Summary
Background:
Tetrahydrobiopterin (BH4) is a potential new orphan drug for the treatment of some patients with phenylketonuria (PKU), mostly mild forms. Numerous studies have confirmed this finding and BH4-responsiveness may be predicted to some extent from the corresponding genotype.
Aim:
To investigate the response to BH4 loading test, the phenylalanine hydroxylase (PAH) mutations and the long-term therapeutic efficacy of BH4 in patients with PKU, and to better define BH4-responsive patients according to phenylalanine (Phe) levels and dietary phenylalanine tolerance.
Methods:
30 Italian PKU patients (age range: 6 months–24 years; 12 female, 18 male) were included in this retrospective study. Eleven out of 30 patients presented with Phe levels below 450 μmol/L and 19 patients with Phe levels between 450 and 900 μmol/L. In the second group, we investigated the effect of long-term (6 months–7 years) oral administration of BH4 on blood Phe levels and daily Phe tolerance.
Results:
In all patients with initial blood Phe levels <450 μmol/L (n = 11), BH4 loading test was positive, but no treatment was introduced. In 12 out of 19 patients with blood Phe levels >450 μmol/L and positive at BH4 loading, the treatment with BH4 (10 mg/kg per day) was initiated. Before BH4 treatment, Phe tolerance was less than 700 mg/day in all patients except for one (patient no. 9), increasing to 2–3-fold (from 498 ± 49 to 1475 ± 155 mg/day) on BH4 treatment. In these patients the amino acid mixture supplementation was stopped and the diet was a combination of low-protein foods and natural proteins, mostly from animal sources.
Conclusion:
Long-term BH4 substitution (up to 7 years) in a group of moderate PKU patients allowed a substantial relaxation of the dietary restrictions or even replacement of the diet with BH4 without any adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperphenylalaninaemia (HPA) is a disorder caused by a deficiency or a decreased activity of phenylalanine-4-hydroxylase (PAH, EC 1.14.16.1) due to either a mutated enzyme protein or a deficiency of its cofactor tetrahydrobiopterin (BH4). HPA due to a mutated PAH produces a spectrum of phenotypes including classic phenylketonuria (PKU), moderate PKU, mild PKU and mild HPA.
Historically, patients with PAH deficiency in whom the oral loading test with BH4 lowered the plasma Phe concentration levels have been observed for many years (Milstien and Kaufman 1975). However, until several years ago when a publication by Kure et al. (1999) reported the first six cases of BH4 responsive PKU, not much attention was given to these phenomena. Since then, numerous studies have confirmed this finding (Bernegger and Blau 2002; Blau and Erlandsen 2004; Boveda et al 2007; Cerone et al 2004; Desviat et al 2004; Dhondt et al 2003; Fiege and Blau 2007; Fiori et al 2005; Gramer et al 2007; Hennermann et al 2005; Lambruschini et al 2005; Lässker et al 2002; Leuzzi et al 2006; Levy et al 2007; Lindner et al 2001, 2003; Lücke et al 2003; Matalon et al 2002, 2005; Mitchell et al 2005; Muntau et al 2002; Okano et al 2004; Perez-Duenas et al 2004; Spaapen and Estela Rubio-Gozalbo 2003; Spaapen et al 2001; Trefz et al 2001; Weglage et al 2002). From all these studies it is clear that the most frequent responders have a mild PKU (Fiege and Blau 2007) and that it may be predicted from the corresponding mutations. Recently, Zurfluh and colleagues (2008) reported the genotypes of 315 patients who responded to the BH4 loading test with lowering of their blood Phe levels. The DNA analysis of these patients identified 57 mutations in the PAH gene and the genotype-predicted prevalence of BH4-responsiveness was found to be higher than had been expected from BH4 loading test data. Thus, BH4 responsiveness cannot be predicted solely on the basis of PAH mutations, but potential non-responders can certainly be excluded.
However, to date, no long-term study has been published on the administration of BH4 and changing of Phe tolerance in patients with PKU. We therefore investigated patients with mild to moderate PKU who responded to BH4 loading test for the long-term (up to 7 years) therapeutic efficacy of BH4 by monitoring the Phe blood levels and dietary phenylalanine tolerance and concluded that this new pharmacological approach is a safe and effective therapy.
Material and methods
Patients
This retrospective study group was formed by 30 patients of the Division of Metabolic Diseases, University Children’s Hospital Padua (age range 6 months–24 years; 12 female, 18 male). Eleven out of 30 patients presented with Phe levels under 450 μmol/L and 19 patients with Phe levels between 450 and 900 μmol/L. All patients had been diagnosed through the newborn screening programme. The inclusion criteria for the study were as follows: (a) known mutations in the PAH gene; (b) normal pterin profile and dihydropteridine reductase activity (no BH4 deficiency); (c) patient or parental agreement with the BH4 loading tests; (d) patients who previously responded positively to the BH4 loading test performed after 6 months of age; (e) patients who do not fully comply with a Phe-restricted diet. All these patients were previously documented with Phe level >450 μmol/L by home monitoring of blood spots and clinic assessments for a period ranging from 6 months to 4 years before starting BH4 therapy. This level identified patients who may benefit from improved Phe control (National Institutes of Health Consensus Development Conference Statement 2001). No guidelines are available at the moment in Italy and for newborns and children up to age 12 years we recommended dietary intervention when blood Phe level was >360 μmol/L.
BH4 loading test
6RBH4 (Schircks Laboratories, Jona, Switzerland), the biologically active form, was used for all tests. BH4 loading test were performed in all 30 patients with mild PKU and mild HPA. Patients were instructed to continue the same dietary practice before and during the test. The BH4 loading test followed the standard procedure (Blau 2008). Basal blood samples were taken from all patients. Patients were given 20 mg/kg BH4 after 3 h of fasting and 30 min before a meal to ensure good GI absorption. For patients who could not swallow the tablets, these were dissolved in 20 ml water in dim light and the suspension was administered within 30 min. Blood sampling was then done before and at 4, 8, 12, and 24 h after BH4 administration. Plasma Phe levels were determined using an amino acid analyser.
Dietary phenylalanine tolerance
Phe tolerance is the amount of Phe that the patient can consume per day while maintaining acceptable blood Phe levels for age (<360 μmol/L for patients <12 years of age and 800 μmol/L for patients >12 years of age) (Gramer et al 2007). Daily Phe intake (mg/day) was monitored by assessment of protein content in the diet (average of several days), as reported by the parents. Owing to the large variability in age (2–16 years), Phe tolerance was evaluated by repeated 3-day dietary protocols before sending the spot. Participants were asked to report any changes in diet on the occasion of clinical visits. The Phe intake was calculated by our computer system (Winfood 2, Medimatica, Italy).
Long-term BH4 treatment
Long-term BH4 treatment was started in 12 BH4-responsive PKU patients with a blood Phe level >450 μmol/L and with non-compliance with the low-Phe prescribed diet. The duration of the treatment varied from 6 months to 7 years and BH4 was given at a dose of 10 mg/kg body weight twice a day. During the treatment the diet was relaxed according to the actual plasma Phe concentration. Regular follow-up blood samples (Guthrie cards) were obtained before the first morning meal and Phe measurement was done by tandem mass spectrometry. No side-effects were observed during the long-term treatment.
Statistical analysis
The BH4 loading test is considered positive when initial plasma Phe concentrations decrease by at least 30% after 8 h. If plasma Phe values decreased <30% after 12–16 h, the patients were classed as partially responsive or slow responders and were not considered in the study. The overall percentage of patients who experienced a response to BH4 loading was calculated and the 95% confidence intervals were determined. Additionally, the percentage of patients who responded to long-term therapy was calculated for a baseline level of 450 μmol/L Phe. This level was chosen arbitrarily. Descriptive statistics were performed using the statistical package WinSTAT for Excel, version 2003.1.
Results
BH4 loading test
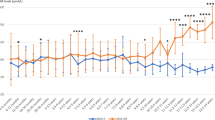
A positive response to BH4 (i.e. Phe reduction of more than 30% after 24 h) was observed in 23 out of 30 patients studied (76%). All 11 patients with Phe levels <450 μmol/L showed a positive response. In these patients BH4 treatment was not initiated. Of the 19 patients with a Phe level >450 μmol/L, 12 (63%) showed a positive response to BH4. In these patients, the plasma Phe decrease after the BH4 loading test was 54.5% ± 15.4%.
All BH4-responsive patients carried at least one mutation known to be BH4 responsive (Table 1), namely p.E390G, p.L48S, p.V388M, or p.R158Q, all of which are associated with residual PAH activity. However, it should be noted that we also detected mutations less frequently reported in BH4-responsive patients such as p.G48S, IVS10–11g>a, and p.I65V.
Long-term BH4 treatment
A summary of the results is shown in Table 1. BH4 loading test was positive in 12 patients with Phe levels >450 mmol/L. Before starting the BH4 therapy, all these patients showed elevated Phe concentration from 433 up to 1215 μmol/L. Only when the patients adhered to the restricted diet (approximately 50 mg Phe per kg/bw in the youngest and 15 mg/kg per day in the older), did Phe drop below defined threshold levels (e.g. 360 μmol/L during the first 12 years of life and 600 μmol/L up to 17 years ). The dietary restrictions were far-reaching and difficult to follow, especially in patients close to puberty when physical development is most demanding. As a consequence, BH4 therapy was started at the dosage of 10 mg/kg. Patients’ ages at the start of treatment ranged from 2 years to 16 years (5.5 ± 4.7) and they were on BH4 for a periods ranging from 6 months to 7 years. In all patients, the treatment with BH4 allowed a substantial relaxation of the dietary restriction with a daily Phe tolerance increased up to 2–3-fold.
In patients nos. 2, 4 and 9, in whom the BH4 loading test showed a marked reduction of Phe levels after 24 h (p < 0.001), BH4 therapy allowed the introduction of high-protein foods such as meat, with Phe levels below 360 μmol/L. Patients nos. 2 and 9 were continued on oral BH4 supplementation for 6 and 7 years respectively, and blood Phe was determined regularly at 14-day intervals. Both patients were clinically evaluated every month during the first 6 months and then every 3 months: their psychomotor development was normal and it has been adequate for each patient’s age. In patients 1, 3, 7, 8 and 12, a combined diet with a Phe intake of 100 mg/kg is still necessary to maintain blood levels below 360 μmol/L. All patients and their families indicate great improvement in their quality of life.
Discussion
Since the publication of Kure and colleagues (1999), various reports have demonstrated that a significant number of patients with PAH deficiency can be successfully treated with BH4. Many patients with HPA whose only treatment option was a life-long low-Phe diet can now benefit from alternative BH4 treatment, increasing their protein tolerance, and in many cases adopt a near-normal diet. Our findings are consistent with these data. Moreover, this is one of the very few studies reporting the effectiveness of long-term BH4 treatment in patients with BH4-responsive HPA.
BH4 loading tests were performed in all patients with mild PKU and mild HPA. In our diagnostic protocol of diagnostic evaluation of HPA we do not perform the BH4 loading test in the neonatal period because, as previously reported, the response to BH4 may be dependent on age (Lässker et al 2002). All the patients were tested after the age of 6 months because the growth rate and protein requirement change over time. The first 4–6 months of life often constitute a ‘honeymoon period’ and the protein requirement including the Phe intake is easily assimilated. In the latter half of first year of life, growth rate decreases and excess of protein including Phe intake is not diverted to growth. Performing the test at this time can avoid false positives due to a natural decline in Phe levels during the neonatal period.
All of our mild HPA patients (Phe <450 μmol/L at the time of BH4 loading test) have proved able to benefit from BH4 treatment, in line with previous studies. Phe concentrations in this group of patients were 2–4 times above normal with a rather high, but still not normal, Phe tolerance. Although there is no evidence that Phe blood concentrations <450 μmol/L may be toxic, there is no international consensus about safe Phe concentrations that may be regarded as ‘harmless’ to the brain. In many instances these patients may not have been treated with a low-Phe diet because it was believed that the possible benefit did not justify the intervention of a difficult and life-long treatment. However, some might wish to lower their blood Phe concentrations and increase their Phe tolerance with a less interventional therapy than diet to avoid even the possibility of a toxic effect from the HPA.
Regarding the genotype–phenotype association, in this group of patients BH4 responsiveness can be reliably predicted from the PAH genotype. This supports the recent report of Zurfluh and colleagues (2008) in which BH4 responsiveness was considered to be more common than previously assumed and a genotype analysis may be predictive.
In the patients with blood Phe levels >450 μmol/L, 12 (63%) showed a positive response to loading and were therefore suitable for long-term treatment. They were treated with BH4 (10 mg/kg per day; 1×) over a period ranging from 6 months to 7 years. All the patients benefited from BH4 substitution, which allowed a substantial relaxation of the dietary restrictions. Before BH4 treatment, Phe tolerance was less than 700 mg/day in all patients except for patient 9, increasing to 2–3-fold on BH4 treatment (Table 1). In these patients the amino acid mixture supplementation was stopped and the diet was a mixture of low-protein foods and natural proteins mostly from animal source. Studies investigating the effect of long-term treatment with BH4 on increased tolerance for natural proteins, making the amino acid mixture dispensable, are still scarce. Only a small number of patients have been reported who were able to stop supplementation with the amino acid mixture as a result of long-term BH4 treatment (Bélanger-Quintana et al 2005; Lambruschini et al 2005). In the study of Lambruschini and colleagues (2005), Phe tolerance (daily Phe intake allowing for blood Phe levels of 120–360 mmol/L) increased significantly (p = 0.004) in 9 patients with mild PKU and in 2 out of 4 patients with moderate PKU, during long-term treatment with 5 mg BH4/kg bw per day. Phe intake was increased by 200 mg/day per week. Mean Phe tolerance increased from 356–172 mg/day to 1546–192 mg/day. The Phe-free amino acid mixture could be completely removed from the patient’s diet. BH4 therapy was discontinued when Phe tolerance could not be increased by more than 400 mg/day and the amino acid mixture could not be completely removed. In another study, increase in Phe tolerance was not sufficient to cover protein requirements from natural protein alone (Hennermann et al 2005).
In conclusion, the incidence of BH4 responsiveness is very high in our patients with Phe levels <450 μmol/L (mostly mild HPA patients) and this correlates with the patients’ genotype and phenotype. The safety levels of Phe for the brain in this group of patients are still a matter of discussion and larger, well-controlled studies are necessary. In our patients with Phe levels of 450–900 μmol/L we found more than 50% to be BH4 responders. Because the restrictive diet is burdensome for many patients and their families, BH4 treatment could improve compliance, especially in adolescence and young adults. For classical PKU the mainstay of the treatment will remain the long-established PKU diet.
Abbreviations
- 6RBH4 :
-
(6R)-L-erythro-5,6,7,8-tetrahydrobiopterin
- BH4:
-
tetrahydrobiopterin
- HPA:
-
hyperphenylalaninaemia
- PAH:
-
phenylalanine hydroxylase
- Phe:
-
phenylalanine
- PKU:
-
phenylketonuria
References
Bélanger-Quintana A, Garcia MJ, Castro M, et al (2005) Spanish BH4-responsive phenylalanine hydroxylase-deficient patients: evolution of seven patients on long-term treatment with tetrahydrobiopterin. Mol Genet Metab 86(Supplement 1): 61–66.
Bernegger C, Blau N (2002) High frequency of tetrahydrobiopterin-responsiveness among hyperphenylalaninemias: a study of 1919 patients observed from 1988 to 2002. Mol Genet Metab 77: 304–313.
Blau N (2008) Defining tetrahydrobiopterin (BH4)-responsiveness in PKU. J Inherit Metab Dis 31(1): 2–3.
Blau N, Erlandsen H (2004) The metabolic and molecular bases of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol Genet Metab 82: 101–111.
Boveda MD, Couce ML, Castineiras DE, et al (2007) The tetrahydrobiopterin loading test in 36 patients with hyperphenylalaninaemia: evaluation of response and subsequent treatment. J Inherit Metab Dis 30(5): 812.
Cerone R, Schiaffino MC, Fantasia AR, Perfumo M, Birk Moller L, Blau N (2004) Long-term follow-up of a patient with mild tetrahydrobiopterin-responsive phenylketonuria. Mol Genet Metab 81: 137–139.
Desviat LR, Pérez B, Bèlanger-Quintana A, et al (2004) Tetrahydrobiopterin responsiveness: results of the BH4 loading test in 31 Spanish PKU patients and correlation with their genotype. Mol Genet Metab 82: 157–162.
Dhondt JL, Ogier H, Benoist JF, Belhesme C, Giraud M (2003) The interpretation of tetrahydrobiopterin loading test in hyperphenylalaninemia. J Inherit Metab Dis 26(Supplement 2): 18.
Fiege B, Blau N (2007) Assessment of tetrahydrobiopterin (BH4) responsiveness in phenylketonuria. J Pediatr 150(6): 627–630.
Fiori L, Fiege B, Riva E, Giovannini M (2005) Incidence of BH4-responsiveness in phenylalanine-hydroxylase-deficient Italian patients. Mol Genet Metab 86(Supplement 1): 67–74.
Gramer G, Burgard P, Garbade SF, Lindner M (2007) Effects and clinical significance of tetrahydrobiopterin supplementation in phenylalanine hydroxylase-deficient hyperphenylalaninaemia. J Inherit Metab Dis 30(4): 556–562.
Hennermann JB, Bührer C, Blau N, Vetter B, Mönch E (2005) Long-term treatment with tetrahydrobiopterin increases phenylalanine tolerance in classic and mild phenylketonuria. Molec Gen Metab 86(Supplement 1): 86–90.
Kure S, Hou DC, Ohura T, et al (1999) Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr 135(3): 375–378.
Lambruschini N, Perez-Duenas B, Vilaseca MA, et al (2005) Clinical and nutritional evaluation of phenylketonuric patients on tetrahydrobiopterin monotherapy. Mol Genet Metab 86(Supplement 1): 54–60.
Lässker U, Zschocke J, Blau N, Santer R (2002) Tetrahydrobiopterin responsiveness in phenylketonuria. Two new cases and a review of molecular genetic findings. J Inherit Metab Dis 25: 65–70.
Leuzzi V, Carducci C, Carducci C, et al (2006) The spectrum of phenylalanine variations under tetrahydrobiopterin load in subjects affected by phenylalanine hydroxylase deficiency. J Inherit Metab Dis 29(1): 38–46.
Levy HL, Milanowski A, Chakrapani A, et al (2007) Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet 370(9586): 504–510.
Lindner M, Haas D, Zschocke J, Burgard P (2001) Tetrahydrobiopterin responsiveness in phenylketonuria differs between patients with the same genotype. Mol Genet Metab 73(1): 104–106.
Lindner M, Steinfeld R, Burgard P, Schulze A, Mayatepek E, Zschocke J (2003) Tetrahydrobiopterin sensitivity in German patients with mild phenylalanine hydroxylase deficiency. Hum Mutat 21(4): 400.
Lücke T, Illsinger S, Aulehla-Scholz C, Sander J, Das AM (2003) BH4-sensitive hyperphenylalaninemia: New case and review of literature. Pediatr Neurol 28(3): 228–230.
Matalon R, Koch R, Michals-Matalon K, Mosley K, Stevens R (2002) Tetrahydrobiopterin-responsive phenylalanine hydroxylase mutations. J Inherit Metab Dis 25(Supplement 1): 23.
Matalon R, Michals-Matalon K, Koch R, Grady J, Tyring S, Stevens RC (2005) Response of patients with phenylketonuria in the US to tetrahydrobiopterin. Mol Genet Metab 86(Supplement 1): S17–21.
Milstien S, Kaufman S (1975) Studies on the phenylalanine hydroxylase system in liver slices. J Biol Chem 250(12): 4777–4781.
Mitchell JJ, Wilcken B, Alexander I, et al (2005) Tetrahydrobiopterin-responsive phenylketonuria: the New South Wales experience. Mol Genet Metab 86(Supplement 1): 81–85.
Muntau AC, Roschinger W, Habich M, et al (2002) Tetrahydrobiopterin as an alternative treatment for mild phenylketonuria. N Engl J Med 347: 2122–2132.
Okano Y, Hase Y, Kawajiri M, et al (2004) In vivo studies of phenylalanine hydroxylase by phenylalanine breath test: diagnosis of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Pediatr Res 56: 714–719.
Perez-Duenas B, Vilaseca MA, Mas A, et al (2004) Tetrahydrobiopterin responsiveness in patients with phenylketonuria. Clin Biochem 37(12): 1083–1090.
Spaapen LJM, Bakker JA, Velter C, et al (2001) Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency in Dutch neonates. J Inherit Metab Dis 24: 325–358.
Spaapen LJ, Estela Rubio-Gozalbo M (2003) Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency, state of the art. Mol Genet Metab 78(2): 93–99.
Trefz FK, Aulehla-Scholz C, Blau N (2001) Successful treatment of phenylketonuria with tetrahydrobiopterin. Eur J Pediatr 160: 315.
Weglage J, Grenzebach M, v.Teeffelen-Heithoff A, et al (2002) Tetrahydrobiopterin responsiveness in a large series of phenylketonuria patients. J Inherit Metab Dis 25: 321–322.
Zurfluh MR, Zschocke J, Lindner M, et al (2008) Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Hum Mutat 29(1): 167–175.
Acknowledgements
Supported by Centro Regionale Malattie Metaboliche Ereditarie, Regione Veneto and COMETA-ASMME, Italy and in part by the Swiss National Science Foundation Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicating editor: John Walter
Competing interests: None declared
References to electronic databases: Phenylalanine-4-hydroxylase: EC 1.14.16.1.
Rights and permissions
About this article
Cite this article
Burlina, A., Blau, N. Effect of BH4 supplementation on phenylalanine tolerance. J Inherit Metab Dis 32, 40–45 (2009). https://doi.org/10.1007/s10545-008-0947-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-008-0947-1