Abstract
The stoichiometric analysis of the metal induced Metallothionein (MT) is pertinent for understanding the metal-MT interactions. Despite innumerable publications on MT, the literature addressing these aspects is limited. To bridge this gap, PIXE and ESI-MS analysis of the commercial rabbit liver MT1 (an isoform of MT), zinc induced isolated rat liver MT1, apo and Arsenic substituted rabbit liver MT1 have been carried out. These techniques in combination provide information about number and the signature of all the metal ions bound to MT. By using ESI-MS in the rabbit MT1, ions of Zn n MT1 (n = 0, 1, 4, 5, 6, 7) whereas, in rat MT1, the Zn1MT1 and Zn5MT1 ions are observed. PIXE analysis shows that some copper along with zinc is also present in the rabbit as well as rat MT1 which could not be assessed with ESI-MS. During As metallation reaction with rabbit MT1, with increase in arsenic concentration, the amount of arsenic bound to MT1 also increases, though not proportionally. The presence of both Zn and Cu in MT1 on Zn supplementation can be related to the role of MT in Zn and Cu homeostasis. Further, the presence of partially metallated MT1 suggests that MT1 may donate fractional amount of metal from it’s fully metallated form to other proteins where Zn acts as a cofactor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metallothioneins (MT) are a family of ubiquitous low molecular weight (6–7 kDa) proteins having different isoforms, with unusually high cysteine (30 %) content. MT binds to a number of heavy metals and plays a crucial role in the homeostasis of essential metals like Zinc and Copper (Bell and Vallee 2009). MT are known to be induced by a variety of agents like heavy metals, glucocorticoid, acute stress and various organic chemicals. Moreover, MT play a critical role in the protection against toxic non-essential metals such Cd, Hg and As etc. (Tandon et al. 2001; Klaassen et al. 2009). Three dimensional structure of MT reveals that it contains two independent domains: (i) M(II)3S9 in the N terminal, named the β domain (ii) M(II)4S11 in the C terminal, named the α domain (M(II) = divalent metal, S = sulphur of cysteine residue) (Arseniev et al. 1988; Robbins et al. 1991).
Arsenic is a well known carcinogen. The arsenic toxicity in the West Bengal, India is well known and has drawn the attention of international scientific community. It has been reported that inorganic arsenite (As (III)) induces MT in rat liver (Albores et al. 1992) and the induction of MT by arsenic is mediated by the metal-activated transcription factor 1 (Ma and He 2009). In the last decade, applying electrospray ionisation mass spectrometry (ESI-MS) and molecular modelling techniques, the group of Stillman has carried number of investigations to understand the reaction of As3+ with recombinant human MT1(rhMT1) and its α and β domains(Ngu and Stillman 2006; Ngu et al. 2010; Irvine et al. 2013). It has been reported that each individual domain fragment of the rhMT1 binds three As3+ ions in a ratio of one As3+ to three S of cysteines i.e. total six Arsenic ions are bound to MT1 (i.e. As6-βα-MT1) (Toyama et al. 2002; Ngu and Stillman 2006). Moreover, As3+ present in As6-βα-MT1 readily transfers to metal free apo-α-MT1 or apo-β-MT1 (Ngu et al. 2010). Recently, Irvine et al. (2013) has reported that during As3+ metallation, the accessibility of the cysteine thiols of the α and β domains of rhMT1 is different. More investigations are required to elucidate the mechanism of binding of As to MT and the role of MT in As detoxification.
MT is usually isolated from the liver of the animals (rat, rabbit) after metal supplementation. The initial NMR and X-ray crystallographic studies to determine the structure of MT were carried out using isolated MT (Arseniev et al. 1988; Robbins et al. 1991). However, isolating milligram quantity of MT, required for spectroscopic studies, from animal tissues is a cumbersome task. This led to shift from the isolated MT to the recombinantly expressed MT for such studies (Blindauer and Leszczyszyn 2009). However, to see the MT response to metal supplementation, isolated MT from animal model is still needed.
With the advancement of techniques like particle induced X-ray emission (PIXE) and ESI-MS which requires only micrograms of sample, it is possible to characterize isolated MT. ESI-MS is an indirect method which determines the metal stoichiometry on the basis of total molecular weight of the protein-metal complex. With PIXE, simultaneous multi elemental analysis (from Al to U) can be done without any ambiguity as the characteristic X-ray of each element is unique and provides the direct signature of its presence in the protein without any sample consumption. The sulphur atoms present in MT (in the cysteine and methionine residues) can act as an internal standard in order to obtain the extent of metallation of MT. A number of metalloproteins have been studied with PIXE for their metal content (Garman and Grime 2005; Nagy et al. 2009; Warner et al. 2010; Solis et al. 2011), but no reports are available in literature regarding the PIXE analysis of MT.
The present study has been designed to understand the metal-MT interactions using complimentary features of PIXE and ESI-MS and, addresses two different problems. In the first part, molecular weight and metal analysis of the commercially available rabbit liver MT1 and the Zn induced isolated MT1 from rat liver have been carried out. It provides information about the presence of elements other than Zn and their stoichiometry in the isolated MT1. In the second part, both these techniques have been applied to follow the binding of As to rabbit apo-MT1 (in vitro), prepared by demetallation of commercial rabbit MT1. This provides information regarding the binding of As3+ to rabbit MT1 and substantiates the similar data available on rhMT1. Such information may be useful for understanding the role of MT in As toxicity.
Materials and methods
Isolation of MT
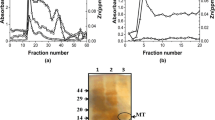
10 Male Wistar rats, weighing 200–250 g, were procured from the Central Animal House, Panjab University, Chandigarh, India, for the induction and isolation of MT. Sephadex G-75 and DEAE-Sephadex A-50 were obtained from Sigma Aldrich Co., USA. Analytical grade chemical reagents were used. The isolation of MT from the rat liver was carried out following the same procedure as used by Bremner and Davies (1975). Zinc (10 mg/kg body weight, as ZnSO4·7H2O per day) was injected subcutaneously for 2 days to rats to induce the synthesis and accumulation of MT. The livers were removed 18 h after the second injection. 20 % liver homogenates in 10 mM of Tris acetate buffer (pH 7.4) were prepared. Homogenates were centrifuged at 10,000×g for 30 min in the REMI C30BL centrifuge. Supernatants were again centrifuged at 100,000×g for 1 h in Brucker ultra centrifuge. The supernatant (cytosol) was fractionated on a Sephadex G-75 column (2.5 × 70 cm) equilibrated with 10 mM Tris acetate buffer (pH 7.4). The Zn containing fractions from low molecular weight region were applied to a DEAE-Sephadex A-50 column pre-equilibrated with 10 mM Tris acetate buffer (pH 7.4). The column was washed with same buffer and bound protein fractions were eluted with a linear gradient of 10–500 mM Tris acetate buffer (pH 7.4). High Zn containing fractions of protein (MT) were then pooled and concentrated using Centricons® (Millipore, 3 kDa MWCO). Zn quantitation of these fractions was carried by Atomic Absorption Spectrometer (Perkin Elmer 3100). Protein estimation of all the above fractions was done at 280 and 205 nm using UV–Vis spectrophotometer (Shimatzu Model UV-1800). The fractionation procedure was repeated multiple times to get required amount of protein for PIXE and ESI-MS studies. About 100–150 μg of MT1 was obtained from the liver of one animal. The experimental design and procedures were approved by the institutional Ethical Committee on Animal experiments.
Preparation of apo-MT1
Rabbit MT1 (95 % pure, procured from United Bo Tai Biotech Co., Ltd., China) was demetallated by adopting the procedure of Ngu and Stillman (2006) with only difference of using buffer of pH 3 instead of 2.7. This pH change was implemented because of the stability range of pH 3–8 for rabbit MT1, as quoted by the supplier. Elution was carried through a Sephadex G-25 column using 25 mM ammonium formate at pH 3 (pH adjusted with HCl) and the fractions were monitored by observing the absorption at 205 nm. Fractions that contained apo-MT1 were pooled and concentrated using centrifugal filter devices (Centricons®, Millipore, 3 kDa MWCO).
Preparation of As-MT1
The apo-MT1 (7.7 μmol) was reacted with increasing stoichiometric ratios (1, 2, 3) of As3+ (100 μM sodium arsenite in 25 mM ammonium formate, pH 3). Each sample was incubated for 2 h at 4 °C. To remove the unbound metal, the samples were loaded on Sephadex G-25 column (equilibrated with 25 mM ammonium formate at pH 3) and eluted with the same buffer. Fractions containing the protein–metal complex were collected by monitoring absorption at 205 nm. These fractions were pooled and concentrated using centrifugal filter devices (Centricons®, Millipore, 3 kDa MWCO).
Particle induced X-ray emission (PIXE)
The samples of isolated rat MT1, rabbit apo MT1 and As substituted rabbit MT1 were lyophilized. 100 μg of lyophilized protein was dissolved in 20 μl buffer. The solution was dispensed and dried on to an 8 μm Kapton® foil mounted on a stainless steel target holder frame (Fig. 1). PIXE analysis of these samples was carried out using the PIXE facilities at the 3 UD pelletron accelerator at the Institute of Physics (IOP), Bhubaneswar, India (Hajivalie et al. 1999) and at the Panjab University Cyclotron, Chandigarh, India (Puri et al., 2006). At the IOP facility, the samples were irradiated in vacuum with a proton beam of energy 3 MeV and the characteristics X-rays were detected by the ORTEC Si(Li) detector (model SLP06165) whereas, at the cyclotron facility, the proton beam energy was 2.7 MeV and the X-rays were detected by the Canberra HPGe detector (model GUL0035).
The thin films of different metals, with respective thickness given in parenthesis, namely, Ag (25.9 μg/cm2), Ni (340 μg/cm2), La (54.5 μg/cm2) and Dy (62.2 μg/cm2), were used for the calibration of the experimental set up. A good agreement was obtained between the measured and the reported values of the thicknesses. Moreover, the sensitivity and the precision of the set up along with the effectiveness of the target preparation technique adopted for the MT samples was verified by performing PIXE analysis of GSH, lysozyme and different concentration of ZnSO4·7H2O targets prepared in the same manner. The presence of unbound metals in the reaction mixtures for As substituted MT1 would substantially diminish the reliability of PIXE results. So, a phantom was prepared using 23.1 μM sodium arsenite in 15 mL of 25 mM ammonium formate buffer (pH 3) and concentrated to 1 mL using centrifugal filter devices (Centricons®, Millipore, 3 kDa MWCO). As no protein was present in the solution, all the As ions are free and should have been removed during the concentration/filtration process. The concentrated sample was then lyophilised, dissolved in 20 μL of 25 mM ammonium formate buffer (pH 3) and dried on Kapton® foil. The PIXE spectra of blank Kapton® foil and the phantom were recorded (Fig. 2). Presence of P, Ca and Fe are observed in the Kapton® foil. In the phantom, K, Cl and Br are observed in addition to the elements of Kapton® foil. The absence of As in phantom verifies that the above followed protocol completely removes the unbound metal ions from the solution. The computer code GUPIX (Campbell et al. 2000) was used to evaluate the elemental concentrations by fundamental parameter method. All the targets used for the current study were thin. For the quantitative analysis of such targets, the GUPIX code required the information regarding the details of experimental geometry, the parameters of the X-ray detectors, the chamber window thickness and composition, the energy of the incident protons and the charge collected.
ESI-MS
ESI-MS experiments were performed with Waters® micromass® Q-TOF Mass Spectrometer at Regional Sophisticated Instrumentation Centre (RSIC), Panjab University, Chandigarh, India. The experimental conditions were as follows: capillary voltage 3973 V, sample cone voltage 21 V and extraction cone voltage 2 V. The spectrometer was calibrated using Leucine enkephalin. The m/z range chosen for the recording of the spectra of MT1 was 500 to 2000 amu. All samples were analyzed thrice and prepared under the environment of argon gas to avoid the oxidation of MT.
Results and discussion
Rabbit MT1 and Rat liver MT1
The PIXE and ESI-MS spectra for the commercial rabbit MT1 are shown in Fig. 3 and for the rat liver MT1 in Fig. 4. In the PIXE spectra, the peaks of S, Cu and Zn are from the MT1, the peaks of Cl and K are from the buffer, and, the peaks of Ca and Fe are from the kapton foil (Fig. 2).
The ratio between S to Zn, S to Cu and Cu to Zn concentrations for both the rabbit MT1 and the rat MT1 are evaluated using GUPIX and given in Table 1. The sulphur to zinc ratio for the rabbit MT1 is 1.60 and for the rat MT1 is 3.23 (Table 1) which reveals that the extent of metallation in rabbit MT1 is higher in comparison to rat MT1. When these S:Zn ratios are compared with that for the fully metallated MT1 (for Zn7MT1, S:Zn is 1.468), it may be observed that the rabbit MT1 is close to fully metallated MT1. The ESI-MS (Fig. 3b) for the rabbit MT1 shows the presence of Zn6MT1 ions carrying +4, +5 and +6 charges (Table 2) as the dominant species, thus supporting PIXE observations. In addition to it, Zn7MT1 ions carrying +4 and +5 charges, Zn5MT1 ions carrying +4 and +5 charges, Zn4MT1 ions carrying +7 charges, Zn1MT1 ions carrying +8 charges and apo-MT1 ions carrying +9 charges are also observed.
The higher sulphur to zinc ratio (3.23) observed for rat MT1 can be due to the presence of a mixture of partially metallated MT1, apo-MT1 and metal saturated MT1. However, the ESI-MS (Table 3) shows only the presence of partially metallated ions of MT1 i.e. Zn5MT1 carrying +9 charges and Zn1MT1 carrying +9 charges. Unlike the rabbit MT1, ions of the fully metallated Zn7MT1 are not observed in the ESI-MS spectrum of the rat MT1. It has been reported that under the normal physiological conditions, 27 % of total MT is in the form of apo-MT (Yang et al. 2001). Our observation of only partially metallated rat MT1 indicates that all of the apoMT1 present in the physiological conditions may have got metallated after Zn supplementation. The charge on the Zn5MT1 ions (i.e. +9) is quite high for a folded protein. Sutherland et al. (2012) have observed the extra stability of Zn5rhMT1. They have proposed that only the terminal sulphurs were involved in the formation of Zn5MT1 which is thermodynamically favourable. The structure of Zn5MT1 would be more open than Zn7MT1 which contains both terminal and bridging sulphurs. The open structure of Zn5MT1 can be responsible for the observed high charge state. The presence of partially metallated MT with varying number of Zn can be related to the capability of MT to donate fractional amount of metal from its fully metallated form to other proteins where Zn acts as a cofactor.
The ratio of Cu to Zn in the commercial rabbit MT1 (0.009) and the rat MT1 (0.35) are found to be differing by a factor of 35. It is interesting to note that on Zn supplementation both Cu and Zn bind to MT1 instead of only Zn as expected. PIXE being a multi-elemental analysis technique also shows that besides Cu and Zn, no other metal binds with MT1 in these conditions. To the best of the authors knowledge, no reports in literature are available on the application of multielemental analysis techniques on isolated MT. Bremner and Davies (1975) observed the presence of Cu along with Zn in the MT fraction of gel filtration chromatography using atomic absorption spectrometry (AAS). The AAS being a single element detection technique can’t rule out the presence of other metals in MT. Moreover, their objective to use Cu and Zn estimation was for the MT isolation and not the metal stoichiometry because the absorbance measured at 280 nm was not suitable for the MT estimation, as it lacks aromatic amino acids. Similarly, while preparing samples for the NMR investigations of Cd induced rat MT1, Nicholson et al. (1983), found the Cd:Zn:Cu proportions (normalized to 7.0 metal ions) to be 4.46:2.35:0.19. Zn and Cu are both essential metals and both have high affinity for MT. Zn and Cu work in synergy for the coactivation of proteins like superoxide dismutase and phospholipase C. Out of various functions proposed for MT, one function is to act as a storage molecule for essential metals in order to maintain the balance between concentration of free Zn and Cu inside the cell. The presence of both Zn and Cu in rat liver MT1 on Zn supplementation can be related to the role of MT1 in Zn and Cu homeostasis.
The calculated masses of metal bound MT1 using the molecular masses of the amino acids and metal are slightly different (up to 10.7 Da) from the experimentally observed masses of ions in the ESI-MS spectra. This can be attributed to the presence of Cu ions in lieu of Zn ions as is evident from PIXE. ESI-MS can’t discriminate between Zn and Cu ions when bound to protein because the atomic mass of Zn and Cu are very close (i.e. 65 and 63 respectively) and a broad isotopic variation in the mass of Zn is also present. The number of Zn and Cu ions bound to MT1 is difficult to evaluate at this stage and require further investigations.
Rabbit apo-MT1 and arsenic substituted MT1
The PIXE and ESI-MS spectra of rabbit apo-MT1 (Fig. 5A) still show the presence of Zn indicating incomplete demetallation of rabbit MT1. The S peak in the PIXE spectrum of apo-MT1 could not be quantified due to strong overlap with the chlorine peak. The chlorine in the sample is due to the hydrochloric acid used to lower the pH of the MT1 solution in order to remove the Zn ions from Zn-MT1. The ESI-MS spectrum of rabbit apo-MT1 shows the presence of +7, +8 and +9 charged ions. The ions of apo-MT1 carrying +9 charges are the dominant species (Table 4). Partially metallated Zn3MT1 and Zn1MT1 ions are also observed. The apo-MT1 exists in random coil structure, with most of its basic amino acids exposed and thus, available for protonation. This might be the reason for getting high charge states of apo-MT1. The presence some of Zn ions in the apo-MT1 prepared from the commercial rabbit MT1 may be due to the higher pH = 3 used in the current protocol instead of 2.7 set by Ngu and Stillman (2006). The binding affinities of all the seven Zn ions present in MT are different (Bell and Vallee 2009). Therefore, it appears that loosely bound Zn ions were released whereas tightly bound ones still remain in MT1 on decreasing pH up to 3, instead of pH 2.7. Hence, a mixture of apo-MT1 and partially-metallated MT1 is observed.
In order to understand the mechanism of As binding to MT1, reactions of sodium arsenite of varying concentration with rabbit apo-MT1 has been carried out. The PIXE spectra (left panel of Fig. 5) of different As substituted MT1 samples also show the presence of Zn in addition to As. Again, we could not determine the sulphur to arsenic ratio could not be estimated due to strong overlap of chlorine peak from buffer. Therefore, the concentration of Zn still bound to MT1 after demetallation has been used as a reference. As the amount of protein in all samples is same, it implies that the amount of bound Zn remains constant. Hence, any change in As/Zn ratio would reflect the amount of As bound MT1. With an increase in the As concentration, the amount of As bound to MT increases as is evident from the As/Zn ratio, which increases from 14.7 to 20.1 to 28.4. The amount of As added to MT1 was in the ratio of 1:2:3 in the three samples; however, the As bound to MT1 was found to be in the ratio of 1:1.37:1.93. The ESI-MS spectra of As substituted MT1 (right panel of Fig. 5) show the presence of low charged ions i.e. (+5, +6) in addition to +7, +8 and +9 charged ions observed for the apo-MT1 (Fig. 5A(b)). In the positive mode of ESI-MS, the positively charged ions arise from the protonation of exposed basic amino acids of the protein. If the pH of the system is kept unchanged, the number of protonable amino acids varies with the extent of metallation as the latter leads to the folding of the MT1. In the present case, the pH of all the samples was kept constant at 3. The appearance of additional charge states of +5 and +6 and the highest abundance of +7 charge state (instead of +9 for apoMT1) on binding of As3+ with MT1 can be due to the folding of MT1 thus exposing lesser number of basic amino acids during ionization. On binding of As3+ with apo-MT1, species like As2MT1, As4MT1, As5MT1 and As6MT1 are observed in all reaction mixtures (Table 5). Irvine et al. (2013) had reported that the probability of subsequent binding of As3+ ion with MT reduces, as the number of already bound ions to MT increases due to the change in the accessibility of remaining cysteines. This may be reason for not observing only As6MT1 ions, which are fully metallated. Peaks of apo-MT1, Zn3MT1 and some unidentified adducts are also seen. The maximum number of bound As is found to be six which is consistent with the observation of Ngu and Stillman (2006). They have also reported the same stoichiometry for As bound to rhMT1a. This suggests that the As and MT stoichiometry remains unchanged, despite the variation in amino acid sequence of the MTs from human to rabbit. The present study supports the observation that the MT1 binds to As in 1:6 stoichiometry which is different from that of MT1 and Zn i.e. 1:7. It suggests that the AsMT1 metal cluster will be different from that of ZnMT1 and its three dimensional structure will be important to throw light in the understanding the role of MT in As toxicity.
Conclusions
The present work shows that PIXE and ESI-MS can be clubbed together in order to extract complimentary information regarding the identity of the metal and its stoichiometry when bound to MT. ESI-MS analysis of isolated rat MT1 shows the presence of only partially metallated MT1 is further substantiated by the high S/Zn ratio (i.e. 3.23, instead of 1.468 for Zn7MT1) observed with PIXE analysis. PIXE results also show that isolated rat MT1 contains significant number of Cu ions along with Zn ions despite being induced by Zn supplementation, suggesting the role of MT in metal homeostasis. By lowering the pH to 3 for rabbit MT1, few Zn ions still remain bound. On As3+ metallation reaction with MT1 at this pH forms As x MT1 (x = 2, 4, 5 and 6) complexes. With an increase in the As3+ ion concentration, the As bound to MT1 increases. To further understand the role of MT in Arsenic toxicity more studies are needed like the isolation of MT induced by As which can then be analyzed by PIXE, ESI-MS and other spectroscopic techniques.
References
Albores A, Koropatnick J, Cherian MG, Zelazowski AJ (1992) Arsenic induces and enhances rat hepatic metallothionein production in vivo. Chem Biol Interact 85:127–140
Arseniev A, Schultze P, Wörgötter E, Braun W, Wagner G, Vasak M, Kägi JHR, Wüthrich KJ (1988) Three-dimensional structure of rabbit liver Cd-metallothionein-2a in aqueous solution determined by nuclear magnetic resonance. Mol Biol 201:637–657
Bell SG, Vallee BL (2009) The metallothionein/thionein system: an oxidoreductive metalbolic zinc link. ChemBioChem 10:55–62
Blindauer CA, Leszczyszyn OI (2009) Metallothioneins: unparalleled diversity in structures and functions for metal ion homeostasis and more. Nat Prod Rep 27:720–741
Bremner I, Davies NT (1975) The induction of metallothionein in rat liver by zinc injection and restriction of food intake. Biochem J 149:733–738
Campbell JL, Hopman TL, Maxwell JA, Nejadly Z (2000) The Guelph PIXE software package III: alternative proton database. Nucl Instrum Methods Phys Res B 170:193–204
Garman EF, Grime GW (2005) Elemental analysis of proteins by microPIXE. Prog Biophys Mol Biol 89:173–205
Hajivalie M, Garg ML, Handa DK, Govil KL, Kakvad T, Vijayan V, Singh KP, Govil IM (1999) PIXE analysis of ancient Indian coins. Nucl Instrum Methods Phys Res B 150:645–650
Irvine GW, Summers KL, Stillman MJ (2013) Cysteine accessibility during As3+ metalation of α- and β-domains of recombinant human MT1a. Biochem Biophy Res Comm 433:477–483
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238:215–220
Ma Q, He X (2009) Induction of metallothionein I by Arsenic via metal-activated transcription factor 1: critical role of C-terminal cysteine residues in arsenic sensing. J of Biol Chem 284:12609–12621
Nagy ZS, Kocsonya A, Kovács I, Hopff D, Lüthje S, Niecke M (2009) High resolution imaging and elemental analysis of PAGE electrophoretograms by scanning proton microprobe. Nucl Instrum Methods Phys Res B 267:2163–2166
Ngu TT, Stillman MJ (2006) Arsenic binding to human metallothionein. J Am Chem Soc 128:12473–12483
Ngu TT, Dryden MDM, Stillman MJ (2010) Arsenic transfer between metallothionein protein at physiological pH. Biochem Biophy Res Comm 401:69–74
Nicholson JK, Sadler PJ, Cain K, Holt DE, Webb M, Hawkes GE (1983) 88 MHz 13Cd-n.m.r. studies of native rat liver metallothioneins. Biochem J 211:251–255
Puri NK, Balouria P, Govil IM, Mohanty BP, Garg ML (2006) Development of regional PIXE facility at Panjab University cyclotron, Chandigarh (India). Int J PIXE 16:7–20
Robbins AH, McRee DE, Williamson M, Collett SA, Xuong NH, Furey WF, Wang BC, Stout CD (1991) Refined crystal structure of Cd, Zn metallothionein at 2.0 Å resolution. J Mol Biol 221:1269–1293
Solis C, Celis H, Romero I, Olivé KI, Andrade E, Rosas FE, Rosales JHM, Moreno RC (2011) Metal/protein ratio determination in the Rhodobacter capsulatus cytoplasmic pyrophosphatase enzyme by particle induced X-ray emission. J Microbiol Methods 84:272–277
Sutherland DEK, Summers KL, Stillman MJ (2012) Noncooperative metalation of metallothionein 1a and its isolated domains with zinc. Biochemistry 51:6690–6700
Tandon SK, Singh S, Prasad S, Mathur N (2001) Hepatic and renal metallothionein induction by an oral equimolar dose of zinc, cadmium or mercury in mice. Food Chem Toxicol 39:571–577
Toyama M, Yamashita M, Hirayama N, Murooka Y (2002) Interactions of Arsenic with human metallothionein-2. J Biochem 132:217–221
Warner JD, DeYoung PA, Ellsworth LA, Kiessel LM, Rycenga MJ, Peaslee GF (2010) Quantitative analysis of a metalloprotein compositional stoichiometry with PIXE and PESA. Nucl Instrum Methods Phys Res B 268:1671–1675
Yang Y, Maret W, Vallee BL (2001) Differential fluorescence labeling of cysteinyl clusters uncovers high tissue levels of thionein. Proc Natl Acad Sci USA 98:5556–5559
Acknowledgments
The authors are thankful to the Pelletron crew of IOP, Bhubneswar and the staff of Panjab University Cyclotron, Chandigarh for the support rendered during PIXE experiment. Department of Science and Technology, New Delhi and Sophisticated Analytical Instrumentation Facility, Panjab University, Chandigarh are gratefully acknowledged for providing ESI-MS facility. This work is funded by University Grant Commission (UGC), New Delhi and UGC-DAE Consortium for Scientific Research, Kolkatta. Roobee Garla and Renuka Ganger are thankful to UGC, New Delhi for providing financial assistance in the form of Junior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garla, R., Mohanty, B.P., Ganger, R. et al. Metal stoichiometry of isolated and arsenic substituted metallothionein: PIXE and ESI-MS study. Biometals 26, 887–896 (2013). https://doi.org/10.1007/s10534-013-9665-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-013-9665-8