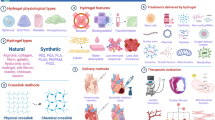
Abstract
Cardiovascular diseases represent a significant public health challenge and are responsible for more than 4 million deaths annually in Europe alone (45% of all deaths). Among these, coronary-related heart diseases are a leading cause of mortality, accounting for 20% of all deaths. Cardiac tissue engineering has emerged as a promising strategy to address the limitations encountered after myocardial infarction. This approach aims to improve regulation of the inflammatory and cell proliferation phases, thereby reducing scar tissue formation and restoring cardiac function. In cardiac tissue engineering, biomaterials serve as hosts for cells and therapeutics, supporting cardiac restoration by mimicking the native cardiac environment. Various bioengineered systems, such as 3D scaffolds, injectable hydrogels, and patches play crucial roles in cardiac tissue repair. In this context, self-healing hydrogels are particularly suitable substitutes, as they can restore structural integrity when damaged. This structural healing represents a paradigm shift in therapeutic interventions, offering a more native-like environment compared to static, non-healable hydrogels. Herein, we sharply review the most recent advances in self-healing hydrogels in cardiac tissue engineering and their potential to transform cardiovascular healthcare.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Cardiovascular diseases are the leading cause of mortality and morbidity worldwide, resulting in the loss of approximately 17.8 million lives in 2017 (Townsend et al. 2016; Mensah et al. 2019). Since 2006, it was projected that the numbers would be alarming, impacting 23.3 million lives by 2023. This would mean that, compared to 2017, there could be a 30% increase in the mortality rate linked to cardiovascular complications (Mathers and Loncar 2006). Heart-related pathologies—such as congenital anomalies, ischemic complications, trauma, and inflammation—compromise cardiac structure and function, often resulting in irreversible acute or chronic heart failure. The limited regenerative capacity of natural cardiac stem cells leaves the heart vulnerable to arrhythmias, as well as significantly decreased ejection fraction and adverse remodeling (Hernandez et al. 2019; Virani et al. 2020; Riggs et al. 2020). Myocardial infarction (MI) and ischemic events further complicate matters, leading to acute loss of myocardial tissue and mechanical instability, resulting in dilation, hypertrophy, and tissue scarring. Noticeably, left ventricular remodeling after MI stands out as the primary driver of heart failure (St. John Sutton and Sharpe 2000). Therefore, there is an urgent need for innovative interventions to support cardiac regenerative approaches and circumvent the drawbacks of these heart-related pathologies. This context sets the stage for exploring the potential of self-healing hydrogels in addressing the structural and functional challenges encountered in cardiac tissue engineering.
Over the past years, various biomaterials have been investigated to address the needs of cardiac tissue regeneration (Nelson et al. 2011; Paul et al. 2014; Wang et al. 2016; Lind et al. 2016; Montgomery et al. 2017; Liang et al. 2018; The et al. 2023), but there is still much more to accomplish. Among those biomaterials, conductive scaffolds and hydrogels have garnered significant attention, given the importance of electrical stimulation in cardiac tissue regeneration. For instance, Furlani et al. have investigated promising electroconductive scaffolds as conductive interfaces. Their study demonstrated that these scaffolds are non-cytotoxic and biocompatible, enabling cardiomyoblasts to interact strongly and form adherent clusters on the scaffolds (Furlani et al. 2023). Additionally, attention has been directed toward distinct manufacturing strategies of cardiac patches predominantly based on those materials, utilizing both natural and synthetic materials (Tian et al. 2012; Montgomery et al. 2017; Izadifar et al. 2018; Yang et al. 2019). In this vein, non-hydrogel and non-self-healable materials have been widely used to create cardiac molds. For example, polydimethylsiloxane (PDMS) was used to produce cardiac molds that maintained their electrical properties without causing irregular heart rhythms, though they faced limitations in establishing electrical connections with host tissue (Shadrin et al. 2017). Likewise, researchers have also combined materials such as GelMA and bioprinted non-self-healable hydrogel-derived scaffolds made from cardiac extracellular matrix and human cardiac progenitor cells (hCPCs) to manufacture cardiac patches, demonstrating high cell viability and enhanced gene expression related to heart tissue development, which improves reconstruction of damaged heart tissue (Bejleri et al. 2018). Additionally, genetically engineered hepatocyte growth factor-expressing MSCs were encapsulated within 3D cardiac patches (Park et al. 2020), nanocellulose-based cardiac patches were investigated with H9c2 cardiomyoblasts (Ajdary et al. 2020), or even a multifunctional epicardial device was synthesized consisting of a biodegradable elastic patch (Huang et al. 2021). Despite all the advancements, non-self-healing materials have inherent limitations, such as poor mechanical properties and durability issues. In particular, non-self-healing biomaterials typically require repeated interventions since they are susceptible to damage during injection or implantation and cannot repair themselves (Jensen et al. 2010a; Jensen et al. 2010b; Diba et al. 2018; Nejadnik et al. 2014). Furthermore, cardiac tissue necessitates a high degree of adaptability to cope with the variable environment post-myocardial infarction (MI) and to withstand the significant mechanical stress and strain it endures. Additionally, a remarkable drawback of non-self-healable hydrogels is their inability to release drugs or cells in a sustained manner due to their rapid degradation or breakage following implantation (Yin et al. 2023).
At the forefront of this field, self-healing hydrogels have emerged as a new promising alternative in cardiac tissue engineering. These hydrogels can restore structural integrity when damaged, representing a paradigm shift in therapeutic intervention. By addressing the vulnerability of surgically configured non-self-healable cardiac patches under mechanical load, self-healing hydrogels offer the potential to enhance the longevity and functionality of these critical interventions. Moreover, self-healing hydrogels offer a more native-like and dynamic milieu for cells compared to their static non-healable counterparts. This is because the cells cannot adapt through remodeling within non-self-healing hydrogels, as these have strong and irreversible crosslinking bonds. Meanwhile, since self-healing hydrogels are made from dynamic bonds that can break and re-bond, they are much easier to remodel and better suited for injection-based therapies due to their high shear-thinning capacity (Sun et al. 2023a).
Consequently, self-healing hydrogels have emerged as versatile tools for advancing cardiac tissue regeneration, such as in minimally invasive clinical therapy approaches. Additionally, they serve as carrier platforms for multiple therapeutic substances (including growth factors and stem cells), facilitating targeted delivery to aid in specific tissue repair and renewal. These hydrogels are crafted to mirror dynamically the active extracellular matrix (ECM) of cardiac tissue, fostering a conducive environment to develop and maintain vital cellular processes such as adhesion, proliferation, and differentiation. Likewise, functioning as supportive scaffolds, they not only offer mechanical reinforcement but also play a pivotal role in orchestrating the assembly of cardiac cells, guiding their organization into functional tissue (Quan et al. 2022).
This review provides a detailed overview of the latest advancements, key challenges, and prospects of self-healing hydrogels in cardiac tissue engineering. By offering a detailed examination of their transformative capabilities, it presents a holistic view of how self-healing hydrogels have the potential to reshape the field of cardiac tissue engineering. Indeed, this exploration of self-healing hydrogels opens new horizons in cardiovascular healthcare, paving the way for innovative solutions and advancements in the treatment of heart-related disorders.
2 Cardiac formation and regeneration – a brief overview
In this section, we will provide a brief explanation of heart anatomy and cardiac tissue, followed by a state-of-the-art description of cardiac tissue engineering.
2.1 Heart anatomy and cardiac tissue
The cardiovascular system originates from mesodermal cells, which differentiate into the myocardium, mesothelial pericardium, and endothelium (endocardium). Between the third and seventh weeks of embryonic development, the primitive heart is formed (Kussman et al. 2023). Cardiogenesis begins around day 20–21 post-fertilization when two parallel endocardial vascular tubes fuse into a single tube. This tube begins to expand and beat by the third week, subsequently undergoing cardiac looping, a process of dextral-looping (rightward twisting) and levo-looping (leftward twisting), initiating the formation of the early four-chamber embryonic heart. Starting in the fourth week, the endocardial cushion tissue located in the mid-central part of the heart expands (from this tissue, the tricuspid and mitral valves, as well as parts of the atrial and ventricular septa, develop) (Romero Flores et al. 2023). Since the early stages of life, the exclusive electrophysiological rhythm, along with the distinct organization of cardiac cells and the extracellular matrix (ECM), generates a mechano-pressure output, producing a hemodynamic force expected to persist throughout the life cycle. The heart features a robust, organized musculature, with cardiomyocytes and the ECM (myocardium) at its core, responsible for generating motion force. This musculature is surrounded by a fluid system known as pericardial fluid, enclosed within a membrane called ‘fibrous pericardium’, which maintains the heart’s anatomical position. The pericardial fluid (located between the heart and the fibrous pericardium) provides external lubrication for cardiac motion. Internally, the endocardium—situated in the intra-heart cavity and comprising endothelial trabeculae—also supports the heart’s pumping action. The anatomy of a fully developed heart, from macro to micro levels, is illustrated in Fig. 1A.
2.2 State-of-the-art in cardiac tissue engineering
Cardiovascular tissue engineering has emerged as a promising approach to tackle the complex challenges posed by cardiovascular diseases and disorders (Fig. 1B), ranging from cardiac muscle to blood vessels and valves. Special attention is devoted to overcoming issues arising from MI; thus, studies have been targeting all phases of post-MI progression, such as inflammatory, proliferative, and maturation phases (Zheng et al. 2021; Schotman and Dankers 2022). Nevertheless, the treatment in early post-MI (the inflammatory phase) is crucial to avoid major inflammation, leading to cell death, and tissue damage. Cellular-based therapies introduced during this phase might inadvertently release pro-inflammatory cytokines and chemoattractants, potentially exacerbating the detrimental effects rather than offering benefits. However, the timing of therapeutic interventions becomes crucial as the MI response evolves (Pupkaite et al. 2020).
Biomaterials play a skeletal role in cardiac tissue engineering, particularly hydrogels that are able to mimic the biological, mechanical, chemical, and electrical cues of the native myocardium. Moreover, hydrogel-based systems can enhance cell viability and enable targeted delivery, thus fostering successful regeneration. Injectable hydrogels, in particular, offer a compelling advantage as they can be delivered through minimally invasive methods, reducing the potential for further tissue damage (Bejleri et al. 2018; Zhang et al. 2022) (see Fig. 1C). For example, when administered shortly after MI, these hydrogels face challenges due to the harsh microenvironment and altered tissue characteristics. Yet, their strategic deployment within the first hours post-MI has been shown to enhance vascularization, mitigate inflammation, and curtail scar expansion (Li et al. 2021). Moving into the proliferative phase, the dynamics shift to reduced inflammation and increased formation of collagen-rich scar tissue initiated by myofibroblast proliferation, making hydrogels more effective. Their capacity to resist degradation aligns with the evolving tissue environment, improving their overall performance (Chin et al. 2022).
Nevertheless, substantial evidence indicates that traditional hydrogels, while useful, present significant challenges that hinder their long-term effectiveness in therapeutic applications. Specifically, traditional hydrogels face durability and mechanical property issues in cardiac tissue engineering. Their limited longevity post-injection and inability to self-repair lead to a shorter lifespan, necessitating repeated injections or replacements (Yin et al. 2023). In addition, traditional hydrogels often lack the dynamic cross-linking mechanisms needed to adapt to mechanical stress and strain, a crucial property for cardiac applications. Biocompatibility is another concern, as traditional hydrogels may degrade quickly and fail to integrate seamlessly with surrounding tissues, potentially triggering immune responses and insufficient support for cardiac tissues (Li et al. 2023a). Furthermore, traditional hydrogels often cannot provide controlled and sustained drug delivery, reducing the effectiveness of therapeutic agents and requiring more frequent interventions, thus increasing procedural risks for patients (Xu and Hsu 2023). In contrast, self-healing hydrogels offer significant advantages. Their ability to repair themselves enhances durability and longevity, making them more efficient for long-term use. Superior mechanical properties and improved biocompatibility make self-healing hydrogels more suitable for cardiac tissue engineering applications, allowing better integration with surrounding tissues and reducing immune responses (Li et al. 2023b). Overall, the unique properties of self-healing hydrogels, including their durability, adaptability, and biocompatibility, make them a superior choice for many medical applications, including cardiac tissue engineering. However, further research is needed to advance the use of hydrogels in cardiac tissue engineering.
In summary, cardiac tissue engineering offers a multifaceted strategy to address the complex challenges of post-myocardial infarction stages. Careful timing of interventions, particularly with injectable hydrogels, holds great promise in influencing the healing process. Continued research and refinements in biomaterial designs, including self-healing approaches, delivery techniques, and understanding of the myocardial microenvironment, will likely enhance the effectiveness of cardiac tissue engineering in promoting post-MI healing and regeneration (Holmes et al. 2005; Ertl and Frantz 2005; Wen et al. 2020).
(A) Cross-sectional heart anatomy illustration showing from macro- to micro-level; (B) Illustration of the most common cardiac-derived diseases and disorders, that in different extends affect the heart tissue and its function; and (C) Explanation of cardiac tissue regeneration following a MI, without early-stage tissue engineering intervention, highlighting the detrimental effects of unbalanced immunomodulation and proliferation processes (excessive myofibroblast proliferation, collagen, and ECM deposition lead to scar tissue maturation and ultimately heart failure). Nevertheless, the intervention of tissue-engineered hydrogels and cardiac patches with self-healing hydrogels improves control over the inflammatory and proliferative phases post-MI, significantly enhancing cardiac tissue regeneration and treatment
3 Self-healing hydrogels in cardiac tissue engineering
In this section, the latest progress on functional self-healing hydrogel design for cardiac tissue engineering applications is explored. Beforehand, an overview of the definition of self-healing hydrogels and types of bonds that conduct to such properties is also provided.
3.1 Definition of self-healing hydrogels and types of bonds
Self-healing hydrogels refer to those with the capacity to restore their structure after being fractured, both micro- or macroscopically. This ability gives self-healing hydrogels long-term stability and makes them durable over time (Wang et al. 2018; Talebian et al. 2019). In this vein, intrinsic self-healing mechanisms can be divided into two groups: physical non-covalent crosslink interactions and chemical covalent crosslink interactions.
However, many times, on their own, none of these mechanisms is enough to fulfill the requirements for self-healing hydrogels, as strength, toughness, fast self-healing ability, or water content are always compromised (Ritchie 2011; Cong et al. 2013; Gong et al. 2016; Karvinen and Kellomäki 2022). Thus, the applications of these self-healing hydrogels are often limited because of their mechanical properties (Sun et al. 2012). For this reason, scientists are investigating new approaches to obtain the best properties for the above-mentioned systems. These approaches include either combining non-covalent and covalent crosslinking systems, as well as aligning nanomaterials or polymers with the hydrogels to enhance their mechanical properties without compromising their self-healing ability (Gong 2010; Henderson et al. 2010; Sun et al. 2013; Zhu et al. 2015; Karvinen and Kellomäki 2022).
In this section, we will describe the main physical noncovalent crosslink interactions and chemical covalent crosslink interactions (Eufrásio-da-Silva et al. 2024). In parallel, Fig. 2 provides an overview of these interactions.
3.1.1 Physical noncovalent interactions
Physical noncovalent interactions are usually based on weak links, which give the hydrogel low mechanical strength but rapid and efficient self-healing capacity. These interactions are listed on the left side of Fig. 2.
Hydrogen bonds are probably the most known of this kind of interaction, as they are very frequent in nature. They are formed when a hydrogen atom is attracted to two atoms (such as N, O, or F), increasing their electronegativity. Hydrogen bonds need to be combined with stronger bonds, forming double-network hydrogels, and contributing to good elastic mechanical properties (Desiraju 2002; Geng et al. 2020).
Hydrophobic interactions are of great interest when designing self-healable hydrogels, especially for delivering hydrophobic drugs (P et al. 2019). These interactions are occurring often in nature, for example in protein folding. They are slightly stronger than H-bonds and can be controlled by modifying the shape or the number of hydrophobic moieties in them. Hydrophobic interactions are easily reversible, due to their unstable configuration (Head-Gordon and Stillinger 1995; Talebian et al. 2019).
Host-guest interactions refer to those consisting of the conjugation of cyclodextrin structures with hydrophobic molecules into their hydrophobic cavities. Thus, they are a more complicated variety of hydrophobic interactions (Kakuta et al. 2013). However, the water uptake needs to be monitored due to the high density of hydrophobic sites in the system (Head-Gordon and Stillinger 1995).
Ionic bonds occur between oppositely charged ions as part of polymers or other structures and nanomaterials. The addition of these nanomaterials can improve the mechanical properties of the system. The most common ionic bonded system is alginate hydrogel, which is formed by crosslinking negatively charged alginate polymers with divalent positively charged ions, usually calcium (Augst et al. 2006; Talebian et al. 2019).
π-π stacking interactions include the links between aromatic rings based on π bonds. There are three possible configurations based on the position of the linked groups: T-shape, parallel displaced, or sandwich configuration. These interactions can also occur within other biological molecules. They are sometimes weaker than hydrogen bonds (Zhao and Zhang 2016; Y et al. 2021).
Lastly, there is one potential interaction, known as crystallization that encompasses different noncovalent interactions between the chains of a soil polymer, such as H-bonds, hydrophobic interactions, or ionic bonds (N et al. 2018).
3.1.2 Chemical covalent interactions
Chemical covalent interactions include both metal coordination bonds (chelation) and dynamic covalent bonds, which in contrast to common covalent bonds, are able to reconnect without physical stimuli, making the reactions that form them reversible when they are under equilibrium control conditions (Rowan et al. 2002; Jin et al. 2013). These interactions occur as a result of the chemical reactions listed on the right side of Fig. 2. They are usually stronger than noncovalent interactions, even so, they create less healable hydrogels.
Within this classificatory framework, firstly, chelation refers to coordinate bonds in which one positively charged transition-metal ion is surrounded by organic molecules acting as ligands, to create complex lattice structures. These bonds are usually stronger than the other covalent interactions, and they are unique for their adhesivity, elasticity, and reversibility, creating hydrogels with high self-healing capacity (Talebian et al. 2019).
Moving on to dynamic covalent bonds, we will focus on those made from imine, acylhydrazone, disulfide, boronate ester, and Diels–Alder reactions:
Imine bonds are the most widely used covalent interactions for the creation of self-healing hydrogels, forming compounds that are also known as Schiff bases. These are synthesized by the reaction of an aldehyde group with a primary amine. An example of this kind of interaction happens between chitosan and benzaldehyde-terminated Pluronic (J et al. 2018).
Acylhydrazone bonds are formed via a condensation reaction between a hydrazine and an aldehyde group. These interactions can be spontaneously created under physiological conditions, making them great candidates to form self-healable hydrogels, as well as injectable hydrogels due to their ideal crosslinking time (Talebian et al. 2019).
Regarding disulfide bonds, on the one hand, they are weaker than the previous ones, and on the other hand, they might be reduced under biological conditions due to their sensitivity to glutathione, a reducing agent present in most human tissues (Østergaard et al. 2004). Thus, they are not very commonly used to form self-healing hydrogels. Essentially, they are thiol-disulfide dynamic exchange reactions.
Boronate esters arise from the interactions between diols and boronic acid, usually when the pH of the medium is higher than the pKa of the boronic acid (8 pKa). Thus, these interactions are highly sensitive to pH modulations and can respond to pH changes in human tissues, whenever they are compatible with them, as shown by Deng C et al. after combining boronic acid monomer with 2-acrylamidophenylboronic acid (Deng et al. 2015).
Lastly, Diels-Alder reactions are essentially electrocyclic reactions that involve π electrons from HOMO and LUMO molecular orbitals that occur in conjugated diene and dienophiles. These reactions are reversible under high temperatures, which do not usually occur in physiological conditions, making them difficult to use in medical applications.
3.2 Functional design of self-healing hydrogels for cardiac tissue engineering applications
In this section, we will discuss the main functional designs that are being investigated to develop self-healing hydrogels for cardiac tissue engineering applications. Furthermore, Table 1 provides an overview description of the latest self-healing hydrogels.
3.2.1 Self-healing hydrogels for bio-inks & 3D printed scaffolds
The progress in engineering strategies within the realm of 3D bioprinting has undergone various stages, initially progressing by using hydrogels alone to 3D-print scaffolds; and more recently, by incorporating active molecules and cells into the hydrogels-based inks in order to form bio-inks (Chimene et al. 2020; Fonseca et al. 2020). The field of 3D bioprinting technology has made significant progress, facilitating the creation of tissue mimetics and organoids. 3D bioprinting also serves as a highly valuable strategy for creating implantable biomaterials and tissue engineering scaffolds, as well as for developing in vitro models for drug testing purposes, aiming to reduce the use of animals in research (Izadifar et al. 2018; Heinrich et al. 2019; Chimene et al. 2020; Fonseca et al. 2020; Lim et al. 2020; Tolabi et al. 2023).
Four primary printing techniques—extrusion-based bioprinting (EBB), inkjet-based bioprinting (a type of droplet-based bioprinting, DBB), laser-assisted bioprinting (LBB), and stereolithography—are extensively employed in the field of bioprinting. Among these, EBB is the most prevalent method for printing self-healing and shear-thinning hydrogels (Karvinen and Kellomäki 2024). Both covalent and physical crosslinking methods have been utilized in EBB. However, despite the variety of self-healing crosslinking methods available, only a limited number have been applied to 3D bioprinting for cardiac biomedical applications. In the context of self-healing covalently crosslinked hydrogels, imine and hydrazone crosslinking are predominantly employed. For extrudable supramolecular hydrogels, crosslinking methods such as host-guest interactions, coordination bonding, peptide-peptide interactions, and hydrophobic interactions have been used. To enhance the mechanical stability of the printed structure, secondary post-crosslinking is often applied. Other less conventional methods of 3D bioprinting used for self-healing hydrogels include inkjet printing and stereolithography (Karvinen and Kellomäki 2024).
Self-healing hydrogels have emerged as the most promising ink materials for 3D bioprinting because, unlike traditional hydrogels, their bonds, as well as their initial structure, functionality, and properties, can be restored after damage (for example, after extrusion). The reversible interactions within hydrogels not only provide desirable mechanical properties, such as mechanical strength and elasticity, but also enhance shear-thinning, which further improves printability (Xu et al. 2019). Consequently, the use of self-healing hydrogels obviates the need for controlling the gelling process for mechanical stabilization purposes.
In the realm of cardiac tissue engineering, the 3D bioprinting of self-healing hydrogels remains relatively unexplored. Nonetheless, a notable study by Daly et al. has made significant strides in this area, as can be seen in Fig. 3A. The researchers developed spheroids composed of self-healing hydrogels, incorporating variations in cell types—specifically, iPSC-derived cardiomyocytes and cardiac fibroblasts in different ratios—to simulate healthy and fibrotic cardiac microtissue. This pioneering work has opened new avenues for research, particularly by creating models that replicate pathological scar environments following myocardial infarction (Daly et al. 2021). Another significant study developed composite hydrogels through a Schiff base reaction between the aldehyde group of oxidized konjac glucomannan (OKGM) and the amino group of branched polyethyleneimine (PEI). This hydrogel exhibited both self-healing properties and shear-thinning capability, rendering it suitable for 3D bioprinting technology. Additionally, the incorporation of carbon nanotubes (CNTs) resulted in electroactive hydrogels, which hold potential for application in cardiac tissue regeneration (Wang et al. 2022). Furthermore, Loebel et al. published a comprehensive protocol in Nature Protocols detailing the preparation methodology for shear-thinning and self-healing hydrogels suitable for 3D printing, with potential applications in cardiac tissue engineering, which outlines the promising future of this technology (Loebel et al. 2017).
3.2.2 Localized injection – strategies of therapeutic delivery
Current research in the area of cardiac regeneration has raised diverse treatment options, such as cell therapy (with the administration of stem cells) and protein therapy, which utilize growth factors and inflammatory mediators; these are among the most effective strategies for promoting cardiac tissue regeneration, although drug delivery platforms and siRNA liberation systems are also being developed (Saludas et al. 2017).
In addition to the treatment modality itself, researchers are also exploring administration methods as an important factor to consider. In this context, minimally invasive approaches are highly desirable. Catheter-based delivery of injectable hydrogels can be achieved through either intravenous or intra-myocardial injection. For intravenous injection, the hydrogels must be in liquid-like state during injection and then transition to gel form upon reaching the target myocardium (Leor et al. 2009). For example, Bastings et al. developed a pH-responsive supramolecular hydrogel carrying growth factors that transitions to a liquid-like state at pH > 8.5 during injection, and upon contact with cardiac tissue, transitions to a solid-like state, thereby adapting well to the injection site (Bastings et al. 2014). However, this intravenous injection approach poses a challenge due to its low treatment retention, and therefore, requires further study (Saludas et al. 2017). Meanwhile, for intramyocardial injection, hydrogels can be deposited directly into the cardiac tissue (Bastings et al. 2014). Injectable self-healing hydrogels are emerging as a particularly attractive option for this purpose, as illustrated in Fig. 3B (Zhang et al. 2022). Table 1 provides a summary of some of the latest self-healing hydrogels used in localized deliveries, targeting therapeutic and regenerative strategies for cardiac tissue.
(A) 3D bioprinted tissue models with self-healing hydrogels: (I) Schematic of 3D bioprinting of scarred cardiac microtissue rings; (II) Activation maps of scarred cardiac microtissues with 1 or 2 scars after 5 days of culture; (III) Quantification of activation delay (ms) (n = 3–4 biologically independent samples, mean ± SD, two-sided student t-test, p = 0.0066); (IV) Contraction profiles of healthy and scarred cardiac microtissues following removal from the support hydrogel after 5 days of culture; and (V) Contraction amplitude (a.u. absolute units) and peak-to-peak time (ms) at 5 days (n = 3 biologically independent samples, mean ± SD, two-sided student t-test). [Reproduced (adapted) from Ref. (Daly et al. 2021) with permission from Copyright Springer Nature]. (B) Injectable conductive self-healable hydrogel: (I) Schematic illustration of the formation of the injectable hydrogel; (II-V) Representative results of injectable self-healable hydrogel showing enhanced performance post-MI regarding: (II) Tissue resistivity of the fibrotic scar areas (n = 9), (III) α-actinin area coverage at 4 weeks post-injection (n = 4), (IV) minimum LV wall thickness (n = 9) and (V) infarct size (n = 9) (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). [Reproduced (adapted) from Ref. (Zhang et al. 2022) with permission from Bioactive Materials (KeWai) Science Direct]. (C) Bi-layer hydrogel cardiac patch: (I) Schematic showing the structure of the patch and experimental methodology for closure of LV-free wall defects in mouse hearts; (II) Self-healing scheme and digital and SEM photographs of a bi-layer hydrogel patch; (III) Representative Sirius-Red and immunofluorescence staining for the studied groups after 7 days post-MI and histograms showing the distribution of fibrosis areas and proliferating cardiomyocytes; and (IV) Quantitative analysis of LV mass on day 7 post-MI. (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.) [Reproduced (adapted) from Ref. (Jiang et al. 2022) with permission from Copyright Clearance Center’s RightsLink by Wiley]
3.2.3 Cardiac patches
Cardiac patches present a promising avenue for addressing the intricate challenges associated with heart disease. For example, left ventricle remodeling after MI has limited ability to regenerate; consequently, the healthy ECM is replaced with scar tissue, impairing the overall functioning of the heart (Gaetani et al. 2012). One of the key advantages of cardiac patches is their ability to provide mechanical support to the damaged heart tissue, thereby reducing the stress on the walls and preventing the enlargement of the heart muscle. This is particularly crucial in the initial stages of disease progression, where the weakening of the walls and the risk of permanent scarring of the heart muscle are significant concerns (Sarig et al. 2016; D’Amore et al. 2016; Lin et al. 2019).
To some extent, cardiac patches facilitate the integration of advanced nanostructure material designs and high-resolution features, which are frequently compromised when conventional injection methods are used. Innovative cardiac patches aim to promote the migration of functional heart muscle cells, repairing heart deficiencies without the formation of scar tissue. The selection of materials and designs to develop cardiac patches significantly affects their characteristics, such as conductivity, modulus, non-toxicity, and drug release capabilities. In this vein, the use of self-healing injectable hydrogels for cardiac patch development is an attractive strategy for cardiac repair, where these hydrogels not only mechanically support infarcted tissue and prevent pathological ventricular remodeling, but also have the ability to repair their own structure and regain their original properties after sustaining damage (Song et al. 2021; Jiang et al. 2022; Hu et al. 2022; Yu et al. 2022) (Fig. 3C).
Some of the notable research focused on patch development for cardiac tissue engineering includes the study by Song et al., which investigates a novel self-healing ionic hydrogel (POG1) developed by incorporating FDA-approved biocompatible polyacrylic acid (PAA) into the hydrogel matrix. This hydrogel exhibits remarkable mechanical properties, including stretchability exceeding 500% strain and compressibility over 85% strain, along with self-healing capabilities and highly stable, ultra-homogeneous conductivity. In vitro studies demonstrate oriented growth and functionalization of cardiomyocytes. In vivo, POG1 provides long-lasting myocardial infarction repair and maintains stable conductivity under cyclic deformation, indicating its potential for advanced cardiac therapy (Song et al. 2021). Another noteworthy article by Yu et al. describes the development of an injectable mechanical-electrical coupling hydrogel patch (MEHP) created through dynamic covalent and noncovalent cross-linking. This MEHP exhibited self-healing and self-adhesive properties, with electrical conductivity and sensitivity tailored to match myocardial tissue. The application of the MEHP showed enhanced electrical connectivity between healthy and infarcted myocardial regions, thereby potentially offering a novel therapeutic approach for cardiac repair post-myocardial infarction (Yu et al. 2022). Hu et al. developed a conductive, self-healing zwitterionic hydrogel by copolymerizing carboxybetaine methacrylate (CBMA) and hydroxyethyl methacrylate (HEMA), both biocompatible monomers. This hydrogel enhances the synchronous contraction of cardiomyocytes in vitro by increasing α-actinin and Connexin-43 (CX-43) expression. In vivo, it promotes electrophysiological signal transmission and improves revascularization in the infarcted area, suggesting its potential for myocardial infarction treatment (Hu et al. 2022).
A more detailed description of self-healing hydrogels designed for cardiac patch applications, along with those intended for other purposes, is presented in Table 1.
4 Final remarks and future perspectives
Although significant progress has been made in the research on self-healing hydrogels for cardiac tissue regeneration, it is worth noting that the number of studies remains limited and none have advanced to clinical trials. Nevertheless, pre-clinical trials have been performed by using animal models and promising outcomes have been reported. In this context, we will now highlight some of the studies mentioned in the previous sections, focusing specifically on their advancement in pre-clinical trials. For example, one of the first studies in a large animal model was the one by Bastings et al., where they delivered a responsive self-healing hydrogel loaded with growth factor for controlled release via intramyocardial injection using NOGA™ catheter, resulting in a reduction of collagen scar (Bastings et al. 2014). However, the majority of the research in the field of self-healing hydrogels for cardiac tissue regeneration has been carried out in small animal models. Jiang et al. 3D-printed a bi-layered cardiac patch formed by a self-healing hydrogel with recombinant functional proteins. The research continued by performing a pre-clinical trial using two mouse animal models: (i) transmural LV puncture model and (ii) MI model. They observed that by replacing the traditional suture method for wound closure by using self-healing hydrogels the heart function improved while fibrosis decreased (Jiang et al. 2022). It is well-known that the electrophysiological rhythm between cardiac cells and ECM is crucial for optimal cardiac function. Most recently, conductive self-healing hydrogels have also been explored in vivo. For example, Song et al. developed and studied in vivo a self-healing ionic conductive hydrogel for cardiac patches, manufactured by combining oxidized alginate, gelatin, and PAAc at ratios of 0, 9.1, or 16.6 mg/ml. The formulation containing 9.1 g/ml of PAAc showed a reduced fibrotic area, thicker LV wall, and higher microvessel density (Song et al. 2021). Meanwhile, Zhang et al. developed a promising self-healing hydrogel to treat post-MI, which has electrical conductivity properties of 5.52 × 10− 4 S/cm, matching the natural cardiac electrophysiology (Zhang et al. 2022). As observed in Fig. 3B, their conductive self-healable hydrogel reduced the arrhythmia susceptibility at 4 weeks post-MI, prevented LV remodeling, and reduced resistivity of the myocardial fibrotic tissue and infarcted size area; moreover, at 4 weeks post-MI, the conductive self-healing hydrogel showed high induction of angiogenesis (Zhang et al. 2022). In vivo research was further performed with another cardiac patch formed using a self-healable and zwitterionic conductive hydrogel —copolymers of carboxybetaine methacrylate (CBMA) and hydroxyethyl methacrylate (HEMA) monomers—matching natural cardiac electrophysiology. The aim was to enhance cell-cell communication and trigger the propagation of electrical impulses in the infarcted area (Hu et al. 2022). Altogether, the self-healable and zwitterionic conductive hydrogel improved cardiac function and revascularization in the infarcted area, thereby repairing tissue and promoting better electrophysiological signals (Hu et al. 2022). Also recently, a self-healable injectable mechanical-electrical-coupled hydrogel patch with interfacial wet adhesion was developed and applied intrapericardially (Yu et al. 2022). Remarkably, self-healing hydrogels and ADSCs were combined, exploring cell therapy for cardiac repair post-MI. Both hydrogels with and without ADSCs showed promising results for cardiac therapy post-MI; nevertheless, hydrogels with ADSCs enhanced performance, demonstrating higher left ventricular (LV) wall thickness, microvessel density, actin area, reduced infarcted area, and improved cardiac function after four weeks of post-MI treatment (Yu et al. 2022).
Indubitably, the outcomes from research on self-healing hydrogels in animal models are promising, and those hydrogels are expected to undergo clinical translation sooner than projected. Those self-healing hydrogels with FDA-approved and natural-based material sources will have a special prevalence, mainly for cardiac cell therapy. Furthermore, self-healing hydrogels with adhesiveness will gain special attention, not only because cardiac-designated systems are intensively subjected to mechanical loading, requiring increased durability, but also due to their potential to enhance the interface between cardiac patches and tissues, thereby improving the accuracy of the bionic translational stages. In addition, adhesive self-healing hydrogels can serve as biomaterials for cardiac wound closure and healing, particularly through minimally invasive approaches such as injection, spray-based techniques, and devices.
In addition to the immediate applications mentioned above, which could achieve faster clinical translation, self-healing hydrogels should also be explored in personalized pathological models and drug screens. Recent advancements in conventional heart-on-a-chip technology, although still using non-self-healable materials, have introduced innovative methods to monitor cardiac function within these devices. This includes microphysiological optical and electrical observations for bionics, achieved by developing heart-on-a-chip devices with integrated approaches aimed at drug screening (Fu et al. 2018; Sun et al. 2023b). Furthermore, bioelectronic materials incorporated into heart-on-a-chip systems have the potential to facilitate the integration of bioelectronic devices, enabling better electrophysiological monitoring of cardiac tissue in vitro models. Lind et al. developed a 3D-printed, self-assembling cardiac microphysiological device using piezo-resistive and highly conductive soft materials. They demonstrated that, in addition to monitoring electrophysiological tissue contractility, these systems could also be used to study drug responses (Lind et al. 2016). In addition, researchers have incorporated mesh nanoelectrodes into cardiac organoids (formed in this case with a non-self-healing material). The use of silicon nanowire-based bioelectronics has provided insights into encoding desired properties. Notably, these advanced approaches can generate 3D maps of cardiac tissue activity over extended periods, making them valuable tools in cardiac research (Tian et al. 2012; Dai et al. 2016; Li et al. 2019; Kalmykov et al. 2019). Building on the previously discussed state-of-the-art in vivo studies, which showed that self-healing conductive hydrogels can significantly restore cardiac function and repair post-MI compared to non-self-healing hydrogels, it is hypothesized that integrating self-healable conductive hydrogels with dynamic organ-on-a-chip systems could open new frontiers in cardiac tissue engineering and bio-microelectromechanical systems (BioMENS). Furthermore, this approach also paves the way for advanced smart theranostic systems, cardiac tissue cyborgs, and innovations in cardiac healthcare. Notably, theranostic self-healable hydrogels could potentially replace non-self-healing wearable skin hydrogels designed to monitor cardiac function (Chun et al. 2022). Moreover, such systems could be minimally invasive, meaning they can be easily delivered and attached to cardiac tissue, while also capable of both regenerating and monitoring heart biophysical signals in real-time. Another noteworthy example of translational technology is a near-infrared irradiation-induced self-healable triboelectric nanogenerator (TENG) developed for potential use in implantable electronic systems. Given the mechanical actuation of heartbeats, these TENGs could pave the way for the clinical translation of implantable systems that enable self-powered wireless cardiac monitoring (Guan et al. 2018). In summary, these are concrete translational examples where smart theranostic self-healing hydrogel systems and BioMENS integration can advance cardiac tissue engineering. This approach can also enhance the development of cardiac tissue cyborgs and improve cardiac healthcare monitoring. Importantly, these advancements rely on collaboration among physicians, materials scientists, and computer scientists to support the evolution of cardiac regeneration monitoring. This collaboration aims to provide personalized treatments for patients and ultimately save lives.
5 Conclusion
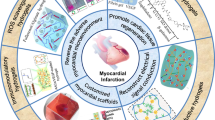
Numerous investigations are being conducted to address the challenges posed by MI. Based on current knowledge, self-healing hydrogels remain an area that warrants further exploration. Currently, the focus is primarily on the development of injectable-based delivery systems and cardiac patches. In vivo studies have demonstrated that the application of self-healing hydrogels reduces fibrotic tissue, improves cardiac function, and aids in repairing damage post-MI. Furthermore, enhanced outcomes have been observed through the use of self-healing conductive hydrogels, both with and without cell therapy integration. The latest advancements in self-healing hydrogels are impacting the field of cardiac tissue engineering and the realm of tissue cyborganics, showcasing promising prospects for addressing cardiovascular disorders (Fig. 4).
Data availability
No datasets were generated or analysed during the current study.
References
R. Ajdary, N.Z. Ezazi, A. Correia et al., Multifunctional 3D-Printed patches for long-term drug release therapies after myocardial infarction. Adv. Funct. Mater. 30, 2003440 (2020). https://doi.org/10.1002/ADFM.202003440
A.D. Augst, H.J. Kong, D.J. Mooney, Alginate Hydrogels as Biomaterials. Macromol. Biosci. 6, 623–633 (2006). https://doi.org/10.1002/MABI.200600069
M.M.C. Bastings, S. Koudstaal, R.E. Kieltyka et al., A fast pH-Switchable and Self-Healing Supramolecular hydrogel carrier for guided, local catheter injection in the Infarcted myocardium. Adv. Healthc. Mater. 3, 70–78 (2014). https://doi.org/10.1002/ADHM.201300076
D. Bejleri, B.W. Streeter, A.L.Y. Nachlas et al., A Bioprinted Cardiac Patch composed of Cardiac-Specific Extracellular Matrix and Progenitor cells for heart repair. Adv. Healthc. Mater. 7, 1800672 (2018). https://doi.org/10.1002/ADHM.201800672
P. Chakraborty, H. Oved, D. Bychenko et al., Nanoengineered peptide-based Antimicrobial Conductive Supramolecular Biomaterial for Cardiac tissue Engineering. Adv. Mater. 33, 2008715 (2021). https://doi.org/10.1002/ADMA.202008715
D. Chimene, R. Kaunas, A.K. Gaharwar, Hydrogel Bioink Reinforcement for Additive Manufacturing: a focused review of emerging strategies. Adv. Mater. 32, 1902026 (2020). https://doi.org/10.1002/ADMA.201902026
I.L. Chin, L. Hool, Y.S. Choi, Correction: interrogating cardiac muscle cell mechanobiology on stiffness gradient hydrogels. Biomater. Sci. 10, 6628–6629 (2022). https://doi.org/10.1039/D2BM90083A
K.Y. Chun, S. Seo, C.S. Han, A wearable All-Gel Multimodal Cutaneous Sensor enabling simultaneous single-site monitoring of Cardiac-related Biophysical signals. Adv. Mater. 34, 2110082 (2022). https://doi.org/10.1002/ADMA.202110082
H.P. Cong, P. Wang, S.H. Yu, Stretchable and self-healing graphene oxide-polymer composite hydrogels: a dual-network design. Chem. Mater. 25, 3357–3362 (2013). https://doi.org/10.1021/CM401919C/SUPPL_FILE/CM401919C_SI_001.PDF
A. D’Amore, T. Yoshizumi, S.K. Luketich et al., Bi-layered polyurethane - extracellular matrix cardiac patch improves ischemic ventricular wall remodeling in a rat model. Biomaterials. 107, 1–14 (2016). https://doi.org/10.1016/J.BIOMATERIALS.2016.07.039
X. Dai, W. Zhou, T. Gao et al., Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol 2016. 119 11, 776–782 (2016). https://doi.org/10.1038/nnano.2016.96
A.C. Daly, M.D. Davidson, J.A. Burdick, 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat. Commun. 12, 1–13 (2021). https://doi.org/10.1038/s41467-021-21029-2
C.C. Deng, W.L.A. Brooks, K.A. Abboud, B.S. Sumerlin, Boronic acid-based hydrogels undergo self-healing at neutral and acidic pH. ACS Macro Lett. 4, 220–224 (2015). https://doi.org/10.1021/ACSMACROLETT.5B00018/SUPPL_FILE/MZ5B00018_SI_001.PDF
G.R. Desiraju, Hydrogen bridges in Crystal Engineering: interactions without Borders. Acc. Chem. Res. 35, 565–573 (2002). https://doi.org/10.1021/AR010054T
M. Diba, S. Spaans, K. Ning et al., Self-healing biomaterials: from molecular concepts to clinical applications. Adv. Mater. Interfaces. 5, 1800118 (2018). https://doi.org/10.1002/admi.201800118
R. Dong, X. Zhao, B. Guo, P.X. Ma, Self-Healing Conductive Injectable hydrogels with Antibacterial Activity as Cell delivery carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces. 8, 17138–17150 (2016). https://doi.org/10.1021/ACSAMI.6B04911/ASSET/IMAGES/LARGE/AM-2016-04911B_0009.JPEG.
G. Ertl, S. Frantz, Healing after myocardial infarction. Cardiovasc. Res. 66, 22–32 (2005). https://doi.org/10.1016/J.CARDIORES.2005.01.011/2/66-1-22-FIG5.GIF
T. Eufrásio-da-Silva, I. Erezuma, A. Dolatshahi-Pirouz, G. Orive, Enhancing regenerative medicine with self-healing hydrogels: a solution for tissue repair and advanced cyborganic healthcare devices. Biomater. Adv. 161, 213869 (2024). https://doi.org/10.1016/J.BIOADV.2024.213869
A.C. Fonseca, F.P.W. Melchels, M.J.S. Ferreira et al., Emulating Human tissues and organs: a Bioprinting Perspective toward Personalized Medicine. Chem. Rev. 120, 11128–11174 (2020). https://doi.org/10.1021/ACS.CHEMREV.0C00342/ASSET/IMAGES/LARGE/CR0C00342_0013.JPEG.
F. Fu, L. Shang, Z. Chen et al., Bioinspired living structural color hydrogels. Sci. Robot. (2018). https://doi.org/10.1126/SCIROBOTICS.AAR8580/SUPPL_FILE/AAR8580_SM.PDF.
F. Furlani, E. Campodoni, N. Sangiorgi et al., Electroconductive scaffolds based on gelatin and PEDOT:PSS for cardiac regeneration. Int. J. Biol. Macromol. 224, 266–280 (2023). https://doi.org/10.1016/J.IJBIOMAC.2022.10.122
R. Gaetani, P.A. Doevendans, C.H.G. Metz et al., Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 33, 1782–1790 (2012). https://doi.org/10.1016/J.BIOMATERIALS.2011.11.003
H. Geng, Q. Dai, H. Sun et al., Injectable and Sprayable Polyphenol-based hydrogels for Controlling Hemostasis. ACS Appl. Bio Mater. 3, 1258-1266 (2020). https://doi.org/10.1021/ACSABM.9B01138
J.P. Gong, Why are double network hydrogels so tough? Soft Matter. 6, 2583–2590 (2010). https://doi.org/10.1039/B924290B
Z. Gong, G. Zhang, X. Zeng et al., High-Strength, tough, fatigue resistant, and Self-Healing Hydrogel based on dual physically cross-linked Network. ACS Appl. Mater. Interfaces. 8, 24030–24037 (2016). https://doi.org/10.1021/ACSAMI.6B05627/SUPPL_FILE/AM6B05627_SI_001.PDF
Q. Guan, Y. Dai, Y. Yang et al., Near-infrared irradiation induced remote and efficient self-healable triboelectric nanogenerator for potential implantable electronics. Nano Energy. 51, 333–339 (2018). https://doi.org/10.1016/J.NANOEN.2018.06.060
B. Guo, J. Qu, X. Zhao, M. Zhang, Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 84, 180–193 (2019). https://doi.org/10.1016/j.actbio.2018.12.008
T. Head-Gordon, H. Stillinger, Is water structure around hydrophobic groups clathrate-like? Communicated Frank. 92, 8308–8312 (1995)
M.A. Heinrich, W. Liu, A. Jimenez et al., 3D bioprinting: from benches to translational applications. Small. 15 (2019). https://doi.org/10.1002/SMLL.201805510
K.J. Henderson, T.C. Zhou, K.J. Otim, K.R. Shull, Ionically cross-linked triblock copolymer hydrogels with high strength. Macromolecules. 43, 6193–6201 (2010). https://doi.org/10.1021/MA100963M/ASSET/IMAGES/MEDIUM/MA-2010-00963M_0010.GIF.
G.A. Hernandez, A. Lemor, D. Clark et al., In Hospital outcomes in adult congenital heart disease patients with Fontan Undergoing Heart Transplantation - A Decade Nationwide Analysis from 2004 until 2014. J. Hear. Lung Transpl. 38, S110 (2019). https://doi.org/10.1016/j.healun.2019.01.257
J.W. Holmes, T.K. Borg, J.W. Covell, Structure and mechanics of healing myocardial infarcts. Annu. Rev. Biomed. Eng. 7, 223–253 (2005). https://doi.org/10.1146/ANNUREV.BIOENG.7.060804.100453
X. Hu, P. Zhang, J. Liu et al., A self-association cross-linked conductive zwitterionic hydrogel as a myocardial patch for restoring cardiac function. Chem. Eng. J. 446, 136988 (2022). https://doi.org/10.1016/J.CEJ.2022.136988
S. Huang, D. Lei, Q. Yang et al., A perfusable, multifunctional epicardial device improves cardiac function and tissue repair. Nat. Med. 27, 480–490 (2021). https://doi.org/10.1038/s41591-021-01279-9
M. Izadifar, D. Chapman, P. Babyn et al., UV-Assisted 3D bioprinting of Nanoreinforced Hybrid Cardiac Patch for myocardial tissue Engineering. Tissue Eng. Part. C Methods. 24, 74–88 (2018). https://doi.org/10.1089/TEN.TEC.2017.0346
T. Jensen, A. Dolatshahi-Pirouz, M. Foss et al., Interaction of human mesenchymal stem cells with osteopontin coated hydroxyapatite surfaces. Colloids Surf. B Biointerfaces. 75, 186–193 (2010b). https://doi.org/10.1016/j.colsurfb.2009.08.029
T. Jensen, T. Jakobsen, J. Baas et al., Hydroxyapatite nanoparticles in poly‐ D L ‐lactic acid coatings on porous titanium implants conducts bone formation. J. Biomed. Mater. Res. A. 95A, 665–672 (2010a). https://doi.org/10.1002/jbm.a.32863
X. Jiang, T. Feng, B. An et al., A Bi-layer Hydrogel Cardiac Patch made of recombinant functional proteins. Adv. Mater. 34 (2022). https://doi.org/10.1002/ADMA.202201411
Y. Jin, C. Yu, R.J. Denman, W. Zhang, Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 42, 6634–6654 (2013). https://doi.org/10.1039/C3CS60044K
N.J. Kaiser, R.J. Kant, A.J. Minor, K.L.K. Coulombe, Optimizing blended collagen-fibrin hydrogels for Cardiac tissue Engineering with Human iPSC-derived cardiomyocytes. ACS Biomater. Sci. Eng. 5, 887–899 (2019). https://doi.org/10.1021/ACSBIOMATERIALS.8B01112/ASSET/IMAGES/LARGE/AB-2018-01112X_0006.JPEG.
T. Kakuta, Y. Takashima, M. Nakahata et al., Preorganized hydrogel: self-healing properties of supramolecular hydrogels formed by polymerization of host-guest-monomers that contain cyclodextrins and hydrophobic guest groups. Adv. Mater. 25, 2849–2853 (2013). https://doi.org/10.1002/ADMA.201205321
A. Kalmykov, C. Huang, J. Bliley et al., Organ-on-e-chip: three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Sci. Adv. 5, 729–752 (2019). https://doi.org/10.1126/SCIADV.AAX0729/SUPPL_FILE/AAX0729_SM.PDF
J. Karvinen, M. Kellomäki, Characterization of self-healing hydrogels for biomedical applications. Eur. Polym. J. 181, 111641 (2022). https://doi.org/10.1016/J.EURPOLYMJ.2022.111641
J. Karvinen, M. Kellomäki, 3D-bioprinting of self-healing hydrogels. Eur. Polym. J. 209, 112864 (2024). https://doi.org/10.1016/J.EURPOLYMJ.2024.112864
B.D. Kussman, A.E. Roberts, W.C. Miller-Hance, Development of the Cardiovascular System. Anesth. Congenit Hear. Dis. 83–115 (2023). https://doi.org/10.1002/9781119791690.CH6
J. Leor, S. Tuvia, V. Guetta et al., Intracoronary injection of in situ forming alginate hydrogel reverses left ventricular remodeling after myocardial infarction in Swine. J. Am. Coll. Cardiol. 54, 1014–1023 (2009). https://doi.org/10.1016/J.JACC.2009.06.010
Q. Li, K. Nan, Le P. Floch et al., Cyborg organoids: implantation of Nanoelectronics via Organogenesis for tissue-wide Electrophysiology. Nano Lett. 19, 5781–5789 (2019). https://doi.org/10.1021/ACS.NANOLETT.9B02512/SUPPL_FILE/NL9B02512_SI_007.PDF
H. Li, B. Yu, P. Yang et al., Injectable AuNP-HA matrix with localized stiffness enhances the formation of gap junction in engrafted human induced pluripotent stem cell-derived cardiomyocytes and promotes cardiac repair. Biomaterials. 279 (2021). https://doi.org/10.1016/J.BIOMATERIALS.2021.121231
R. Li, J. Ren, M. Li et al., Self-healing, self-adhesive, stretchable and flexible conductive hydrogels for high-performance strain sensors. Soft Matter. 19, 5723–5736 (2023a). https://doi.org/10.1039/D3SM00581J
W. Li, Y. Wu, X. Zhang et al., Self-healing hydrogels for bone defect repair. RSC Adv. 13, 16773–16788 (2023b). https://doi.org/10.1039/D3RA01700A
S. Liang, Y. Zhang, H. Wang et al., Paintable and rapidly Bondable Conductive hydrogels as Therapeutic Cardiac patches. Adv. Mater. 30, 1704235 (2018). https://doi.org/10.1002/ADMA.201704235
K.S. Lim, J.H. Galarraga, X. Cui et al., Fundamentals and Applications of Photo-Cross-linking in Bioprinting. Chem. Rev. 120, 10662–10694 (2020). https://doi.org/10.1021/ACS.CHEMREV.9B00812/ASSET/IMAGES/MEDIUM/CR9B00812_0010.GIF.
X. Lin, Y. Liu, A. Bai et al., A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng. 3, 632–643 (2019). https://doi.org/10.1038/s41551-019-0380-9
J.U. Lind, T.A. Busbee, A.D. Valentine et al., Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing. Nat. Mater. 16, 303–308 (2016). https://doi.org/10.1038/nmat4782
C. Loebel, C.B. Rodell, M.H. Chen et al., Shear-thinning and self-healing hydrogels as injectable therapeutics and for 3D-printing. Nat. Protoc. 12, 1521–1541 (2017). https://doi.org/10.1038/nprot.2017.053
C.D. Mathers, D. Loncar, Projections of global mortality and Burden of Disease from 2002 to 2030. PLoS Med. 3, e442 (2006). https://doi.org/10.1371/journal.pmed.0030442
G.A. Mensah, G.A. Roth, V. Fuster, The Global Burden of Cardiovascular diseases and Risk factors: 2020 and Beyond. J. Am. Coll. Cardiol. 74, 2529–2532 (2019). https://doi.org/10.1016/J.JACC.2019.10.009
M. Montgomery, S. Ahadian, L. Davenport Huyer et al., Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017. 16, 1038–1046 (2017). https://doi.org/10.1038/nmat4956
M.R. Nejadnik, X. Yang, M. Bongio et al., Self-healing hybrid nanocomposites consisting of bisphosphonated hyaluronan and calcium phosphate nanoparticles. Biomaterials. 35, 6918-6929 (2014). https://doi.org/10.1016/j.biomaterials.2014.05.003
D.M. Nelson, Z. Ma, K.L. Fujimoto et al., Intra-myocardial biomaterial injection therapy in the treatment of heart failure: materials, outcomes and challenges. Acta Biomater. 7, 1–15 (2011). https://doi.org/10.1016/J.ACTBIO.2010.06.039
H. Østergaard, C. Tachibana, J.R. Winther, Monitoring disulfide bond formation in the eukaryotic cytosol. J. Cell. Biol. 166, 337–345 (2004). https://doi.org/10.1083/JCB.200402120
P.L. B G, S W, et al., A thermo-responsive and self-healing liposome-in-hydrogel system as an antitubercular drug carrier for localized bone tuberculosis therapy. Int. J. Pharm. 558, 101–109 (2019). https://doi.org/10.1016/J.IJPHARM.2018.12.083
B.W. Park, S.H. Jung, S. Das et al., In vivo priming of human mesenchymal stem cells with hepatocyte growth factor–engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci. Adv. 6 (2020). https://doi.org/10.1126/SCIADV.AAY6994/SUPPL_FILE/AAY6994_SM.PDF
A. Paul, A. Hasan, H. Kindi, Al et al., Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 8, 8050–8062 (2014). https://doi.org/10.1021/NN5020787/SUPPL_FILE/NN5020787_SI_001.PDF
J. Pupkaite, V. Sedlakova, C. Eren Cimenci et al., Delivering more of an Injectable Human recombinant collagen III hydrogel does not improve its therapeutic efficacy for treating myocardial infarction. ACS Biomater. Sci. Eng. 6, 4256–4265 (2020). https://doi.org/10.1021/ACSBIOMATERIALS.0C00418/SUPPL_FILE/AB0C00418_SI_001.PDF
L. Quan, Y. Xin, X. Wu, Q. Ao, (2022) Mechanism of Self-Healing Hydrogels and Application in Tissue Engineering. Polym 2022, Vol 14, Page 2184 14:2184. https://doi.org/10.3390/POLYM14112184
K.W. Riggs, F. Zafar, Y. Radzi et al., Adult congenital heart disease: current early expectations after Cardiac Transplantation. Ann. Thorac. Surg. 109, 480–486 (2020). https://doi.org/10.1016/J.ATHORACSUR.2019.06.067
R.O. Ritchie, The conflicts between strength and toughness. Nat. Mater. 10, 817–822 (2011). https://doi.org/10.1038/NMAT3115
C.B. Rodell, M.E. Lee, H. Wang et al., Injectable Shear-Thinning hydrogels for minimally invasive delivery to Infarcted myocardium to Limit Left ventricular remodeling. Circ. Cardiovasc. Interv. 9 (2016). https://doi.org/10.1161/CIRCINTERVENTIONS.116.004058/-/DC1
B.G. Romero Flores, L. Villavicencio Guzmán, M. Salazar García et al., Normal development of the heart: a review of new findings. Bol. Med. Hosp. Infant Mex. 80, 79–93 (2023). https://doi.org/10.24875/BMHIM.22000138
S.J. Rowan, S.J. Cantrill, G.R.L. Cousins et al., Dynamic Covalent Chemistry. Angew Chemie Int. Ed. 41, 898–952 (2002). https://doi.org/10.1002/1521-3773(20020315)41:6%3C898::AID-ANIE898%3E3.0.CO;2-E
L. Saludas, S. Pascual-Gil, F. Prósper et al., Hydrogel based approaches for cardiac tissue engineering. Int. J. Pharm. 523, 454–475 (2017). https://doi.org/10.1016/J.IJPHARM.2016.10.061
N. Samadi, M. Sbzi, M. Babaahmadi, Self-healing and tough hydrogels with physically cross-linked triple networks based on Agar/PVA/Graphene. Int. J. Biol. Macromol. 107:2291–2297 (2018). https://doi.org/10.1016/J.IJBIOMAC.2017.10.104
U. Sarig, H. Sarig, E. de-Berardinis et al., Natural myocardial ECM patch drives cardiac progenitor based restoration even after scarring. Acta Biomater. 44, 209–220 (2016). https://doi.org/10.1016/J.ACTBIO.2016.08.031
M.J.G. Schotman, P.Y.W. Dankers, Factors influencing Retention of Injected Biomaterials to treat myocardial infarction. Adv. Mater. Interfaces. 9, 2100942 (2022). https://doi.org/10.1002/ADMI.202100942
I.Y. Shadrin, B.W. Allen, Y. Qian et al., Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8, 1–15 (2017). https://doi.org/10.1038/s41467-017-01946-x
X. Song, X. Wang, J. Zhang et al., A tunable self-healing ionic hydrogel with microscopic homogeneous conductivity as a cardiac patch for myocardial infarction repair. Biomaterials. 273, 120811 (2021). https://doi.org/10.1016/J.BIOMATERIALS.2021.120811
J.-Y. Sun, X. Zhao, W.R.K. Illeperuma et al., Highly stretchable and tough hydrogels. Nature. 489, 133 (2012). https://doi.org/10.1038/NATURE11409
T.L. Sun, T. Kurokawa, S. Kuroda et al., Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013. 1210 12, 932–937 (2013). https://doi.org/10.1038/nmat3713
F. Sun, Q. Yao, Y. Wang et al., Self-healing injectable stimuli responsive polysaccharide hydrogel constructed with dynamic covalent bonds. J. Appl. Polym. Sci. 140, e54304 (2023a). https://doi.org/10.1002/APP.54304
L. Sun, Y. Wang, F. Bian et al., Bioinspired optical and electrical dual-responsive heart-on-a-chip for hormone testing. Sci. Bull. 68, 938–945 (2023b). https://doi.org/10.1016/J.SCIB.2023.04.010
S.J. Sutton, M.G. Sharpe N, Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 101, 2981–2988 (2000). https://doi.org/10.1161/01.CIR.101.25.2981
S. Talebian, M. Mehrali, N. Taebnia et al., Self-Healing hydrogels: the next paradigm shift in tissue Engineering? Adv. Sci. 6, 1801664 (2019). https://doi.org/10.1002/ADVS.201801664
C. The, X. Han, Y. He et al., (2023) The Role of Hydrogel in Cardiac Repair and Regeneration for Myocardial Infarction: Recent Advances and Future Perspectives. Bioeng. 10, 165 (2023) https://doi.org/10.3390/BIOENGINEERING10020165
B. Tian, J. Liu, T. Dvir et al., Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012). https://doi.org/10.1038/nmat3404
H. Tolabi, N. Davari, M. Khajehmohammadi et al., Progress of Microfluidic Hydrogel-based scaffolds and Organ-on-chips for the cartilage tissue Engineering. Adv. Mater. 35, e2208852 (2023). https://doi.org/10.1002/ADMA.202208852
N. Townsend, L. Wilson, P. Bhatnagar et al., Cardiovascular disease in Europe: epidemiological update 2016. Eur. Heart J. 37, 3232–3245 (2016). https://doi.org/10.1093/EURHEARTJ/EHW334
S.S. Virani, A. Alonso, E.J. Benjamin et al., Heart Disease and Stroke Statistics-2020 update: a Report from the American Heart Association. Circulation. 141, E139–E596 (2020). https://doi.org/10.1161/CIR.0000000000000757
L. Wang, J. Jiang, W. Hua et al., Mussel-Inspired Conductive Cryogel as Cardiac tissue Patch to Repair Myocardial Infarction by Migration of Conductive nanoparticles. Adv. Funct. Mater. 26, 4293–4305 (2016). https://doi.org/10.1002/ADFM.201505372
L.L. Wang, Y. Liu, J.J. Chung et al., Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat. Biomed. Eng. 112, 983–992 (2017). https://doi.org/10.1038/s41551-017-0157-y
Y. Wang, C.K. Adokoh, R. Narain, Recent development and biomedical applications of self-healing hydrogels. Expert Opin. Drug Deliv. 15, 77–91 (2018). https://doi.org/10.1080/17425247.2017.1360865
Y.L. Wang, L. Han, X.L. Zhang et al., 3D bioprinting of an electroactive and self-healing polysaccharide hydrogels. J. Tissue Eng. Regen Med. 16, 76–85 (2022). https://doi.org/10.1002/TERM.3238
Y. Wen, X. Li, Z. Li et al., Intra-myocardial Delivery of a Novel Thermosensitive Hydrogel Inhibits Post-infarct Heart Failure After Degradation in Rat. J Cardiovasc Transl Res 13, 677–685 (2020). https://doi.org/10.1007/S12265-019-09941-X/FIGURES/3
J.Q. X Z, Y L, et al., Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials. 183, 185–199 (2018). https://doi.org/10.1016/J.BIOMATERIALS.2018.08.044
J. Xu, S. Hsu, Self-healing hydrogel as an injectable implant: translation in brain diseases. J. Biomed. Sci. 2023. 301 30, 1–19 (2023). https://doi.org/10.1186/S12929-023-00939-X
C. Xu, G. Dai, Y. Hong, Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater. 95, 50–59 (2019). https://doi.org/10.1016/J.ACTBIO.2019.05.032
Y.L. CW, W. SW, C. SH H, An injectable, self-healing phenol-functionalized chitosan hydrogel with fast gelling property and visible light-crosslinking capability for 3D printing. Acta Biomater. 122, 211–219 (2021). https://doi.org/10.1016/J.ACTBIO.2020.12.051
Y. Yang, D. Lei, S. Huang et al., Elastic 3D-Printed Hybrid Polymeric Scaffold improves Cardiac Remodeling after myocardial infarction. Adv. Healthc. Mater. 8 (2019). https://doi.org/10.1002/ADHM.201900065
H. Yin, F. Liu, T. Abdiryim, X. Liu, Self-Healing hydrogels: from synthesis to multiple applications. ACS Mater. Lett. 5, 1787–1830 (2023). https://doi.org/10.1021/ACSMATERIALSLETT.3C00320/ASSET/IMAGES/MEDIUM/TZ3C00320_0022.GIF.
C. Yu, Z. Yue, M. Shi et al., An Intrapericardial Injectable Hydrogel Patch for mechanical-electrical coupling with Infarcted Myocardium. ACS Nano. 16, 16234–16248 (2022). https://doi.org/10.1021/ACSNANO.2C05168/SUPPL_FILE/NN2C05168_SI_002.ZIP
L. Zhang, T. Li, Y. Yu et al., An injectable conductive hydrogel restores electrical transmission at myocardial infarct site to preserve cardiac function and enhance repair. Bioact Mater. 20, 339–354 (2022). https://doi.org/10.1016/J.BIOACTMAT.2022.06.001
R. Zhao, R.Q. Zhang, A new insight into π–π stacking involving remarkable orbital interactions. Phys. Chem. Chem. Phys. 18, 25452–25457 (2016). https://doi.org/10.1039/C6CP05485D
Z. Zheng, Y. Tan, Y. Li et al., Biotherapeutic-loaded injectable hydrogels as a synergistic strategy to support myocardial repair after myocardial infarction. J. Control Release. 335, 216–236 (2021). https://doi.org/10.1016/J.JCONREL.2021.05.023
H. Zhu, S. Zhu, Z. Jia et al., Anomalous scaling law of strength and toughness of cellulose nanopaper. Proc. Natl. Acad. Sci. U S A 112, 8971–8976 (2015). https://doi.org/10.1073/PNAS.1502870112/SUPPL_FILE/PNAS.201502870SI.PDF
Acknowledgements
We gratefully appreciate of the support for this work from the Spanish Ministry of Economy, Industry, and Competitiveness (PID2022-139746OB-I00/AEI/10.13039/501100011033) and technical support from the ICTS NANBIOSIS (Drug Formulation Unit, U10) at the University of the Basque Country. Lidia Maeso thanks the Basque Government for the Ph.D. grant (PRE_2022_1_0053). Alireza Dolatshahi-Pirouz acknowledges the VIDI research programme (Project number R0004387), which is (partly) financed by The Netherlands Organisation for Scientific Research (NOW), as well as the Danish Council for Independent Research (Technology and Production Sciences, 8105–00003B); further, extend the acknowledgment for other funding which this work has also received financial support, as European Union’s Horizon 2020 research and innovation programme (under grant agreement No 951747), and Novo Nordisk Foundation (under grant agreement No NNF22OC007994). Besides, we would like to thank BioRender.com for the schematic designs that we could prepare for this work.
Funding
We gratefully appreciate the support for this work from the Spanish Ministry of Economy, Industry, and Competitiveness (PID2022-139746OB-I00/AEI/https://doi.org/10.13039/501100011033) and technical support from the ICTS NANBIOSIS (Drug Formulation Unit, U10) at the University of the Basque Country. Lidia Maeso thanks the Basque Government for the Ph.D. grant (PRE_2022_1_0053). Alireza Dolatshahi-Pirouz acknowledges the VIDI research program (Project number R0004387), which is (partly) financed by The Netherlands Organization for Scientific Research (NOW), as well as the Danish Council for Independent Research (Technology and Production Sciences, 8105–00003B); further, extend the acknowledgment for other funding which this work has also received financial support, as European Union’s Horizon 2020 research and innovation program (under grant agreement No 951747), and Novo Nordisk Foundation (under grant agreement No NNF22OC007994). Besides, we would like to thank BioRender.com for the schematic designs used in this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the review. Literature search and data analysis were performed by [Lidia Maeso], [Tatiane Eufrásio-da-Silva], and [Enes Deveci]. The first draft of the manuscript was written by [Tatiane Eufrásio-da-Silva] and [Lidia Maeso] and was critically revised by [Tatiane Eufrásio-da-Silva], [Alireza Dolatshahi-Pirouz] and [Gorka Orive]. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maeso, L., Eufrásio-da-Silva, T., Deveci, E. et al. Latest progress of self-healing hydrogels in cardiac tissue engineering. Biomed Microdevices 26, 36 (2024). https://doi.org/10.1007/s10544-024-00716-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-024-00716-z