Abstract
Plants suffer from combined stress of sulfur deficiency and cadmium toxicity in some agricultural lands. However, little is known about the reaction in plants, such as responses in antioxidant enzymes and non-protein thiol compounds, to such combined stress. Therefore, in this study, four treatments, S-sufficiency (TS−Cd), S-deficiency (T−S−Cd), Cd stress (TS+Cd) and combined stress of S-deficiency and Cd stress (T−S+Cd), were set up to investigate (1) the effects of sulfur deficiency or sulfur sufficiency on Cd toxicity to kidney bean cultivar seedlings and the related mechanisms, and (2) the responses of two kidney bean cultivars to combined stress of S-deficiency and Cd-tolerance. The results showed significant increases in hydrogen peroxide (H2O2) and malondialdehyde contents and significant increases in antioxidant enzyme (superoxide dismutase, catalase, peroxidase, and glutathione S-transferase) activities and non-protein thiol compounds (non-protein thiols, reduced glutathione, phytochelatins) synthesis in the plants in TS+Cd and T−S+Cd. On the tissue level, higher proportion of Cd was found to be immobilized/deposited in roots, while on the sub-cell level, higher proportion of Cd was located in cell walls and vacuole fractions with lower in cell organelles. Taken together, the results indicated that Cd detoxification was achieved by the two kidney bean cultivars through antioxidant enzyme activation, non-protein thiol compound synthesis and sub-cellular compartmentalization. In addition, the results indicated that sufficient S supply helped to relieve Cd toxicity, which is of special significance for remediation or utilization of Cd-contaminated soils as S is a plant essential nutrient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a naturally-occurring trace element and a highly toxic pollutant as well (Nazar et al. 2012). Cadmium contamination in agricultural soils has been dramatically increasing worldwide as a result of anthropogenic activities, such as improper disposal of industrial effluent and mining waste, and abuse of phosphate fertilizers, pesticides and sewage sludge (Li et al. 2014). According to the report of the Ministry of Environmental Protection and the Ministry of Land and Resources of the People’s Republic of China (2017), Cd pollution area of agricultural soils in China has reached more than 2.8 × 105 hm2. Cadmium is highly mobile in soil and readily taken up by plants. Once into plants, it can cause oxidative stress, increase membrane permeability, disturb mineral nutrition, and inhibit photosynthesis and structural changes when in high concentration (Gallego et al. 2012; Gill et al. 2012; Nazar et al. 2012; Rui et al. 2016) and poses a serious threat to human health through the food chain (Nazar et al. 2012).
To prevent the deleterious effect of Cd, plants employ various strategies such as sub-cellular Cd compartmentalization, antioxidation enzyme recovery and Cd chelation and/or sequestration. Nazar et al. (2012) observed higher activities of antioxidative enzymes, such as catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), peroxidase (POD) and superoxide dismutase (SOD), in plants suffered from Cd stress. Likewise, more CAT, SOD, POD and GR were produced under Cd stress (Singh and Shah 2014).
Sulfur is an essential element for plant growth. Research shows that sulfur plays an important role in Cd tolerance of plants (Bashir et al. 2013). At the tissue level, S can decrease Cd accumulation in shoots and alleviate Cd toxicity in plants via the formation and precipitation of Cd-S (Nocito 2002). At the cellular level, S supply can decrease the proportion of Cd in cell walls but increase the proportion in vacuoles, a metabolically inactive fraction, thus, reducing Cd biotoxicity (Zhang et al. 2014). In addition, plant defense mechanisms, such as improved growth, increased antioxidative capacity and reduced reactive oxygen species (ROS) and lipid peroxidation, can be initiated by S (Gill and Tuteja 2011; Khan et al. 2015; Mostofa et al. 2015). Sulfur might lead to increases in the synthesis of non-protein thiols (NPT) substances, including phytochelatins (PCs) and reduced glutathione (GSH), which directly chelate and sequestrate Cd in vacuoles (Gallego et al. 2012; Sharma et al. 2016). Zhang et al. (2013) reported that S supply protected rice from Cd toxicity by increasing the synthesis of the thiol substances (such as PCs and GSH) to decrease Cd availability.
However, sulfur deficiency is one of the major problems in agriculture production (Tombuloğlu et al. 2016). In Chinese agricultural soils, the likeliness of the occurrence of sulfur deficiency has increased (Li et al. 2001; Zhou et al. 2005). Kidney bean (Phaseolus vulgaris), a leguminous plant widely cultivated in South China, has high nutritional value, grows rapidly, and is of low production cost. As a leguminous plant, kidney bean needs more sulfur for growth as compared with non-leguminous plants, and in turn is more likely to be faced with sulfur deficiency. On the other hand, agricultural soils in South China have been severely polluted by Cd due to nonferrous metal smelting operations without strict pollution control (Hu et al. 2013). Therefore, kidney bean might suffer from the combined stress of sulfur deficiency and cadmium toxicity, and sulfur deficiency might exacerbate cadmium toxicity. This study was aimed to investigate (1) the effects of sulfur deficiency/sufficiency on Cd toxicity to kidney bean plants and the related mechanisms, and (2) the responses of two kidney bean cultivars to combined stress of S-deficiency and Cd-tolerance. The results from this study are expected to be helpful in kidney bean production in the Cd-contaminated soils in South China.
Materials and methods
Treatment set up and sampling
Seeds of two kidney bean (P. vulgaris) cultivars, Youxuan and Texuan, were sterilized with 10% H2O2 for 10 min and germinated at 25 °C in an artificial climate incubator in plastic pots (length × wide × height: 20 cm × 10 cm × 8 cm) filled with sterilized sands. After cotyledons fully stretched, uniform seedlings were transferred into plastic pots (25 cm in diameter) filled with 1 kg reconstituted sterilized sands and divided into two groups: + S and − S. The + S group was watered with an S-sufficient nutrient medium containing 102.20 g/L KNO3, 189.00 g/L Ca(NO3)·4H2O, 98.00 g/L MgSO4·7H2O, 27.20 g/L NH4H2PO4, 2.86 g/L H3BO3, 1.55 g/L MnSO4·4H2O, 0.22 g/L ZnSO4·7H2O, 0.08 g/L CuSO4·5H2O, 8.42 g/L EDTA-Fe–Na, 0.03 g/L CoCl2·6H2O, and 0.08 g/L MoO3. In contrast, the − S group was watered with the same nutrient medium but with the SO4 2− salts being replaced by equimolar amounts of corresponding Cl− salts. After 7 days, three pots from each of the two groups were sacrificed for seedling collecting for later analyses, and then each of the two groups was further divided into another two groups: +Cd and −Cd, obtaining a total of 4 treatments of TS+Cd, TS−Cd, T−S+Cd, and T−S−Cd. For the T+S+Cd and T−S+Cd treatments, Cd (in the form of CdCl2·2.5H2O) was added at a rate of 8 mg/kg soil on a dry weight basis. After another 7 days, plants were harvested from all the treatments.
For the plants harvested before and after Cd treatment, the roots and shoots were separated. The roots were soaked in 20 mM EDTA for 20 min to desorb the metal ions bound to the roots. Then, the roots were washed with deionized water, blot-dried, frozen in liquid nitrogen and kept at −80 °C in an ultra-low temperature freezer (Haier, DW-86L338, China) before being used for biochemical assays and cadmium sub-cellular accumulation measurements within a week after harvesting and freezing.
Determination of antioxidant enzyme activities
Fresh samples of leaves (0.2 g) were ground in a chilled mortar and enzymes were extracted with 4 mL ice-cold 50 mM potassium phosphate buffer (pH 7.2) containing 1% (w/v) polyvinylpyrrolidone. The homogenate was centrifuged at 12,000×g and 4 °C for 15 min. The supernatants were collected for enzyme assays. The activities of SOD, POD, and CAT were determined using an ultraviolet spectrophotometer UV-2501PCS (Shimadzu, Japan) as described by Chen and Wang (2006). Glutathione S-transferase (GST) activity was determined using a kit from Nanjing Jiancheng Bioengineering Research Institute. Determination of protein content was carried out using Coomassie brilliant blue, with bovine serum albumin (BSA) as standard.
Assessment of oxidative damage
To assess the extent of membrane damage in leaves, lipid peroxidation was measured and expressed as malondialdehyde (MDA) content (μmol/g FW) and H2O2 content (μmol/g FW). MDA was determined according to Chen and Wang (2006). Briefly, fresh leaf samples (0.2 g) were homogenized in 2 mL solution that contained 0.5% (w/v) thiobarbituric acid (TBA) and 10% (w/v) trichloroacetic acid (TCA). The mixture was subjected to a hot water bath at 95 °C for 20 min and then immediately to an ice bath to stop the reaction. The mixture was then centrifuged at 3000×g and 4 °C for 10 min, and MDA concentration was measured spectrophotometrically at 532 nm, 600 nm and 450 nm. H2O2 in leaves was determined following the procedure of Loreto and Velikova (2001). Point 5 g of fresh samples was homogenized with 5 mL of 0.1% (w/v) TCA in an ice bath. The homogenate was centrifuged at 12,000×g for 15 min; then, 1 mL of the supernatant was added to a mixture of 0.5 mL of 10 mM potassium phosphate buffer (pH 7.0) and 1 mL of 1 M potassium iodide. The absorbance at 390 nm was read. H2O2 concentration was calculated according to the standard curve.
Determination of NPT, GSH and PCs contents
Fresh leaf samples (1 g) were ground in liquid nitrogen and homogenized in 8 mL 5% sulfosalicylic acid (SSA). The homogenate was centrifuged at 10,000×g and 4 °C for 15 min and the supernatant was stored at −80 °C before used for NPT analyses. NPT were determined following the method described by Rama Devi and Prasad (Rama Devi and Prasad 1998). One millilitre of the supernatant was added to 2 mL of a reaction medium consisting of 0.5 mL 0.2 M Tris–HCL (pH 8.2) and 0.15 mL 10 mM 2-nitrobenzoic acid (DTNB). The absorbance at 412 nm was read after 20 min. For control, no DTNB was added. GSH was determined also using a kit from Nanjing Jiancheng Bioengineering Research Institute. The amount of PCs was estimated using the following formula: PCs = NPT-GSH (Bhargava et al. 2005).
Tissue fractionation and Cd analysis
Frozen roots,stems and leaves were homogenized in a pre-cold extraction buffer containing 50 mM Tris–HCl (pH 7.5), 250 mM sucrose and 1.0 mM C4H10O2S4 and 10 mM cysteine. Cells were separated into the following three fractions: cell wall, organelle and vacuole, using the differential centrifugation technique (Wang et al. 2008). The homogenate was centrifuged at 3000×g for 15 min and the precipitation was designated as ‘cell wall’ mainly consisting of cell walls and cell wall debris. The resulting supernatant was further centrifuged at 15,000×g for 30 min. The resulting deposition and supernatant were the “organelle” fraction and the “vacuole” fraction, respectively. The entire separation procedure was performed at 4 °C. The cell wall, organelle and vacuole fractions were digested with concentrated HNO3–HClO4 (3:1, v/v), and Cd concentration was determined by atomic absorption spectrophotometry (ZEEnit700P, Analytik Jena AG, Germany). Cd partition coefficient in sub-cellular fractions was estimated using the following formula:
where Cd i is the Cd concentration in sub-cellular fraction i, i can be cell wall, organelle or vacuole.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) with SPSS 17.0 and Microsoft Excel 2003, and figures were plotted using Sigma Plot 12.0. The results were presented as mean ± S.E. (standard error) in tables and figures. Significance of differences was determined at the 0.05 confidence level and treatment means were separated by Duncan’s multiple range test.
Results
Oxidative damage
H2O2 increased significantly by 133 and 110% in T−S+Cd and TS+Cd, respectively for Youxuan and significantly by 129 and 140% in T−S+Cd and TS+Cd, respectively for Texuan after Cd treatment as compared with before Cd treatment (Fig. 1). In addition, H2O2 content in T−S+Cd and TS+Cd was significantly higher than that in T−S−Cd and TS−Cd, respectively: 86 and 68%, respectively for Youxuan and 50 and 64%, respectively for Texuan. H2O2 content in TS−Cd and T−S−Cd did not increased significantly after 7 days of Cd treatment. It was not significantly different between T−S+Cd and TS+Cd though it was higher in the former and was not significantly different between T−S−Cd and TS−Cd, either. The two cultivars showed similar H2O2 contents and changing patterns.
The changes of hydrogen peroxide (H2O2) content in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
MDA showed a similar increasing pattern to that of H2O2 (Fig. 2). It increased significantly by 183 and 92% in T−S+Cd and TS+Cd, respectively for Youxuan and significantly by 188 and 109% in T−S+Cd and TS+Cd, respectively for Texuan after Cd treatment as compared with before Cd treatment. In addition, MDA content in T−S+Cd and TS+Cd was significantly higher than that in T−S−Cd and TS−Cd, respectively: 71 and 101%, respectively for Youxuan and 57 and 47%, respectively for Texuan. MDA content did not increased significantly in TS−Cd but increased significantly in T−S−Cd after 7 days of Cd treatment. MDA content was not significantly different between T−S+Cd and TS+Cd though it was higher in the former and was not significantly different between T−S−Cd and TS−Cd, either. The two cultivars showed similar H2O2 contents and changing patterns.
The changes of malondialdehyde (MDA) content in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
ROS scavenging antioxidant system
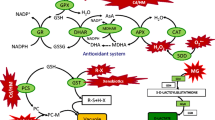
In order to prevent or minimize lipid peroxidation damage, activities of various antioxidant enzymes in plants are increased to scavenge ROS. In this study, the activities of the following antioxidant enzymes were focused on: SOD, CAT, POD, and GST.
SOD activity in Youxuan (Fig. 3a) and Texuan (Fig. 3b) increased significantly by 125 and 194%, respectively in T−S+Cd and by 67 and 108%, respectively in TS+Cd after Cd treatment as compared with before Cd treatment. SOD activity in Youxuan (364.41 U/g FW) and Texuan (510.85 U/g FW) was higher after Cd treatment in T−S+Cd than in TS+Cd where SOD activity was 349.73 U/g FW and 399.52 U/g FW, respectively. SOD activity increased by 125% for Youxuan and 194% for Texuan after Cd treatment compared with before Cd treatment in T−S+Cd. For TS+Cd, SOD activity increased by 67% for Youxuan and 108% for Texuan after Cd treatment compared with before Cd treatment.
The changes of superoxide dismutase (SOD) activity in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
POD activity was induced by Cd and increased significantly after Cd treatment in the two cultivars, especially when sulfur was limited (T−S+Cd) (Fig. 4). However, activity increase was more pronounced in Youxuan than in Texuan, by 229 and 351% in Youxuan, and just 131 and 249% in Texuan after Cd treatment in the TS+Cd and T−S+Cd treatments, respectively as compared with before Cd treatment. Compared with that in TS−Cd and T−S−Cd, POD activity in TS+Cd and T−S+Cd was 66 and 154%, respectively higher in Youxuan and 47 and 100%, respectively higher in Texuan after Cd treatment.
The changes of peroxidase (POD) activity in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
Figure 5 shows that CAT activity in the two cultivars increased significantly after Cd treatment in all treatments but TS−Cd. CAT activity in both cultivars was greater in S-deficient treatments (T−S+Cd, T−S−Cd) than in S-sufficient treatments (TS+Cd, TS−Cd) after Cd treatment. And there were no significant differences among T−S+Cd, TS+Cd and T−S−Cd for Youxuan while for Texuan, CAT activity in T−S+Cd was significantly higher than in TS+Cd, T−S−Cd and TS−Cd. CAT activity increased by 272 and 409% in Texuan and 419 and 602% in Youxuan after Cd treatment compared with that before Cd treatment in TS+Cd and T−S+Cd, respectively. Compared with that in TS−Cd and T−S−Cd, CAT activity after Cd treatment in TS+Cd and T−S+Cd increased by 231 and 38%, respectively in Youxuan, and by 215 and 186%, respectively in Texuan.
The changes of catalase (CAT) activity in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
Similarly, GST activity was greater in the treatments with Cd addition than in those without Cd addition (Fig. 6). But unlike other enzyme activities, GST activity was larger in S-sufficient treatments (TS+Cd/TS−Cd) than in the corresponding S-deficient ones (T−S+Cd/T−S−Cd) for both cultivars. GST activity increased much more in Texuan (by 218% in TS+Cd and 176% in T−S+Cd) than in Youxuan (by 95% in TS+Cd and 98% in T−S+Cd) after Cd treatment as compared with before Cd treatment. GST activity after Cd treatment in TS+Cd and T−S+Cd was 17 and 195% higher than in TS−Cd and T−S−Cd, respectively for Youxuan, and 69 and 56% higher, respectively for Texuan.
The changes of glutathione S-transferase (GST) activity in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
Sulfur-assimilation compounds
For both cultivars after Cd treatment, NPT content was higher in TS−Cd than in T−S−Cd and was significantly higher in TS+Cd than in T−S+Cd (Fig. 7). For TS+Cd, NPT content increased more in Youxuan (by 434%) than in Texuan (by 358%), but for T−S+Cd, more in Texuan (by 320%) than in Youxuan (by 272%) after Cd treatment as compared with before Cd treatment. NPT content after Cd treatment in TS+Cd and T−S+Cd was 146 and 57%, respectively higher in Youxuan and 221 and 56%, respectively higher in Texuan than in TS−Cd and T−S−Cd, respectively.
The changes of non-protein thiols (NPT) content in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
GSH content (Fig. 8) and PCs content (Fig. 9) showed similar increasing trends to that of NPT for both cultivars.
The changes of reduced glutathione (GSH) content in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
The changes of phytochelatins (PCs) content in the leaves of Youxuan (a), and Texuan (b). The same capital letters indicate no significant differences (P ≥ 0.05) between the treatments for a same sampling time, while the same lowercase letters indicate no significant differences (P ≥ 0.05) between sampling times for a same treatment. The values are mean ± SE and n = 3. S + Cd, Cd stress; S − Cd, S-sufficient; − S − Cd, S-deficient; − S + Cd, combined stress of S-deficiency and Cd stress
Cd accumulation in tissues and its sub-cellular distribution
After 7 days of Cd exposure, more Cd accumulated in plant tissues in T−S+Cd than in TS+Cd (Table 1). More Cd was deposited in the roots of Youxuan than in the roots of Texuan whether in T−S+Cd or TS+Cd, while an opposite trend was found in the stems of the two cultivars (Table 1). A larger proportion of Cd was translocated from roots to shoots in Texuan than in Youxuan at different periods with or without the supply of sulfur (Table 2).
Cd sub-cellular distribution in the two cultivars in different treatments are exhibited in Fig. 10. It can be seen that in the plant leaves in any of the four treatments, Cd was predominantly (approximately 80%) immobilized/deposited in cell walls, while in the roots and stems, comparable amounts of Cd were deposited in cell walls and vacuoles. This result suggests that vacuolar sequestration played a vital role in Cd detoxification in both cultivars. The proportion of Cd in the organelles of different tissues in treatment T−S+Cd was greater than in TS+Cd.
Partition coefficient of Cd in the tissues of Youxuan with Cd stress (TS+Cd) (a), Youxuan under combined stress of S-deficiency and Cd stress (T−S+Cd) (b), Texuan under Cd stress (TS+Cd) (c), and Texuan under combined stress of S-deficiency and Cd stress (T−S+Cd) (d). The values are mean ± SE and n = 3
Discussion
In stressful environments, reactive oxygen species (ROS), e.g., superoxide anion (O2 −), hydroxyl radicals (·OH), and hydrogen peroxide (H2O2), are commonly produced. Significant increase of H2O2 content in T−S+Cd and Ts+Cd (Fig. 1) indicated a Cd pollution level of 8 mg/kg had caused a stressful effect on the plants. In contrast, no significant changes of H2O2 content in T−S−Cd implied that S deficiency did not play a significant role in H2O2 production. ROS such as H2O2 are strong oxidant agents and they can cause lipid peroxidation and subsequent membrane injury as well as protein and nucleic acid damage (Ercal et al. 2001). MDA content, a direct measure of lipid peroxidation, is a good indicator for oxidative stresses (Armas et al. 2015). Similarly, significant increases of MDA content in T−S+Cd and Ts+Cd (Fig. 2) indicated occurrence of lipid peroxidation in the plants under Cd stress. But it was worthy of noting that in the plants not subjected to Cd stress, significant increase in MDA content was observed in those in T−S−Cd but not in TS−Cd, implying that S deficiency might also lead to lipid peroxidation due to production of other ROS but H2O2. Increased generation of MDA and H2O2 indicated that Cd provoked cellular disruption in the two cultivars through the induction of oxidative damage. The most severe oxidative damage was observed in Texuan under Cd stress (Figs. 1, 2).
Antioxidation enzyme recovery is one of the various protective strategies employed by plants subjected to environmental stress. Antioxidation enzymes including SOD, CAT, POD and GST are very effective in scavenging the harmful ROS. SOD is induced primarily and responsible for the detoxification of O2 −; CAT and POD are two major systems to promote H2O2 conversion to H2O and O2 in plant cells (Torres et al. 2008); GST is a protein superfamily of multifunctional enzymes, whose members play a crucial role in protecting plants against Cd toxicity (Corticeiro et al. 2013). Under Cd stress, production of CAT, SOD and POD was reported to be more significantly triggered with increasing Cd concentration (Singh and Shah 2014). Deng et al. (2010) observed higher CAT, APX, GR, POD and SOD activities with increasing Cd stress (Deng et al. 2010). Findings from this study were consistent with those reported in the literature: Antioxidation enzyme activities increased significantly in plants under Cd stress (Figs. 3, 4, 5, 6).
Cadmium rapidly induces the synthesis of PCs’ thiol-based complexing substances through the up-regulation of GSH biosynthesis by PCs synthase (Cobbett 2000), which explains the significant increase in NPT substances in the treatments with Cd application (Figs. 7, 8, 9). This is one of the detoxification mechanisms adopted by plants under Cd as NPT substances can directly chelate Cd and sequestrate it into vacuoles, a metabolically inactive fraction of plant cells (Gallego et al. 2012; Sharma et al. 2016), thus reducing the biotoxicity of Cd. Unlike H2O2 content, MDA content and the antioxidation enzyme activities, the contents of NPT compounds were significantly higher in Ts+Cd than in T−S+Cd. This is because S is an element in these compounds, and sufficient S was clearly favorable for their synthesis. As a result, Cd bioavailability was reduced, less ROS were produced, and lower antioxidation enzyme activities were found in Ts+Cd compared with in T−S+Cd.
For the two cultivars tested in this study, higher Cd accumulation was found in roots than in other tissues (Table 1), and S-deficiency caused more Cd to be transported from the roots to the above-ground parts (Table 2), which is consistent with the findings of others (Gallego et al. 2012; Liang et al. 2016; Sharma et al. 2016). The results indicated that the application of S can reduce Cd translocation and relieve the oxidative damage of kidney bean leaves during Cd exposure in this study. Cd accumulation in Youxuan roots was higher than in Texuan roots. In contrast, Texuan had a higher translocation factor than Youxuan (Table 2). This suggests that Cd accumulation and translocation are not only related to S nutrient status, but also to genotype.
Sub-cellular partitioning of metals is one of the possible internal mechanisms for heavy metal detoxification in plants (Sharma et al. 2016). Cd mainly accumulated in the metabolically inactive fractions of cell walls and vacuoles, with an extremely small percentage of Cd deposited in the metabolically active cell fraction of organelles (Fig. 10). There are polyose (including cellulose, hemicellulose, and pectin) and proteins within cell walls to bind Cd ions and stop Cd from entering protoplasm (Fu et al. 2011; Van Belleghem et al. 2007; Zhang et al. 2014). For the part of Cd that does enter protoplasms, it would be transferred into vacuoles and converted into inactive chemical forms, thus achieving Cd detoxification (Su et al. 2014; Whiting et al. 2000). The organelles are very metal-sensitive (Zhang et al. 2014). For both cultivars, a higher proportion of Cd was found in organelles in the S-deficient treatment compared with the S-sufficient treatment, further supporting the Cd detoxification mechanism of NPT chelation and subsequent compartmentalization of the Cd-PCs complexes in vacuoles (Nocito 2002; Van Belleghem et al. 2007).
Conclusions
Cadmium stress led to significant increase in ROS (i.e., H2O2) content and prominent lipid peroxidation in both cultivars no matter S was sufficient or not. In response to Cd stress, mainly three detoxification mechanisms were adopted by the cultivars: (1) increasing the activities of antioxidation enzymes (i.e., SOD, CAT, POD and GST) to scavenge the ROS so as to minimize lipid peroxidation and membrane damage; (2) synthesizing more NPT to chelate and compartmentalize Cd especially when S was sufficient; (3) on the tissue level, keeping Cd from translocating from roots to the above-ground parts; on the sub-cell level, keeping Cd in cell walls and vacuoles instead of organelles.
The results clearly showed that sufficient S supply can alleviate Cd toxicity in plants. This is of special significance as S is an essential nutrient for plants and in addition, it is of low cost compared with other soil amendments used to remediate soil Cd contamination or other remediation technologies.
Additionally, the two cultivars showed some differences in their responses to Cd stress. Genotype difference can be further explored in the future.
References
Armas T, Pinto AP, de Varennes A, Mourato MP, Martins LL, Gonçalves MLS, Mota AM (2015) Comparison of cadmium-induced oxidative stress in Brassica juncea in soil and hydroponic cultures. Plant Soil 388:297–305. doi:10.1007/s11104-014-2330-3
Bashir H, Ahmad J, Bagheri R, Nauman M, Qureshi MI (2013) Limited sulfur resource forces Arabidopsis thaliana to shift towards non-sulfur tolerance under cadmium stress. Environ Exp Bot 94:19–32
Bhargava P, Kumar Srivastava A, Urmil S, Chand Rai L (2005) Phytochelatin plays a role in UV-B tolerance in N2-fixing cyanobacterium Anabaena doliolum. J Plant Physiol 162:1220–1225. doi:10.1016/j.jplph.2004.12.006
Chen JX, Wang XF (2006) The Experimental guide of plant physiology. South China University of Technology Press, Guangzhou
Cobbett CS (2000) Phytochelatins and their roles in heavy metal detoxification. Plant Physiol 123:825–832
Corticeiro S, Freitas R, Figueira E (2013) The role of GSTs in the tolerance of Rhizobium leguminosarum to cadmium. Biometals 26:879–886. doi:10.1007/s10534-013-9664-9
Deng X, Xia Y, Hu W, Zhang H, Shen Z (2010) Cadmium-induced oxidative damage and protective effects of N-acetyl-l-cysteine against cadmium toxicity in Solanum nigrum L. J Hazard Mater 180:722–729. doi:10.1016/j.jhazmat.2010.04.099
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms Involved in Metal-induced oxidative damage. Curr Top Med Chem 1:529–539. doi:10.2174/1568026013394831
Fu X, Dou C, Chen Y, Chen X, Shi J, Yu M, Xu J (2011) Subcellular distribution and chemical forms of cadmium in Phytolacca americana L. J Hazard Mater 186:103–107. doi:10.1016/j.jhazmat.2010.10.122
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Gill SS, Tuteja N (2011) Cadmium stress tolerance in crop plants. Plant Signal Behav 6:215–222. doi:10.4161/psb.6.2.14880
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120. doi:10.1016/j.plantsci.2011.04.018
Hu Y, Liu X, Bai J, Shih K, Zeng EY, Cheng H (2013) Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environ Sci Pollut Res 20:6150–6159. doi:10.1007/s11356-013-1668-z
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Li S, Lin B, Zhou W (2001) Soil organic sulfur mineralization in the presence of growing plants under aerobic or waterlogged conditions. Soil Biol Biochem 33:721–727
Li Y, Wang L, Yang L, Li H (2014) Dynamics of rhizosphere properties and antioxidative responses in wheat (Triticum aestivum L.) under cadmium stress. Ecotoxicol Environ Safe 102:55–61. doi:10.1016/j.ecoenv.2014.01.004
Liang T, Ding H, Wang G, Kang J, Pang H, Lv J (2016) Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulfur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol Environ Safe 124:129–137
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Mostofa MG, Rahman A, Ansary MMU, Watanabe A, Fujita M, Phan Tran L (2015) Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci Rep 5:14078. doi:10.1038/srep14078
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Nocito FF (2002) Cadmium-induced sulfate uptake in maize roots. Plant Physiol 129:1872–1879
Rama Devi S, Prasad MNV (1998) Copper toxicity in Ceratophyllum demersum L. (Coontail), a free floating macrophyte: response of antioxidant enzymes and antioxidants. Plant Sci 138:157–165
Rui H, Chen C, Zhang X, Shen Z, Zhang F (2016) Cd-induced oxidative stress and lignification in the roots of two Vicia sativa L. varieties with different Cd tolerances. J Hazard Mater 301:304–313
Sharma SS, Dietz K, Mimura T (2016) Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ 39:1112–1126. doi:10.1111/pce.12706
Singh I, Shah K (2014) Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 108:57–66
Su Y, Liu J, Lu Z, Wang X, Zhang Z, Shi G (2014) Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ Exp Bot 97:40–48
The Ministry of Environmental Protection (2017) The Ministry of Land and Resources Report on the national soil contamination survey. http://www.mep.gov.cn/gkml/hbb/qt/201404/t20140417_270670.htm. Accessed 17 Apr 2017
Tombuloğlu H, Ablazov A, Filiz E (2016) Genome-wide analysis of response to low sulfur (LSU) genes in grass species and expression profiling of model grass species Brachypodium distachyon under S deficiency. Turk J Biol 40:934–943. doi:10.3906/biy-1508-32
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, Colepicolo P (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol Environ Safe 71:1–15. doi:10.1016/j.ecoenv.2008.05.009
Van Belleghem F, Cuypers A, Semane B, Smeets K, Vangronsveld J, D’Haen J, Valcke R (2007) Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol 173:495–508. doi:10.1111/j.1469-8137.2006.01940.x
Wang X, Liu Y, Zeng G, Chai L, Song X, Min Z, Xiao X (2008) Subcellular distribution and chemical forms of cadmium in Bechmeria nivea (L.) Gaud. Environ Exp Bot 62:389–395. doi:10.1016/j.envexpbot.2007.10.014
Whiting SN, Leake JR, Mcgrath SP, Baker A (2000) Positive responses to Zn and Cd by roots of the Zn and Cd hyperaccumulator Thlaspi caerulescens. New Phytol 145:199–210
Zhang W, Lin K, Zhou J, Zhang W, Liu L, Han X (2013) Spatial distribution and toxicity of cadmium in the joint presence of sulfur in rice seedling. Environ Toxicol Pharmacol 36:1235–1241
Zhang W, Lin K, Zhou J, Zhang W, Liu L, Zhang Q (2014) Cadmium accumulation, sub-cellular distribution and chemical forms in rice seedling in the presence of sulfur. Environ Toxicol Pharmacol 37:348–353
Zhou W, He P, Li S, Lin B (2005) Mineralization of organic sulfur in paddy soils under flooded conditions and its availability to plants. Geoderma 125:85–93. doi:10.1016/j.geoderma.2004.07.005
Acknowledgements
This work was supported by the National High-Tech R&D Program of China (Program 863, 2013AA102402), and the Science & Technology Planning Project of Guangdong Province, China (2015A020208012, 2015B090903077, 2014A030304036), and China Postdoctoral Science Foundation (2016M602479). We thank also Prof. Shiming Luo from South China Agricultural University for his helpful reviews and discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Li, D., Chen, G., Lu, Q. et al. Responses of two kidney bean (Phaseolus vulgaris) cultivars to the combined stress of sulfur deficiency and cadmium toxicity. Biometals 31, 17–28 (2018). https://doi.org/10.1007/s10534-017-0052-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-017-0052-8