Abstract
Janus particles are popular in recent years due to their anisotropic physical and chemical properties. Even though there are several established synthesis methods for Janus particles, microfluidics-based methods are convenient and reliable due to low reagent consumption, monodispersity of the resultant particles and efficient control over reaction conditions. In this work a simple droplet-based microfluidic technique is utilized to synthesize magnetically anisotropic TiO2-Fe2O3 Janus microparticles. Two droplets containing reagents for Janus particle were merged by using an asymmetric device such that the resulting droplet contained the constituents within its two hemispheres distinct from each other. The synthesized Janus particles were observed under the optical microscope and the scanning electron microscope. Moreover, a detailed in vitro characterization of these particles was completed, and it was shown that these particles have a potential use for biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Janus microparticles possess different physical and chemical properties leading to a growing field of scientific research. In comparison to conventional microparticles with uniform physical/chemical properties, Janus particles exhibit hybrid characteristics providing several advantages in applications such as cell encapsulation and assembly (Zhang et al. 2018), DNA assays (Lan et al. 2016), biological multiplexing (Nisisako et al. 2006), targeted drug delivery (Khan et al. 2014; Xie et al. 2012; Feng et al. 2019; Seo et al. 2013; Indalkar et al. 2013; Yang et al. 2012), noninvasive imaging (Song et al. 2018), theragnostics (Rahiminezhad et al. 2020; Zhang et al. 2019), microlenses (Nisisako et al. 2014, 2015; Zou et al. 2024), removal of organic and metal pollutants (Guix et al. 2018; Shin et al. 2023), and water decontamination (Chen et al. 2017; Ren et al. 2019).
Several methods have been reported for the synthesis of Janus particles (Safaie and Ferrier 2020; Saqib et al. 2022). Categorized broadly, the synthesis techniques are of two types: batch wise synthesis techniques and microfluidic based techniques. Under the batch wise category, Janus particles are generally synthesized via masking, phase separation and self-assembly techniques (Safaie and Ferrier 2020). Although these batch wise synthesis techniques are promising, they have inherent disadvantages that limit their applicability, such as polydispersity, limited control over reactions conditions, and wasteful consumption of reagents. In contrast microfluidic-based techniques provide superior control over reaction conditions, narrow size distribution of synthesized particles and minimal consumption of reagents (Saqib et al. 2022). Moreover, it allows precise control over the reaction conditions and efficient transfer of heat into and out of the system due to the small size of the microreactor. Microfluidic reactors can be categorized as either continuous flow or droplet-based flow systems. In continuous flow microfluidic systems, the reaction occurs within the fluid flowing in the channel and due to the direct interaction of reagents with channel walls, there is a possibility of cross contamination, and clogging problems. Moreover, continuous flow displays a parabolic flow profile which develops as a result of the no-slip boundary condition at the wall, which causes fluid at the wall to be slower than the fluid in the center of the channel. This leads to non-uniform residence time inside the microchannels which is not desirable in chemical syntheses and biological analyses. Furthermore, unless there is a stimulus to enhance mixing, parallel flowing fluids do not diffuse into each other before the characteristic diffusion length which depends on the microchannel geometry.
In droplet-based microfluidics, reagents are contained within droplets that are carried by an immiscible carrier fluid within the channel. This type of flow also allows rapid mixing due to the chaotic advection as a result of the recirculation zones produced by the two-phase flow inside a microchannel (Suh and Kang 2010; Baroud et al. 2010; Ozkan and Erdem 2015). Droplets in a microfluidic system act as individual microreactors that can be used to perform reactions with higher efficiency as compared to non-droplet based (continuous) microfluidic systems (Erdem et al. 2014; Shang et al. 2017; Wahab and Erdem 2020). For these reasons microfluidic droplet-based synthesis techniques have gained popularity not only for isotropic particles but also for Janus particle synthesis (Saqib et al. 2022). There are several microfluidic-based techniques for the synthesis of Janus particles reported in the literature that can be broadly classified into four categories namely co-flowing streams (Yu et al. 2012, 2016; Nisisako and Torii 2007; Marquis et al. 2012), phase separation (Jeong et al. 2013; Pang et al. 2014; Shah et al. 2009; Lone et al. 2011; Kim et al. 2013; Li et al. 2015), and double-emulsion (Chen et al. 2009a, b; Thiele and Seiffert 2011; Perro et al. 2022).
Even though microfluidic-based techniques provide a superior pathway for synthesizing Janus particles, the techniques available in literature are faced with fundamental limitations. Co-flow technique relies heavily on the diffusion length scale to avoid mixing and for the maintenance of a clear interface between the two parallel flowing phases. In order to avoid mixing the viscosities and velocities of the parallel flowing fluids need to be identical which is not possible in Janus particle synthesis since the two adjacent parts are inherently different. Secondly, in case of the phase separation method, the constantly changing volume of the droplet due to solvent evaporation does not allow the system to achieve thermal equilibrium, and therefore predicting the shape of the synthesized particles is difficult (Jeong et al. 2013). Furthermore, majority of the phase separation techniques utilize off-chip crosslinking leading to potential merging of droplets while being stored in off-chip containers. In case of double emulsion method, the series of two or three consecutive droplet generation junctions presents a challenge to fine tune the flow rate combinations to generate encapsulated droplets continuously. Apart from that the density difference between the two droplets causes the inner droplet to be pushed towards the back of the encapsulating droplet but this only happens during the flow of the droplet in the continuous phase fluid while majority of the techniques utilize off-chip curing. Due to this the resulting particles might exhibit limited to no anisotropy.
In this research paper we demonstrate a droplet based microfluidic device for the simple and efficient synthesis of magnetically anisotropic TiO2/Fe3O4 Janus particles where all steps of the synthesis are carried out within the microreactor. TiO2/Fe3O4 Janus particles have dual property due to TiO2 content, which is suitable to be used in application such as medical implants, in production of healthcare products, as well as bioimaging, whereas due to the Fe3O4 content, it will have a magnetic property which enables it to be manipulated in a magnetic field. In addition, we provide a detailed biocompatibility analysis of these particles to show their suitability for future biological applications.
2 Design
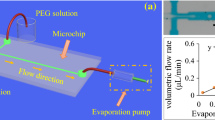
Janus particles with magnetic anisotropy could be useful for many applications, and adding another anisotropy to the particle would allow the particles to be employed for specialized applications. Therefore, the research idea was extended towards attaining Janus particles with dual properties provided by two different types of nanoparticles. For this purpose, the most important step was to bring together the two types of nanoparticles in a controlled manner. The schematic of the device used for this synthesis is shown in Fig. 1.
In droplet-based devices, droplets are used as micro-reactors therefore the aim is to merge two droplets having different types of nanoparticles in the device. For this purpose, four potential droplet merging geometries were designed, fabri- cated and tested and the device with the best performance was chosen for the experiments.
In this design, two dispersed phase inlet channels were designed to be at a distance of L = 560 μm apart, so that as the droplet of the first dispersed phase is generated it coalesces with the droplet of the second phase while it is forming in the channel. This process is shown in Fig. 2. A wider segment is also added just after the second dispersed phase channel to facilitate droplet merging (Sivasamy et al. 2010; Jang et al. 2018; Tan and Takeuchi 2006).
Droplet merging sequence in the asymmetric cross-junction merging device. Please see Video S1
Although the device exhibited great merging efficiency it was important to ensure that the Janus orientation of the droplets was maintained during and after the merging process. For this reason, the orientation of droplet interaction during the merging process was observed. Figure 3(a) and (b) show how the angle of droplet interaction affects the mixing procedure in the droplets. In Fig. 3(b) the angle of interaction is low and therefore the droplets merge in an almost sideways orientation resulting in a Janus configuration. This Janus configuration is further maintained by the chaotic advection mixing inside the droplet due to the two-phase flow which is brought about by the presence of the immiscible interface between the continuous phase and the dispersed phase. The velocity profile of a droplet flowing inside an immiscible fluid is different from a single-phase flow velocity profile. The droplet experiences recirculation zones due to this modified flow profile. The recirculation zones inside the droplet are symmetric if the flow conditions are symmetric with reference to the center of the droplet as show in Fig. 3(a). These symmetric recirculation zones ensure that the two constituents of the droplet, which are separated along the center of the droplet, do not merge.
On the other hand, if the angle of interaction is high as shown in Fig. 3(b), the droplets merge in an almost head on orientation and the constituents mix in a chaotic manner till the contents of the droplet become homogenous. This chaotic mixing is also assisted by the recirculation zones but since the contents of the droplet were not symmetrically separated at the beginning, they are mixed homogenously unlike the first case.
The angle of interaction is governed by the ratio (\(\varnothing ={Q}_{d}/{Q}_{c}\)) of the volumetric flow rates of the dispersed phase (\({Q}_{d}\)) and the continuous phase (\({Q}_{c}\)) fluids. If the value of \(\varphi\) is low, the droplet size is smaller therefore giving the second droplet to completely exit the channel without interacting with the first droplet and finally the two droplets interact just at the edge of the main channel as shown in Fig. 3(a). In contrast when the value of \(\varphi\) is high the droplet size is larger, and the second droplet is blocked by the first droplet and the second droplet is unable to exit the main channel. As a result, the two droplets interact almost head-on at a high interaction angle as shown in Fig. 3(b).
3 Experimental methods
3.1 Materials
A mixture of olive oil 64% (v/v), hexadecane 30% (v/v) and hexane 6% (v/v) is used as the continuous phase with 2wt% Span80 surfactant which is added to avoid droplet merging in the downstream channel.
Both of the dispersed phase fluids are composed of the monomer Poly(ethylene glycol) diacrylate (PEGDA 575) 80.6% (v/v), photo initiator -hydroxy-2-methylpropiophe- (HMP) 13% (v/v) and Tween 20 3.2% (v/v). One of the dispersed phases contains ferrofluid with 0.25 g/mL SPIONs suspended in deionized water 3.2% (v/v) and the other contains titanium dioxide nanoparticles (sigma Aldrich-914576) 0.2% (v/v).
Poly(ethylene glycol) diacrylate (PEGDA) average molecular weight (Mn) 575, n-hexane, hexadecane Reagent Plus 99%, Tween 20, Span 80 surfactant, and 2-hydroxy-2-methylpropiophe-(HMP), TiO2 nanoparticles were purchased from Sigma-Aldrich. A polydimethylsiloxane (PDMS) SYLGARD 184 silicone elastomer kit was purchased from Dow Corning GmbH, Germany. Photoresist SU-8 2050 and the SU-8 developer were purchased from Microresist Technology Germany.
3.2 Methods
The microfluidic chip was created using the traditional soft lithography method. Initially, an SU-8 master mold was produced through photolithography and subsequently developed with a resist developer. A mixture of PDMS base and curing agent was prepared in a 10:1 ratio. This blend was then poured onto the SU-8 mold and subjected to vacuum degassing to eliminate air bubbles. The mixture was cured in an 80 °C oven for 2 h. After curing, the PDMS layer was detached from the SU-8 mold, and inlet and outlet holes were created using a 2.0 mm microfluidic chip hole puncher. To seal the channels, the PDMS was bonded to another flat PDMS surface using oxygen plasma treatment for 60 s. The finished devices were placed in an 80 °C oven overnight to restore the hydrophobic properties of the channel walls.
The experimental setup consisted of an optical microscope, two syringe pumps, a microfluidic device, and an ultraviolet source. The Ultraviolet radiation exposure setup consisted of a UV lamp and an aperture placed on top of the device to expose only the specific parts of the device to UV radiation. After the particles were collected in a centrifuge tube, excess oil was removed, and the particles were washed with pure ethanol (to remove oil) multiple times (at least five times) and DI water (to remove uncured monomer) alternately at a centrifuge speed of 500 rpm. The particles were transferred on top of a SEM stub covered with conducting tape using a micropipette. The sample was then coated with around 5 nm of gold and then observed under the SEM.
3.3 In vitro characterizations of the TiO2/Fe3O4 Janus particles
L-929 fibroblasts (Şap Enstitüsü, 92123004) were used to assess biological characterizations of the Janus particles, in accordance with ISO 10993 standards. The cells were cultured in DMEM (Dulbecco's Modified Eagle F-12) medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin under standard cell culture conditions (37 °C, 5% CO2). Simultaneously, TiO2/Fe3O4 Janus particles were sterilized in 70% ethanol and UV-C irradiation each for 1 h. Then, these particles were weighed and incubated in the culture medium at 37 °C for 72 h to obtain extracts having 1 and 0.01 mg/ml concentrations. 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), lactate dehydrogenase (LDH), and total protein assays, as well as morphological analysis were performed.
To assess cellular proliferation, fibroblasts were seeded in 96-well plates at a density of 10,000 cells/well and cultured under standard conditions for 24 h. At the end of 24 h, the culture medium was replaced with the Janus particle extracts (0.01 and 1 mg/ml) and fresh culture medium as the control group. Experiments were performed for 5 days, and the media were changed every 2 days. At the end of the 1st, 3rd, and 5th days, the media was removed, and the cells were rinsed with 1xPBS. MTT solution was added to the cells and incubated for 4 h (37 °C, 5% CO2). Then, formazan crystals formed by the reduction of MTT by metabolically active cells were dissolved with 37% isopropyl alcohol containing 0.77% HCl, and optical density (OD) values were measured at 570 nm. Standard curves were drawn to correlate the optical density values to cellular proliferation.
The cytotoxic of the Janus particles was assessed using an LDH assay kit. For these experiments, cells were seeded in 96-well plates at a density of 10,000 cells/well and interacted with the regular culture medium for 24 h. Then, the culture medium was replaced with the Janus particle extracts (0.01 and 1 mg/ml), and fresh culture medium for the control group. LDH measurements were performed at 24, 48, and 72 h of culture. For low control measurements, the amount of LDH released from the healthy cells using standard culture medium was measured. For high control measurements, standard media were replaced with media containing 1% Triton-X. Absorbance measurements were made at 492 nm following the manufacturers’ instruction (Cytotoxicity Detection Kit (LDH)-11644793001 Roche).
Total protein measurements indirectly assessed the Janus particles’ effect on cellular activity. Similar to MTT and LDH experiments, cells were seeded in 96-well plates using a standard medium at a density of 5,000 cells/well, and cells were allowed to adhere and proliferate for 24 h. Then, the culture medium was replaced with the Janus particle extracts (0.01 and 1 mg/ml), and fresh culture medium for the control group. At the end of the 3rd day, the cells were rinsed with 1xPBS. Total protein measurements were carried out using the Bradford kit (Ozbiosciences) according to the manufacturer’s instructions.
To examine the effect of Janus particles on cellular morphology, cells were seeded in 96-well plates at a density of 2,000 cells/well and cultured for 24 h. Then, the cells were exposed to the Janus particle extracts at 1 mg/ml concentration for 3 days. At the end of the 3rd day, optical microscope images of the cells were captured. Then, the media was removed, and the cells were rinsed with 1xPBS, followed by fixing them with 4% paraformaldehyde solution for 20 min. Afterward, they were permeabilized with 1xPBS containing 0.1% Triton-X for 10 min. Then, the cells were stained with rhodamine-phalloidin (stains f-actin filaments) and DAPI (stains cell nuclei), respectively. Stained cells were examined under a confocal microscope (Leica TCS SP8 DMI8), and cellular spreading was quantified using ImageJ software. The in vitro experiments were repeated three times, and the statistical analyses were carried out using t-test.
4 Results and discussion
4.1 Synthesis of TiO2/Fe3O4 Janus particles
Experiments were performed to utilize the asymmetric cross-junction geometry for Janus particle synthesis using monomer solutions mixed with SPIONs (brown colored) and titanium dioxide nanoparticles (white colored) as the two dispersed phases. It can be clearly seen that the two semicircular parts of the droplet are clearly distinct from each other giving the droplet its Janus configuration. The merged droplets then continue to flow in the wider channel in a synchronized manner while maintaining the Janus configuration as shown in Fig. 4. At the same time a UV lamp, placed on the device, exposes the droplets to UV radiation which initiates polymerization of the droplets into solid particles due to the presence of photo-initiator in the dispersed phase. The average size of particles calculated from the experiments was 95.4 ± 1.4 μm. The synthesized Fe2O3/TiO2 Janus particles are imaged under SEM as shown in Fig. 5. The EDX spectra, shown in the figure confirm that the particles observed under are indeed PEGDA polymer particles since the EDX is characteristic of PEGDA. Unfortunately, EDX is not capable of showing the content of the particles as the measurements are made from the surface, therefore it is not possible to see the Fe3O4 or TiO2 content.
The volumetric flow rates used in Janus particle synthesis are \({Q}_{d}=0.2 \mu L/min\) for the dispersed phases and \({Q}_{d}=4.5 \mu L/min\) for the continuous phase. Under these conditions the frequency of droplet generation is 180 droplets/min. This corresponds to approximately 13 \(\mu L\) of Janus formation per minute.
5 In vitro characterizations of the Janus particles
The biological properties of the TiO2/Fe3O4 Janus particles were assessed using L929 fibroblasts. To do this, Janus particle extracts were prepared using a regular cell culture medium. The particles were immersed in the medium for 72 h at 37ºC at 0.01 and 1 mg/ml concentrations (regular cell culture medium was used as the control group). Then, the Janus particle extracts’ effect on the fibroblast proliferation was assessed using an MTT assay kit up to 5 days of culture. The results are shown in Fig. 6(a). The Janus particles did not cause any adverse effect on fibroblast proliferation both at 0.01 and 1 mg/ml concentrations up to 5 days of culture. An LDH assay kit was used to assess the cytotoxicity of the Janus particles. LDH assay is a common method to determine cytotoxicity by measuring the cytoplasmic enzyme activity released by the damaged cells (Smith et al. 2011). In the experiments, fibroblasts were exposed to Janus particle extracts having 0.01 and 1 mg/ml concentrations for up to 72 h, and the results are shown in Fig. 6(b). The %LDH release from the cells exposed to Janus particle extracts was comparable with the control group. This result indicated that the Janus particles did not show any cytotoxic effect against fibroblasts at the designated time points and concentrations. The cellular activity of the fibroblasts exposed to the Janus particle extracts was also assessed indirectly by measuring the total protein content of the cells after 3 days of culture. The results, shown in Fig. 6(c), indicated no significant difference in the total protein content of the cells cultured with the Janus particle extracts and the regular culture medium.
The Janus particles’ effect on cellular morphology was assessed using optical and confocal microscopy (Fig. 7). The morphologies of cells exposed to the regular cell culture medium and 1 mg/ml Janus particle extract were characterized on the 3rd day of the culture. The captured images showed that fibroblast morphologies were similar for both groups. In addition, the average spreading of cells exposed to the regular medium and the Janus particle extract were measured as 857 ± 170 µm and 870 ± 217 µm, respectively, similar to our previous findings (Durukan et al. 2024). This result showed that the Janus particles did not lead to any adverse effect on cellular spreading. Similar cellular morphologies and spreading, together with the aforementioned proliferation, and total protein content results suggested that the cellular functions were not negatively affected by the Janus particles in the designated time points and concentrations. Collectively, in vitro cellular interactions of the TiO2/Fe3O4 Janus particles showed that these particles have potential for use in biomedical applications and can further be investigated for in vivo studies.
6 Conclusions
In this work a microfluidic device for the synthesis of magnetically anisotropic Janus particles was reported. Janus particles are known to provide multi- functionality and orientation dependent physical and chemical properties that are much superior to conventional isotropic particles. The microfluidic device had an asymmetric cross-junction geometry having an expanded section to facilitate the merging of droplets. The distance between the two asymmetric droplet junctions was fixed such that the first droplet approached the second droplet just as it got pinched off from its dispersed phase stream. The separation of the contents of the two hemispheres was achieved by having the droplets merge in a sideways orientation instead of a head-on orientation. The angle of merging was the deciding factor in maintaining the Janus orientation of the droplets. As the droplets were transported further downstream the contents of the two hemispheres remained separated. During this time the droplets were exposed to UV radiation to initiate polymerization of the droplets into solid particles. Particles were then collected at the outlet and imaged under the optical microscope and the SEM. The obtained results show that the synthesized particles underwent complete polymerization and were magnetically anisotropic in nature. Furthermore, a detailed in vitro characterization of these particles was carried out and it was shown that the TiO2/Fe3O4 Janus particles have potential for use in biomedical applications.
Availability of data and materials
No datasets were generated or analysed during the current study.
References
C.N. Baroud, F. Gallaire, R. Dangla, Dynamics of microfluidic droplets. Lab Chip 10(16), 2032–2045 (2010)
C.-H. Chen, R.K. Shah, A.R. Abate, D.A. Weitz, Janus particles templated from double emulsion droplets generated using microfluidics. Langmuir 25(8), 4320–4323 (2009a)
C.-H. Chen, A.R. Abate, D. Lee, E.M. Terentjev, D.A. Weitz, Microfluidic assembly of magnetic hydrogel particles with uniformly anisotropic structure. Adv. Mater. 21(31), 3201–3204 (2009b)
A. Chen, X.-H. Ge, J. Chen, L. Zhang, J.-H. Xu, Multi-functional micromotor: microfluidic fabrication and water treatment application. Lab Chip 17(24), 4220–4224 (2017)
M.B. Durukan, D. Keskin, Y. Tufan, O. Incer, M.O. Cicek, B. Yildiz, . . . H.E. Unalan, An Edible Supercapacitor Based on Zwitterionic Soy Sauce‐Based Gel Electrolyte. Adv. Funct. Mater. 34(6), 2307051 (2024)
E.Y. Erdem, J.C. Cheng, F.M. Doyle, A.P. Pisano, Multi-temperature zone, droplet-based microreactor for increased temperature control in nanoparticle synthesis. Small 10(6), 1076–1080 (2014)
Z.-Q. Feng, K. Yan, J. Li, X. Xu, T. Yuan, T. Wang, J. Zheng, Magnetic janus particles as a multifunctional drug delivery system for paclitaxel in efficient cancer treatment. Mater. Sci. Eng. C 104, 110001 (2019)
M. Guix, S.M. Weiz, O.G. Schmidt, M. Medina-Sanchez, Self-propelled micro/nanoparticle motors. Part. Part. Syst. Charact. 35(2), 1700382 (2018)
Y. Indalkar, S. Gaikwad, A. Ubale, Janus particles recent and novel approach in drug delivery: an overview. J. Curr. Pharm. Res. 3(4), 1031 (2013)
Y. Jang, C. Cha, J. Jung, J. Oh, Interfacial compression-dependent merging of two miscible microdroplets in an asymmetric cross-junction for in situ microgel formation. Macromol. Res. 26(12), 1143–1149 (2018)
J. Jeong, E. Um, J.-K. Park, M.W. Kim, One-step preparation of magnetic janus particles using controlled phase separation of polymer blends and nanoparticles. RSC Adv. 3(29), 11801–11806 (2013)
I.U. Khan, C.A. Serra, N. Anton, X. Li, R. Akasov, N. Messaddeq, I. Kraus, T.F. Vandamme, Microfluidic conceived drug loaded janus particles in side-by-side capillaries device. Int. J. Pharm. 473(1–2), 239–249 (2014)
J. Kim, J. Joo, S.-Y. Park, Preparation of asymmetric porous janus particles using microfluidics and directional uv curing. Part. Part. Syst. Charact. 30(11), 981–988 (2013)
J. Lan, J. Chen, N. Li, X. Ji, M. Yu, Z. He, Microfluidic generation of magnetic-fluorescent janus microparticles for biomolecular detection. Talanta 151, 126–131 (2016)
W. Li, H. Dong, G. Tang, T. Ma, X. Cao, Controllable microfluidic fabrication of janus and microcapsule particles for drug delivery applications. RSC Adv. 5(30), 23181–23188 (2015)
S. Lone, S.H. Kim, S.W. Nam, S. Park, J. Joo, I.W. Cheong, Microfluidic synthesis of janus particles by uv-directed phase separation. Chem. Commun. 47(9), 2634–2636 (2011)
M. Marquis, D. Renard, B. Cathala, Microfluidic generation and selective degradation of biopolymer-based janus microbeads. Biomacromolecules 13(4), 1197–1203 (2012)
T. Nisisako, T. Torii, Formation of biphasic janus droplets in a microfabricated channel for the synthesis of shape-controlled polymer microparticles. Adv. Mater. 19(11), 1489–1493 (2007)
T. Nisisako, T. Torii, T. Takahashi, Y. Takizawa, Synthesis of monodisperse bicolored janus particles with electrical anisotropy using a microfluidic co-flow system. Adv. Mater. 18(9), 1152–1156 (2006)
T. Nisisako, T. Ando, T. Hatsuzawa, Capillary-assisted fabrication of biconcave polymeric microlenses from microfluidic ternary emulsion droplets. Small 10(24), 5116–5125 (2014)
T. Nisisako, H. Suzuki, T. Hatsuzawa, Biconvex polymer microlenses with tunable imaging properties designed by janus droplet microfluidics. Micromachines 6(10), 1435–1444 (2015)
A. Ozkan, E.Y. Erdem, Numerical analysis of mixing performance in sinusoidal microchannels based on particle motion in droplets. Microfluid. Nanofluid. 19, 1101–1108 (2015)
X. Pang, C. Wan, M. Wang, Z. Lin, Strictly biphasic soft and hard Janus structures: synthesis, properties, and applications. Angew. Chem. Int. Ed. 53(22), 5524–5538 (2014)
A. Perro, N. Coudon, J.-P. Chapel, N. Martin, L. B´even, J.-P. Douliez, Building micro-capsules using water-in-water emulsion droplets as templates. J. Colloid Interf. Sci. 613, 681–696 (2022)
Z. Rahiminezhad, A.M. Tamaddon, S. Borandeh, S.S. Abolmaali, Janus nanoparticles: new generation of multifunctional nanocarriers in drug delivery, bioimaging and theranostics. Appl. Mater. Today 18, 100513 (2020)
M. Ren, W. Guo, H. Guo, X. Ren, Microfluidic fabrication of bubble propelled micromotors for wastewater treatment. ACS Appl. Mater. Interfaces 11(25), 22761–22767 (2019)
N. Safaie, R.C. Ferrier Jr., Janus nanoparticle synthesis: Overview, recent developments, and applications. J. Appl. Phys. 127, 170902 (2020)
M. Saqib, P.A. Tran, B. Ercan, E.Y. Erdem, Microfluidic Methods in Janus Particle Synthesis. Int. J. Nanomedicine 17, 4355–4366 (2022)
K.D. Seo, J. Doh, D.S. Kim, One-step microfluidic synthesis of janus microhydrogels with anisotropic thermo-responsive behavior and organophilic/hydrophilic loading capability. Langmuir 29(49), 15137–15141 (2013)
R.K. Shah, J.-W. Kim, D.A. Weitz, Janus supraparticles by induced phase separation of nanoparticles in droplets. Adv. Mater. 21(19), 1949–1953 (2009)
L. Shang, Y. Cheng, Y. Zhao, Emerging droplet microfluidics. Chem. Rev. 117(12), 7964–8040 (2017)
S. Shin, S. Cho, R. Song, H. Kim, J. Lee, Oil pollution remediation with mass-producible and recyclable amphiphilic magnetic Janus particles. Chem. Eng. J. 471, 144734 (2023)
J. Sivasamy, Y.C. Chim, T.-N. Wong, N.-T. Nguyen, L. Yobas, Reliable addition of reagents into microfluidic droplets. Microfluid. Nanofluid. 8, 409–416 (2010)
S.M. Smith, M.B. Wunder, D.A. Norris, Y.G. Shellman, A simple protocol for using a LDH-based cytotoxicity assay to assess the effects of death and growth inhibition at the same time. PLoS ONE 6(11), e26908 (2011)
G. Song, M. Chen, Y. Zhang, L. Cui, H. Qu, X. Zheng, M. Wintermark, Z. Liu, J. Rao, Janus iron oxides@ semiconducting polymer nanoparticle tracer for cell tracking by magnetic particle imaging. Nano Lett. 18(1), 182–189 (2018)
Y.K. Suh, S. Kang, A review on mixing in microfluidics. Micromachines 1(3), 82–111 (2010)
W.-H. Tan, S. Takeuchi, Timing controllable electrofusion device for aqueous droplet-based microreactors. Lab Chip 6(6), 757–763 (2006)
J. Thiele, S. Seiffert, Double emulsions with controlled morphology by microgel scaffolding. Lab Chip 11(18), 3188–3192 (2011)
M.A. Wahab, E.Y. Erdem, Multi-step microfludic reactor for the synthesis of hybrid nanoparticles. J. Micromech. Microeng. 30(8), 085006 (2020)
H. Xie, Z.-G. She, S. Wang, G. Sharma, J.W. Smith, One-step fabrication of polymeric janus nanoparticles for drug delivery. Langmuir 28(9), 4459–4463 (2012)
Z. Yang, D.A. Aarts, Y. Chen, S. Jiang, P. Hammond, I. Kretzschmar, H.-J. Schneider, M. Shahinpoor, A.H. Muller, C. Xu, et al., Janus particle synthesis, self-assembly and applications. R. Soc. Chem. (2012)
Z. Yu, C.-F. Wang, L. Ling, L. Chen, S. Chen, Tri phase microfluidic directed self-assembly: anisotropic colloidal photonic crystal supraparticles and multicolor patterns made easy. Angew. Chem. 10(124), 2425–2428 (2012)
X. Yu, C. Zhang, S. You, H. Liu, L. Zhang, W. Liu, S.-S. Guo, and X.-Z. Zhao, Microfluidic synthesis of multiferroic janus particles with disk-like compartments. Appl. Phys. Lett. 108(7) (2016)
L. Zhang, K. Chen, H. Zhang, B. Pang, C.-H. Choi, A.S. Mao, H. Liao, S. Utech, D.J. Mooney, H. Wang et al., Microfluidic templated multicompartment microgels for 3d encapsulation and pairing of single cells. Small 14(9), 1702955 (2018)
Y. Zhang, K. Huang, J. Lin, P. Huang, Janus nanoparticles in cancer diagnosis, therapy and theranostics. Biomater. Sci. 7(4), 1262–1275 (2019)
P. Zou, C. Xu, L. Zhi, S. Melinte, Z. Wang, C. Zuo, R. Ye, Photonic Hook-Assisted Contrast-Enhanced Super-Resolution Imaging Using Janus Microspheres. IEEE Photonics Technol. Lett. 36(5), 353–356 (2024)
Funding
This work was funded by the Turkish Scientific and Technological Research Council (TÜBİTAK) 1001 grant (project ID: 219M480).
Author information
Authors and Affiliations
Contributions
M. S. designed and fabricated the microfluidic device and performed the synthesis experiments, wrote the related part of the manuscript, Y.T. designed and performed the in vitro experiments, wrote the related part of the manuscript, Z.C.O. helped with the characterization experiments, B.E. and E.Y.E. supervised the work and proofread the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This work also does not require any ethical approval as it did not involve any human or animal studies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (WMV 4065 KB)
Supplementary file2 (WMV 3158 KB)
Supplementary file3 (WMV 3963 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saqib, M., Tufan, Y., Orsel, Z.C. et al. Biocompatible Janus microparticle synthesis in a microfluidic device. Biomed Microdevices 26, 31 (2024). https://doi.org/10.1007/s10544-024-00711-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s10544-024-00711-4