employed. The association between neuropsychological tests and the MRS variables in the patients with AD were analyzed using partial correlations, covarying for age. We used logistic regression to assess whether MRS metabolite concentrations could reliably classify individuals according to AD status, compared to the MMSE. Statistical analyses were performed using SPSS version 11.0.1 (SPSS Inc, Chicago, Ill).
Results
We studied 98 subjects; 39 with AD, 21 with MCI and 38 healthy elderly controls. The three groups were not significantly different with respect to age, years of education or male to female ratio. The subject group with AD had a MMSE score indicating mild dementia (mean 23; range 16–30; Table 1). Composition of the MRS voxel did not differ between the groups with respect to grey or white matter, but the proportion of CSF was greater in the patients with AD than in the normal elderly controls (F = 12.01, P = 0.0004).
People with AD had a substantial reduction in [NAA] compared to normal controls (F = 13.33, P = 0.0005) and a significant reduction compared to those with MCI (P < 0.05). There was a non-significant trend towards a difference in NAA between MCI and normal controls (P = 0.06; Fig. 2). [Cr + PCr] was reduced in both AD (F = 4.33, P < 0.005) and MCI (P < 0.05) relative to controls but there was no difference in [Cr + PCr] between those with AD and MCI. We found no significant between-group differences in [mI] or [Cho] (Table 2) Fig. 3.
Hippocampal [NAA] within the hippocampi of healthy controls subjects, patients with Alzheimer’s disease and subjects with mild cognitive impairment. One-way ANOVA indicated that people with Alzheimer’s disease had significantly lower [NAA] compared to the healthy control subjects (P = 0.0004) and subjects with mild cognitive impairment (P = 0.024). † differs from controls ‡ differs from MCI
Hippocampal [Cr + PCr] within the hippocampi of healthy controls subjects, patients with Alzheimer’s disease and subjects with mild cognitive impairment. The healthy control subjects had higher [Cr + PCr] than people with Alzheimer’s disease (P = 0.003) and subjects with mild cognitive impairment (P = 0.023). † differs from controls ‡ differs from MCI
Logistic regression analysis showed that mean hippocampal [NAA] and [Cr + PCr] correctly categorised 75% of normal controls with a negative predictive probability of 73% and correctly categorised 72% of the patients with AD with a positive predictive probability of 74%.
Within the combined AD and MCI groups we carried out a planned exploration of the relationship between overall cognitive ability (as measured by the MMSE) and hippocampal [NAA]. The correlation between mean [NAA] and overall cognitive ability showed a trend which did not reach significance (r = 0.255, P = 0.056). However, there were significant correlations between other neuropsychological parameters and [NAA], with hippocampal [NAA] correlating with both delayed recall of a learned word list (r = 0.330, P = 0.018) and delayed praxis (r = 0.341, P = 0.015). These correlations remained significant after correcting for age (r = 0.345, P = 0.016 and r = 0.334, P = 0.020, respectively).
Discussion
In this study we have found that changes in [NAA] and [Cr + PCr], measured by 1H-MRS, differ between mild AD, MCI and unaffected elderly controls and correlates with neuropsychological measures of cognition in the impaired groups. We chose to study MRS changes in the hippocampus as this area is affected early in the Alzheimer’s disease process and studied only subjects early in the disease process as these present the most clinically relevant group with respect to a diagnostic biomarker.
Previously a decrease in [NAA] in AD has been well established in different brain regions (reviewed in Valenzuela and Sachdev 2001) as well as in whole brain (Falini et al. 2005). Many of these studies have used MRS in regions of the brain other than the hippocampus. Obtaining a reliable MRS spectrum in hippocampus in AD can be challenging as this is the site of earliest pathology and hence shows increased atrophy. However, some previous studies have focused on hippocampus and these also have shown a decrease in NAA (Ackl et al. 2005; Dixon et al. 2002; Schuff et al. 1997). Our results confirm and extend this work, demonstrating that hippocampal [NAA] is reduced even in very mild AD compared to both healthy controls and to those with MCI. We have shown that mean hippocampal NAA values in MCI fall between those of mild AD and normal elderly controls.
This finding of an intermediate value of [NAA] in MCI also mirrors previous studies. Thus, Chantal et al. (2004) reported a difference in the NAA/Cr + PCr ratio between subjects with MCI and controls in the left medial temporal lobe and Ackl et al. (2005) found a similar difference in hippocampus. However, neither found a difference between subjects with MCI and patients with AD. A decrease in NAA/Cr + PCr in MCI relative to controls was also found by (Kantarci et al. 2006) but (Catani et al. 2001) and (Kantarci et al. 2000) found no difference in NAA/Cr + PCr between subjects with MCI and controls. Indeed NAA/Cr + PCr was found to be the most sensitive measure distinguishing between MCI and AD (Kantarci et al. 2002).
It is clear from these studies that measures of NAA and NAA/Cr + PCr are decreased in established AD but the findings from very mild AD and from MCI are less certain. One possible reason for this discrepancy is the regions of brain examined in different studies—it is possible that changes in MCI are confined to those regions of the brain affected early in the AD process. Our finding of an intermediate NAA value in MCI in hippocampus is in line with this hypothesis.
Another source of variability in studies of MCI is the means of subject recruitment. In a previous systematic review (Bruscoli and Lovestone 2004) we noted the substantial difference in conversion rates from MCI to AD—ranging from less than 5% to more than 30% per year. The only variable distinguishing high from low conversion rates was the source of the recruitment to the study, with memory clinic attendees showing a conversion rate twice that of community dwelling volunteers. This finding suggests that MCI subjects entering studies via memory clinics are substantially different to subjects with MCI in the community even though clinical and neuropsychological assessments fail to distinguish between these groups. Most, if not all, previous 1H-MRS studies of MCI have recruited subjects through memory clinics or similar specialist services raising questions as to the generalisability of these findings as well as perhaps explaining some of the apparent discrepancies in the literature. All of the subjects with MCI in our study were recruited through direct contact and not through self or professional referral to a memory clinic. The only difference between controls and MCI subjects in the present study was the MCI inclusion criteria and therefore we are confident that this group represents, more fully than in previous studies, those people with a mild cognitive impairment as it occurs frequently in the community. As such the group of people with MCI represented in this study closely resemble an important target group in the community—those concerned about their memory but not yet acting upon these concerns. We find that in this representative group MRS metabolites are indeed altered in the hippocampus with a reduction in [Cr + PCr] and a trend towards a reduction in [NAA]. Thus, MCI seems to be an intermediate state and in line with this we found that within the impaired group hippocampal [NAA] was related to overall cognitive ability, as measured by the MMSE, and more specifically to memory, as measured by the delayed recall of a learnt word list and the delayed praxis subtests of the CERAD neuropsychological battery.
[NAA] has also been used to discriminate between patients with AD and controls. We found that we could best distinguish between our patients and controls using a model including hippocampal [NAA] and [Cr + PCr] (74% classified correctly). These findings are in line with others (Dixon et al. 2002; Schuff et al. 1997) although these previous studies included more severely affected patients. Despite this we found similar classification accuracy even in very mild AD.
We also found a reduction in [Cr + PCr] in the subjects with AD and MCI compared to normal controls. The Cr + PCr peak measures creatine and phosphocreatine, which are present in both neurons and glial cells and are thought to reflect energy use and storage by neurons, and are relatively stable over time and largely unaffected by disease states. However, our finding of a significantly lower [Cr + PCr] in people with AD and those with MCI may suggest an impairment in energy metabolism which in AD is in accordance with some (Adalsteinsson et al. 2000; Ernst et al. 2004) but not all (Huang et al. 2001), previous findings.
We found no difference in hippocampal [mI] between AD, controls and MCI. Previously, increases in mI in AD have been reported in parietal lobe (Rose et al. 1999) and temporal lobe (Chantal et al. 2002; Parnetti et al. 1997). Only one study has measured mI levels within the hippocampus, however, and this reported no change in mI/NAA or mI/Cho + Cr + PCr (Dixon et al. 2002).
Kantarci et al. (2000) found that mI/Cr + PCr was different between MCI and control groups in posterior cingulate gyrus and paratrigonal white matter and Chantal et al. (2004), between MCI and AD in parietotemporal cortex. No one has previously examined hippocampal mI in people with MCI.
Conclusions
We have demonstrated that there is a significant reduction in hippocampal [NAA] and [Cr + PCr] in early AD, and that people with MCI are intermediate between early AD and control groups. The test characteristics of the MRS data do not meet the criteria for a definitive stand-alone biomarker (The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and, National Institute on Aging Working Group 1998) but nonetheless this data is promising considering that we have studied a group of patients in the severity range where diagnosis can be difficult and in people not yet referred by themselves or by their physicians to specialist services. These findings suggest that in this group of patients MRS, alone or in combination with other imaging and non-imaging biomarkers may prove to be useful in the diagnostic process and may also be valuable in assessing treatments for dementia.
References
Ackl N, Ising M, Screiber YA et al (2005) Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 384:23–28
Adalsteinsson E, Sullivan EV, Kleinhans N et al (2000) Longitudinal decline of the neuronal marker N-acetyl aspartate in Alzheimer’s disease. Lancet 355:1696–1697
Bruscoli M, Lovestone S (2004) Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 16:129–140
Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, Gobbi G, Senin U, Mecocci P (2001) 1H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport 12(11):2315-2317
Chantal S, Braun CMJ, Bouchard RW et al (2004) Similar 1H magnetic resonance spectroscopic metabolic pattern in the medial temporal lobes of patients with mild cognitive impairment and Alzheimer disease. Brain Res 1003:26–35
Chantal S, Labelle M, Bouchard RW, Braun CM, Boulanger Y (2002) Correlation of regional proton magnetic resonance spectroscopic metabolic changes with cognitive deficits in mild Alzheimer disease. Arch Neurol 59:955-962
Christiansen P, Toft P, Larsson HBW et al (1993) The concentration of N-acetyl asparate, creatine + phosphocreatine and choline in different parts of the brain in adulthood and senium. Magn Reson Imaging 11:799–806
Dixon RM, Bradley KM, Budge MM et al (2002) Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in Alzheimer’s disease. Brain 125:2332–2341
Ernst T, Chang L, Melchor R et al (2004) Frontotemporal dementia and early Alzheimer disease: differentiation with frontal lobe H-1 MR spectroscopy. Radiology 203:829–836
Falini A, Bozzali M, Magnani G et al (2005) A whole brain MR spectroscopy study from patients with Alzheimer’s disease and mild cognitive impairment. Neuroimage 26:1159–1163
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Fox NC, Warrington EK, Freeborough PA, Hartikainen P, Kennedy AM, Stevens JM, Rossor MN (1996) Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain 119(Pt 6):2001–2007
Huang W, Alexander GE, Chang L et al (2001) Brain metabolite concentration and dementia severity in Alzheimer’s disease: a 1H MRS study. Neurology 57:626–632
Jack CR Jr, Petersen RC, O’Brien PC, Tangalos EG (1992) MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology 42:183–188
Jack CR Jr, Petersen RC, Xu YC, Waring SC, O’Brien PC, Tangalos EG, Smith GE, Ivnik RJ, Kokmen E (1997) Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 49:786–794
Juottonen K, Laakso MP, Partanen K, Soininen H (1999) Comparative MR analysis of the entorhinal cortex and hippocampus in diagnosing Alzheimer disease. AJNR Am J Neuroradiol 20:139–144
Kantarci K (2007) 1H magnetic resonance spectroscopy in dementia. Br J Radiol 80(Spec No 2):146–152
Kantarci K, Jack CRJ, Xu YCM et al (2000) Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology 55:210–217
Kantarci K, Xu Y, Shiung MM et al (2002) Comparative diagnostic utility of different MR modalities in mild cognitive impairment and Alzheimer’s disease. Dement Geriatr Cogn Disord 14:198–207
Kantarci K, Weigand SD, Petersen RC et al (2006) Longitudinal (1)H MRS changes in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 28:1330–1339
McKhann G, Drachman D, Folstein M et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34:939–944
Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C (1989) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39(9):1159–1165
Parnetti L, Tarducci R, Presciutti O, Lowenthal DT, Pippi M, Palumbo B, Gobbi G, Pelliccioli GP, Senin U (1997) Proton magnetic resonance spectroscopy can differentiate Alzheimer's disease from normal aging. Mech Ageing Dev 97:9–14
Petersen RC, Stevens JC, Ganguli M et al (2001) Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the quality standards subcommittee of the American academy of neurology. Neurology 56:1133–1142
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Robertson DM, van Amelsvoort TM, Daly EB, Simmons A, Whitehead MM, Morris R, Murphy KC, Murphy DGM (2001) Effects of estrogen replacement therapy on human brain aging: an in vivo 1H MRS study. Neurology 57:2114–2117
Rose SE, de Zubicaray GI, Wang D, Galloway GJ, Chalk JB, Eagle SC, Semple J, Doddrell DM (1999) A 1H MRS study of probable Alzheimer's disease and normal aging: implications for longitudinal monitoring of dementia progression. Magn Reson Imaging 17:291–299
Schuff N, Amend D, Ezekiel F et al (1997) Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology 49:1513–1521
Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ, Alzheimer’s Disease Neuroimaging Initiative (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65(4):403–413
Simmons A, Arridge SR, Barker G et al (1996) Simulation of MRI cluster plots and application to neurological segmentation. Magn Reson Imaging 14:73–92
Simmons A, Smail M, Moore E, Williams SCR (1998) Serial precision of metabolite peak area ratios and water referenced metabolite peak areas in proton MR spectroscopy of the human brain. Magn Reson Imaging 16(3):319–330
Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund L-O, Kinsey A, Guentert A, Proitsi P, Powell J, Causevic M, Killick R, Lynham S, Broadstock M, Tunnard C, Leung R, Foy C, O’Brien D, Breen G, Furney S, Ward M, Kloszewska I, Mecozzi P, Soininen H, Tsolaki M, Vellas B, Williams J, Murphy D, Parkins I, Resnick S, Ferucci L, Wong D, Zhou Y, Muehlboeck S, Evans A, Francis P, Spenger C, Lovestone L for the AddNeuroMed consortium (2010) Clusterin, an amyloid chaperone protein in plasma, is associated with severity, pathology and progression in Alzheimer’s disease. Arch Gen Psych 67(7):739–748
The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and, National Institute on Aging Working Group (1998) Consensus report of the working group on: “molecular and biochemical markers of Alzheimer’s disease”. Neurobiol Aging 19:109–116
Valenzuela MJ, Sachdev P (2001) Magnetic resonance spectroscopy in AD. Neurology 56:592–598
Xu Y, Jack CR Jr, O’Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC (2000) Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology 54:1760–1767
Yesavage JA (1988) Geriatric depression scale. Psychopharmacol Bull 24:709–711
Acknowledgments
This study was supported by the Alzheimer’s Research Trust and the NIHR Specialist Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and King’s College London, Institute of Psychiatry, London, United Kingdom. We thank the participants and their relatives for taking part.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foy, C.M.L., Daly, E.M., Glover, A. et al. Hippocampal Proton MR Spectroscopy in Early Alzheimer’s Disease and Mild Cognitive Impairment. Brain Topogr 24, 316–322 (2011). https://doi.org/10.1007/s10548-011-0170-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-011-0170-5