Abstract
The bacterium Pseudomonas aeruginosa is commonly isolated from the general environment and also infects the lungs of patients with cystic fibrosis (CF). Iron in mammals is not freely available to infecting pathogens although significant amounts of extracellular iron are available in the sputum that occurs in the lungs of CF patients. P. aeruginosa has a large number of systems to acquire this essential nutrient and many of these systems have been characterised in the laboratory. However, which iron acquisition systems are active in CF is not well understood. Here we review recent research that sheds light on how P. aeruginosa obtains iron in the lungs of CF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cystic fibrosis and Pseudomonas aeruginosa
The genetic condition cystic fibrosis (CF) is the most common lethal disease amongst the Caucasian population, occurring at an incidence of approximately 1 in 2,500 live births. Individuals with CF experience a range of clinical manifestations but chronic lung disease is well accepted as the leading cause of morbidity and mortality amongst these patients (Davis 2006; Davies et al. 2007). The morbidity and mortality associated with cystic fibrosis is predominantly related to an increased and relentless rate of lung function decline, interspersed by sporadic declines termed pulmonary exacerbations that serve to accelerate the process. Exacerbations are characterised by increases in coughing, sputum volume and breathlessness and may cause fever and weight loss.
The lungs of CF patients contain thick, viscous mucus that lines the airways and is difficult to clear. The resulting environment is conducive to bacterial infection with Pseudomonas aeruginosa being the most common isolate, followed by Staphylococcus aureus and Hemophilus influenzae (Ratjen and Doring 2003). Burkholderia cepacia and related species are also important pathogens in CF (Mahenthiralingam et al. 2005; Chiarini et al. 2006). The microflora in CF is very complex, with multiple species often present (Harrison 2007). A recent study identified over 100 different bacterial species in bronchoalveolar lavage fluid from a total of 28 patients (Harris et al. 2007). The population of organisms present in each patient differed considerably. There is significant variation between the strains and species present in the upper and lower airways, and even between different lobes of the lung (Smith et al. 1998; Nixon et al. 2001).
P. aeruginosa has been a major focus of research attention because it is present in the lungs of the majority of CF patients, often in large numbers (over 108 cfu per ml of sputum) (e.g. Aaron et al. 2004; Reid et al. 2007) and infection with P. aeruginosa is correlated with a progressive and relentless decline in patient health (Burns et al. 2001; Emerson et al. 2002; Konstan et al. 2007). P. aeruginosa is a saprophytic bacterium that can be isolated from a range of environmental niches (Palleroni 1981). In CF, infection with P. aeruginosa persists despite a robust humoral and cellular chronic immune response and extensive antibiotic treatment. Strains of P. aeruginosa that colonise CF patients are genetically as diverse as those found in the general environment (Wiehlmann et al. 2007), suggesting that all strains of P. aeruginosa have the ability to infect CF patients, although there is also evidence for infectious clones (“epidemic strains”) that have an increased ability to cause infection (reviewed in Govan et al. 2007). Once infection is established the same strain usually remains present in patients although it is likely to undergo genetic adaptation to the CF lung environment (Smith et al. 2006). Some instances of superinfection by other strains have been reported (McCallum et al. 2001).
There is considerable evidence that in CF P. aeruginosa exists primarily in biofilms rather than in a free-floating planktonic state (Lam et al. 1980; Singh et al. 2000; Yang et al. 2008). Biofilms are highly structured bacterial communities that are encased in a biopolymer matrix and gene expression in biofilms is very different from that of planktonic bacteria (Costerton et al. 1999; Stoodley et al. 2002; Hall-Stoodley et al. 2004). Biofilms confer significant antibiotic resistance and are difficult to eradicate (VanDevanter and Van Dalfsen 2005). Oxygen availability in biofilms in the CF lung may be low (Worlitzsch et al. 2002; Yoon et al. 2002) which would be expected to significantly affect the physiology of the bacteria.
Iron and infection
Bacteria require iron as a cofactor for numerous enzymes essential for metabolism. Free iron in biological solutions at pH 7.0 is present as oxidised Fe3+ and is present at concentrations of 10−9 M or less which is too low to be sufficient for bacterial growth (Bullen et al. 1978; Ratledge and Dover 2000). In mammals, most iron is bound by or incorporated into proteins (primarily ferritin, transferrin, lactoferrin and hemoglobin) with high affinity (Kd ~ 1020 for the extracellular proteins transferrin and lactoferrin). This has been termed “iron withholding” as it reduces the bioavailability of iron to infecting bacteria and it is an important component of innate immunity against bacterial infection (Weinberg 1984; Ratledge and Dover 2000). Its importance is emphasized by experiments with very many species showing that addition of iron in models of infection increases bacterial pathogenicity (reviewed in Ratledge and Dover 2000); for example, addition of iron reduced the LD50 of a strain of P. aeruginosa by up to 1,000-fold in a mouse model of infection (Forsberg and Bullen 1972). Iron saturation of host proteins is reduced during infection as part of the systemic inflammatory response, further lowering the amount of iron available to infecting bacteria (Weinberg 1984).
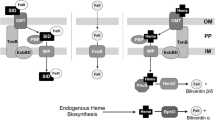
Many bacteria acquire iron through the secretion of siderophores, which are iron-scavenging molecules with high affinities for Fe3+ ions (typically with formation constants in the range 1022 to 1035, though siderophores with both higher and lower affinities are known; Matzanke 2005). There are numerous examples of siderophores made by pathogenic bacteria that have been shown to be required for normal infection (reviewed in Ratledge and Dover 2000; Bullen et al. 2005). P. aeruginosa makes two kinds of well-characterised siderophores, pyoverdines and pyochelin (Fig. 1). Pyoverdines have iron formation constants between 1024 and 1027 at pH 7.0 (Budzikiewicz 2004). Strains of P. aeruginosa make one of three different pyoverdines designated Type I, II and III (Meyer et al. 1997). Each pyoverdine has a specific receptor for its uptake (FpvAI, FpvAII or FpvAIII) (Poole et al. 1993; de Chial et al. 2003) and each strain only expresses the FpvA receptor for the type of pyoverdine that it produces. A second receptor for uptake of Type I pyoverdine (FpvB) enables Type II and Type III strains to use Type I pyoverdine (Ghysels et al. 2004).
Siderophores synthesised by Pseudomonas aeruginosa. Each pyoverdine is composed of a fluorescent dihydroxyquinoline chromophore attached to an acyl chain (R) and a Type-specific peptide. Fe3+ is bound by the catecholate and hydroxamate groups (Teintze et al. 1981; Tappe et al. 1993; Wasielewski et al. 2008). Pyochelin is thought to bind iron in a 1:1 ratio (Schlegel et al. 2004; Tseng et al. 2006) but the structure of the ferripyochelin complex has not been determined
Pyoverdine synthesis and ferripyoverdine uptake is best characterised for P. aeruginosa strain PAO1 that makes Type I pyoverdine (reviewed in Schalk 2007; Visca et al. 2007). Synthesis requires the coordinated action of at least 15 enzymes located in the cytoplasm and the periplasm of the bacteria. Uptake of ferripyoverdine is primarily mediated by an outer membrane protein FpvA, acting in conjunction with the TonB energy-transducing protein to import ferripyoverdine into the periplasm. Synthesis of pyochelin by P. aeruginosa has also been well studied (Crosa and Walsh 2002) with uptake of ferripyochelin being via the cell surface receptor FptA (Ankenbauer and Quan 1994).
Pyoverdine is generally considered the primary siderophore for P. aeruginosa. It has a higher affinity for iron than pyochelin (2.5 × 105 M−1; Cox and Graham 1979) and is more effective than pyochelin at releasing iron from transferrin (Sriyosachati and Cox 1986). Pyoverdine was necessary for infection in an animal model of severe burn wounds (Meyer et al. 1996) and was of more importance than pyochelin for infection in immunosuppressed mice (Takase et al. 2000). However pyochelin also contributed to bacterial virulence in this study, mirroring earlier findings (Cox 1982).
Pyoverdine-mediated iron transport is important for biofilm development (Banin et al. 2005; Patriquin et al. 2008). Mutant strains of P. aeruginosa that do not synthesize pyoverdine made defective biofilms under conditions of iron starvation and addition of pyoverdine restored normal biofilm formation. Pyochelin was not sufficient to enable complete biofilm development in the absence of pyoverdine, but addition of heterologous siderophores desferrioxamine and ferric citrate to the pyoverdine-deficient mutant restored biofilm phenotype to that of wild-type bacteria (Banin et al. 2005). Iron starvation is associated with changes in quorum sensing and twitching motility, both of which are important factors in biofilm development (Patriquin et al. 2008). These data, demonstrating the need for siderophore-mediated iron transport in normal biofilm development, are consistent with earlier findings that iron sequestration by lactoferrin inhibits P. aeruginosa biofilm development and affects twitching motility that is essential for biofilm development (Singh et al. 2002). Conversely, high amounts of iron also inhibit biofilm formation (Musk et al. 2005; Yang et al. 2007; Musk and Hergenrother 2008).
P. aeruginosa has the capacity to use a wide range of siderophores synthesized by other organisms (reviewed in Cornelis and Matthijs 2002; Poole and McKay 2003) and has at least two systems for uptake of heme (Ochsner et al. 2000). Genome analysis indicates that P. aeruginosa also has the capacity to acquire Fe2+ ions through a FeoAB transport system (reviewed in Cartron et al. 2006) and through an EfeU-type system (Grosse et al. 2006; Cao et al. 2007) but this has not been examined experimentally.
How does P. aeruginosa obtain iron in CF lungs?
Within the healthy airway, iron is predominantly found in iron binding proteins such as transferrin, lactoferrin and ferritin, whilst only a minute amount is present in a freely available form (Mateos et al. 1998). However, sputum from CF patients contains significant amounts of iron (average of four studies of 1.2 μg/ml; Stites et al. 1998, 1999; Reid et al. 2004, 2007). Some of this is in the form of ferritin (average of 1.7 μg/ml in the four studies, significantly higher than in control samples). Elevated iron levels were positively correlated with P. aeruginosa bacterial load in stable infected patients although the relationship in exacerbating patients was less clear (Reid et al. 2007). These data show that CF airways are not fully iron-deplete environments and suggest that iron is present in sufficient quantities to support bacterial growth. They are consistent with findings that lung epithelial cells with a mutation in the CF gene accumulate and release significant amounts of extracellular iron when grown in culture, whereas cells with the functional gene do not (Moreau-Marquis et al. 2008).
As well as ferritin, the human airway contains lactoferrin and transferrin. Pyoverdine can acquire iron from both of these proteins (Sriyosachati and Cox 1986; Xiao and Kisaalita 1997). Furthermore, proteases that are secreted by P. aeruginosa can degrade lactoferrin and transferrin and increase the ability of pyoverdine to acquire iron from these molecules (Doring et al. 1988; Wolz et al. 1994). Transferrin and lactoferrin undergo proteolysis in CF (Britigan et al. 1993) providing a potential supply of iron to the bacteria.
Iron availability for P. aeruginosa in CF is complicated by the presence of other infecting organisms that may compete for available iron, but may also increase its bioavailability. Microbial inter-species iron transactions have been little studied but competition for iron may have a major influence on bacterial growth. In one in vitro study that is relevant to CF the presence of S. aureus caused reduced expression of iron uptake genes of P. aeruginosa, implying that P. aeruginosa was able to obtain iron from S. aureus (Mashburn et al. 2005). Conversely, co-culture of P. aeruginosa with Burkholderia spp. caused increased expression of iron-responsive genes in P. aeruginosa, including those involved in pyoverdine and pyochelin synthesis, because of iron sequestration by the Burkholderia siderophore ornibactin (Weaver and Kolter 2004). Burkholderia species from patients with CF also make pyochelin (Sokol 1986; Darling et al. 1998) that could deliver iron to P. aeruginosa. B. cepacia and P. aeruginosa can form mixed biofilms (Eberl and Tummler 2004), providing intimate interactions between the bacteria that have a high potential for competition for iron but also for exchange of siderophores and iron cross-feeding.
In light of the above we can ask the question, do pyoverdine and pyochelin contribute to iron acquisition by P. aeruginosa in CF? An initial approach to this question involved purification of pyoverdine from CF sputa (Haas et al. 1991a). Pyoverdine was successfully purified from six out of twelve sputum samples, in amounts corresponding to a mean concentration of just under 1 μmol in the sputa. The majority of the pyoverdine (54–88%) was ferrated. Spectral analysis indicated that pyoverdine was present in the remaining six samples but the amounts were too low for purification. We have refined these methods and directly measured the amounts of pyoverdine in CF sputum and detected pyoverdine in sputum from 25 of 28 patients in amounts ranging from 0.7 to 51 μmol (I. Lamont, L. Martin and D. Reid, manuscript in preparation). The presence of pyoverdine in most CF sputa implies that is has a role in iron acquisition by the bacteria.
Six isolates of P. aeruginosa from CF patients were all able to make pyochelin (Haas et al. 1991b), but this compound has a much lower affinity for iron than pyoverdine, or the host iron-binding proteins lactoferrin and transferrin, so that its significance as an iron-scavenging agent during infection is not clear. So far as we are aware, pyochelin has not been identified in CF sputum.
A second approach to attempting to understand iron acquisition by P. aeruginosa in CF is to analyze the effects of sputum on gene expression. Microarray analysis showed that the presence of sputum in growth medium induced expression of a wide range of iron-acquisition genes including those involved in synthesis and uptake of pyoverdine and pyochelin and the hasA gene that enables heme uptake (Palmer et al. 2005). Microarray analysis has also been carried out using RNA extracted directly from sputum from a CF patient (Son et al. 2007). Bacteria in the sputum had increased expression of pyochelin synthesis genes but not pyoverdine synthesis genes, relative to bacteria grown in laboratory minimal medium.
Most characterised CF strains of P. aeruginosa make and use Type I or Type II pyoverdine (Meyer et al. 1997; De Vos et al. 2001) although an epidemic strain uses Type III pyoverdine (de Chial et al. 2003). However, in genotyping analysis of P. aeruginosa from CF the three FpvA receptor types occurred with similar frequencies (Wiehlmann et al. 2007). Intriguingly, some isolates from patients who had been chronically infected for a long time had lost the ability to make pyoverdine, although they retained the ability to take up ferripyoverdine (De Vos et al. 2001). A similar finding was made in a longitudinal study of chronic P. aeruginosa infection in a single patient (Smith et al. 2006). These bacteria may utilize pyoverdine made by other P. aeruginosa in CF or may acquire iron through a different uptake system. The concentrations of iron in some CF sputa are greater than 10 μM, which is sufficient to suppress pyoverdine-mediated transport in vitro (Meyer and Abdallah 1978). If P. aeruginosa is exposed to such concentrations for a prolonged period in CF, mutations in the pyoverdine system could occur without being biologically disadvantageous. Many strains of P. aeruginosa in CF have a high rate of mutation (Oliver et al. 2000) increasing the likelihood of mutations in genes that are not necessary for survival and growth in chronic infections that span many years.
P. aeruginosa may also acquire iron via siderophore-independent pathways in CF. Pulmonary exacerbations may lead to haemolysis resulting in the presence of heme in sputum. This would be a potential source of iron for P. aeruginosa via heme uptake pathways. In addition, under low oxygen conditions such as may be experienced by the bacteria in biofilms in CF iron may be in the ferrous (Fe2+) form. This could be taken up by P. aeruginosa via the FeoABC (and EfeU) pathways. There are also suggestions that the airways in CF are abnormally acidified (Tate et al. 2002), potentially increasing the pool of Fe2+ as acidic conditions protect Fe2+ ions against oxidation.
Conclusions and future challenges
Siderophore-mediated iron uptake by P. aeruginosa in the laboratory is well understood and there is reasonable knowledge of other iron uptake pathways. The challenge is to relate knowledge gained in vitro to iron acquisition in CF. Recent research has shown that the amount of extracellular iron in CF sputum is much higher than in other biological fluids but the form of this iron needs to be determined before we can properly understand how it might be acquired by P. aeruginosa. The presence of pyoverdine in sputum from CF patients indicates that this siderophore plays a role in iron acquisition in CF but its absence from some samples, coupled to the occurrence of pyoverdine-deficient mutants in CF, show that it is not the only mechanism of iron uptake and indeed, other iron-acquisition pathways may play a dominant role in some patients. The iron acquisition pathways used by P. aeruginosa in CF may be quite variable and different in individual patients, depending on the exact nature of the environment and the form(s) and amount of iron that is present. In addition to better understanding of the forms of iron present, it will be a major challenge to understand the complex interplay between P. aeruginosa and other bacteria and how this affects iron acquisition. How all of these factors interact to influence the biofilm mode of growth that is thought to occur in CF will require extensive study in vitro as well as in vivo. However, it is clear that iron acquisition is crucial to the survival and growth of P. aeruginosa in CF and a proper understanding of the mechanisms used holds the prospect of identifying new and effective ways of treating infections by this key pathogen.
References
Aaron SD et al (2004) Adult cystic fibrosis exacerbations and new strains of Pseudomonas aeruginosa. Am J Respir Crit Care Med 169:811–815. doi:10.1164/rccm.200309-1306OC
Ankenbauer RG, Quan HN (1994) FptA, the Fe(III)-pyochelin receptor of Pseudomonas aeruginosa: a phenolate siderophore receptor homologous to hydroxamate siderophore receptors. J Bacteriol 176:307–319
Banin E, Vasil ML, Greenberg EP (2005) Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA 102:11076–11081. doi:10.1073/pnas.0504266102
Britigan BE, Hayek MB, Doebbeling BN, Fick RB Jr (1993) Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect Immun 61:5049–5055
Budzikiewicz H (2004) Siderophores of the Pseudomonadaceae sensu stricto (fluorescent and non-fluorescent Pseudomonas spp.). Fortschr Chem Org Naturst 87:81–237
Bullen JJ, Rogers HJ, Griffiths E (1978) Role of iron in bacterial infection. Curr Top Microbiol Immunol 80:1–35
Bullen JJ, Rogers HJ, Spalding PB, Ward CG (2005) Iron and infection: the heart of the matter. FEMS Immunol Med Microbiol 43:325–330. doi:10.1016/j.femsim.2004.11.010
Burns JL et al (2001) Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis 183:444–452. doi:10.1086/318075
Cao J, Woodhall MR, Alvarez J, Cartron ML, Andrews SC (2007) EfeUOB (YcdNOB) is a tripartite, acid-induced and CpxAR-regulated, low-pH Fe2+ transporter that is cryptic in Escherichia coli K-12 but functional in E. coli O157:H7. Mol Microbiol 65:857–875. doi:10.1111/j.1365-2958.2007.05802.x
Cartron ML, Maddocks S, Gillingham P, Craven CJ, Andrews SC (2006) Feo-transport of ferrous iron into bacteria. Biometals 19:143–157. doi:10.1007/s10534-006-0003-2
Chiarini L, Bevivino A, Dalmastri C, Tabacchioni S, Visca P (2006) Burkholderia cepacia complex species: health hazards and biotechnological potential. Trends Microbiol 14:277–286. doi:10.1016/j.tim.2006.04.006
Cornelis P, Matthijs S (2002) Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ Microbiol 4:787–798. doi:10.1046/j.1462-2920.2002.00369.x
Costerton JW, Stewart PS, Greenberg EP (1999) Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1328. doi:10.1126/science.284.5418.1318
Cox CD (1982) Effect of pyochelin on the virulence of Pseudomonas aeruginosa. Infect Immun 36:17–23
Cox CD, Graham R (1979) Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol 137:357–364
Crosa JH, Walsh CT (2002) Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev 66:223–249. doi:10.1128/MMBR.66.2.223-249.2002
Darling P, Chan M, Cox AD, Sokol PA (1998) Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect Immun 66:874–877
Davies JC, Alton EW, Bush A (2007) Cystic fibrosis. BMJ 335:1255–1259. doi:10.1136/bmj.39391.713229.AD
Davis PB (2006) Cystic fibrosis since 1938. Am J Respir Crit Care Med 173:475–482. doi:10.1164/rccm.200505-840OE
de Chial M et al (2003) Identification of type II and type III pyoverdine receptors from Pseudomonas aeruginosa. Microbiology 149:821–831. doi:10.1099/mic.0.26136-0
De Vos D et al (2001) Study of pyoverdine type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch Microbiol 175:384–388. doi:10.1007/s002030100278
Doring G, Pfestorf M, Botzenhart K, Abdallah MA (1988) Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun 56:291–293
Eberl L, Tummler B (2004) Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: genome evolution, interactions and adaptation. Int J Med Microbiol 294:123–131. doi:10.1016/j.ijmm.2004.06.022
Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL (2002) Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34:91–100. doi:10.1002/ppul.10127
Forsberg CM, Bullen JJ (1972) The effect of passage and iron on the virulence of Pseudomonas aeruginosa. J Clin Pathol 25:65–68. doi:10.1136/jcp.25.1.65
Ghysels B et al (2004) FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology 150:1671–1680. doi:10.1099/mic.0.27035-0
Govan JR, Brown AR, Jones AM (2007) Evolving epidemiology of Pseudomonas aeruginosa and the Burkholderia cepacia complex in cystic fibrosis lung infection. Future Microbiol 2:153–164. doi:10.2217/17460913.2.2.153
Grosse C, Scherer J, Koch D, Otto M, Taudte N, Grass G (2006) A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol Microbiol 62:120–131. doi:10.1111/j.1365-2958.2006.05326.x
Haas B, Kraut J, Marks J, Zanker SC, Castignetti D (1991a) Siderophore presence in sputa of cystic fibrosis patients. Infect Immun 59:3997–4000
Haas B, Murphy E, Castignetti D (1991b) Siderophore synthesis by mucoid Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. J Microbiol 37:654, 657
Hall-Stoodley L, Costerton JW, Stoodley P (2004) Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. doi:10.1038/nrmicro821
Harris JK et al (2007) Molecular identification of bacteria in bronchoalveolar lavage fluid from children with cystic fibrosis. Proc Natl Acad Sci USA 104:20529–20533. doi:10.1073/pnas.0709804104
Harrison F (2007) Microbial ecology of the cystic fibrosis lung. Microbiology 153:917–923. doi:10.1099/mic.0.2006/004077-0
Konstan MW et al. (2007) Risk factors for rate of decline in forced expiratory volume in one-second in children and adolescents with cystic fibrosis. J Pediatr 151:134–139, 139.e1
Lam J, Chan R, Lam K, Costerton JW (1980) Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun 28:546–556
Mahenthiralingam E, Urban TA, Goldberg JB (2005) The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3:144–156. doi:10.1038/nrmicro1085
Mashburn LM, Jett AM, Akins DR, Whiteley M (2005) Staphylococcus aureus serves as an iron source for Pseudomonas aeruginosa during in vivo coculture. J Bacteriol 187:554–566. doi:10.1128/JB.187.2.554-566.2005
Mateos F, Brock JH, Perez-Arellano JL (1998) Iron metabolism in the lower respiratory tract. Thorax 53:594–600
Matzanke BF (2005) Iron transport: siderophores. In: King RB (ed) Encyclopedia of Inorganic Chemistry, 2nd edn. Wiley, New York
McCallum SJ, Corkill J, Gallagher M, Ledson MJ, Hart CA, Walshaw MJ (2001) Superinfection with a transmissible strain of Pseudomonas aeruginosa in adults with cystic fibrosis chronically colonised by P. aeruginosa. Lancet 358:558–560. doi:10.1016/S0140-6736(01)05715-4
Meyer J-M, Abdallah MA (1978) The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J Gen Microbiol 107:319–328
Meyer JM, Neely A, Stintzi A, Georges C, Holder IA (1996) Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun 64:518–523
Meyer J-M et al (1997) Use of siderophores to type pseudomonads: the three Pseudomonas aeruginosa pyoverdine systems. Microbiology 143:35–43
Moreau-Marquis S et al (2008) The DeltaF508-CFTR mutation results in increased biofilms formation by Pseudomonas aeruginosa by increasing iron bioavailability. Am J Physiol Lung Cell Mol Physiol 295:L25–L37. doi:10.1152/ajplung.00391.2007
Musk DJJ, Hergenrother PJ (2008) Chelated iron sources are inhibitors of Pseudomonas aeruginosa biofilms and distribute efficiently in an in vitro model of drug delivery to the human lung. J Appl Microbiol 105:380–388
Musk DJ, Banko DA, Hergenrother PJ (2005) Iron salts perturb biofilm formation and disrupt existing biofilms of Pseudomonas aeruginosa. Chem Biol 12:789–796. doi:10.1016/j.chembiol.2005.05.007
Nixon GM et al (2001) Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 138:699–704. doi:10.1067/mpd.2001.112897
Ochsner A, Johnson Z, Vasil ML (2000) Genetics and regulation of two distinct haem-uptake systems, phu and has, in Pseudomonas aeruginosa. Microbiology 146:185–198
Oliver A, Canton R, Campo P, Baquero F, Blazquez J (2000) High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251–1254. doi:10.1126/science.288.5469.1251
Palleroni NJ (1981) Introduction to the family Pseudomonadaceae. In: Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG (eds) The prokaryotes. A handbook on habitats. Isolation and identification of bacteria. Springer-Verlag, Berlin, pp 655–665
Palmer KL, Mashburn LM, Singh PK, Whiteley M (2005) Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187:5267–5277. doi:10.1128/JB.187.15.5267-5277.2005
Patriquin GM, Banin E, Gilmour C, Tuchman R, Greenberg EP, Poole K (2008) Influence of quorum sensing and iron on twitching motility and biofilm formation in Pseudomonas aeruginosa. J Bacteriol 190:662–671. doi:10.1128/JB.01473-07
Poole K, McKay GA (2003) Iron acquisition and its control in Pseudomonas aeruginosa: many roads lead to Rome. Front Biosci 8:d661–d686. doi:10.2741/1051
Poole K, Neshat S, Krebes K, Heinrichs D (1993) Cloning and nucleotide analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J Bacteriol 175:4597–4604
Ratjen F, Doring G (2003) Cystic fibrosis. Lancet 361:681–689. doi:10.1016/S0140-6736(03)12567-6
Ratledge C, Dover LG (2000) Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54:881–941. doi:10.1146/annurev.micro.54.1.881
Reid DW, Lam QT, Schneider H, Walters EH (2004) Airway iron and iron-regulatory cytokines in cystic fibrosis. Eur Respir J 24:286–291. doi:10.1183/09031936.04.00104803
Reid DW, O’May C, Champion A, Kirov SM (2007) Increased airway iron as a potential factor in the persistence of Pseudomonas aeruginosa infection in cystic fibrosis. Eur Respir J 30:286–292. doi:10.1183/09031936.00154006
Schalk IJ (2007) Metal trafficking via siderophores in Gram-negative bacteria: specificities and characteristics of the pyoverdine pathway. J Inorg Biochem 102:1159–1169
Schlegel K, Taraz K, Budzikiewicz H (2004) The stereoisomers of pyochelin, a siderophore of Pseudomonas aeruginosa. Biometals 17:409–414. doi:10.1023/B:BIOM.0000029437.42633.73
Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP (2000) Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762–764. doi:10.1038/35037627
Singh PK, Parsek MR, Greenberg EP, Welsh MJ (2002) A component of innate immunity prevents bacterial biofilm development. Nature 417:552–555. doi:10.1038/417552a
Smith DL, Smith EG, Pitt TL, Stableforth DE (1998) Regional microbiology of the cystic fibrosis lung: a post-mortem study in adults. J Infect 37:41–43. doi:10.1016/S0163-4453(98)90475-3
Smith EE et al. (2006) Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci USA 103:8487–8492
Sokol PA (1986) Production and utilization of pyochelin by clinical isolates of Pseudomonas cepacia. J Clin Microbiol 23:560–562
Son MS, Matthews WJ Jr, Kang Y, Nguyen DT, Hoang TT (2007) In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun 75:5313–5324. doi:10.1128/IAI.01807-06
Sriyosachati S, Cox CD (1986) Siderophore-mediated iron acquisition from transferrin by Pseudomonas aeruginosa. Infect Immun 52:885–891
Stites SW, Walters B, O’Brien-Ladner AR, Bailey K, Wesselius LJ (1998) Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest 114:814–819. doi:10.1378/chest.114.3.814
Stites SW, Plautz MW, Bailey K, O’Brien-Ladner AR, Wesselius LJ (1999) Increased concentrations of iron and isoferritins in the lower respiratory tract of patients with stable cystic fibrosis. Am J Respir Crit Care Med 160:796–801
Stoodley P, Sauer K, Davies DG, Costerton JW (2002) Biofilms as complex differentiated communities. Annu Rev Microbiol 56:187–209. doi:10.1146/annurev.micro.56.012302.160705
Takase H, Nitanai H, Hoshino K, Otani T (2000) Impact of siderophore production on Pseudomonas aeruginosa infections in immunocompromised mice. Infect Immun 68:1834–1839. doi:10.1128/IAI.68.4.1834-1839.2000
Tappe R, Taraz K, Budzikiewicz H, Meyer J-M, Lefevre LF (1993) Structure elucidation of a pyoverdine produced by Pseudomonas aeruginosa ATCC 27853. J Prakt Chem 335:83–87. doi:10.1002/prac.19933350113
Tate S, MacGregor G, Davis M, Innes JA, Greening AP (2002) Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax 57:926–929. doi:10.1136/thorax.57.11.926
Teintze M, Hossain MB, Barnes CL, Leong J, van der Helm D (1981) Structure of ferric pseudobactin, a siderophore from a plant growth promoting Pseudomonas. Biochemistry 20:6446–6457. doi:10.1021/bi00525a025
Tseng CF et al (2006) Bacterial siderophores: the solution stoichiometry and coordination of the Fe(III) complexes of pyochelin and related compounds. J Biol Inorg Chem 11:419–432. doi:10.1007/s00775-006-0088-7
VanDevanter DR, Van Dalfsen JM (2005) How much do Pseudomonas biofilms contribute to symptoms of pulmonary exacerbation in cystic fibrosis? Pediatr Pulmonol 39:504–506. doi:10.1002/ppul.20220
Visca P, Imperi F, Lamont IL (2007) Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol 15:22–30. doi:10.1016/j.tim.2006.11.004
Wasielewski E et al (2008) Multiple conformations of the metal-bound pyoverdine PvdI, a siderophore of Pseudomonas aeruginosa: a nuclear magnetic resonance study. Biochemistry 47:3397–3406. doi:10.1021/bi702214s
Weaver VB, Kolter R (2004) Burkholderia sp. alter Pseudomonas aeruginosa physiology through iron sequestration. J Bacteriol 186:2376–2384. doi:10.1128/JB.186.8.2376-2384.2004
Weinberg ED (1984) Iron withholding: a defense against infection and neoplasia. Physiol Rev 64:65–102
Wiehlmann L et al (2007) Population structure of Pseudomonas aeruginosa. Proc Natl Acad Sci USA 104:8101–8106. doi:10.1073/pnas.0609213104
Wolz C et al (1994) Iron release from transferrin by pyoverdin and elastase from Pseudomonas aeruginosa. Infect Immun 62:4021–4027
Worlitzsch D et al (2002) Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109:317–325
Xiao R, Kisaalita WS (1997) Iron acquisition from transferrin and lactoferrin by Pseudomonas aeruginosa pyoverdin. Microbiology 143:2509–2515
Yang L, Barken KB, Skindersoe ME, Christensen AB, Givskov M, Tolker-Nielsen T (2007) Effects of iron on DNA release and biofilm development by Pseudomonas aeruginosa. Microbiology 153:1318–1328. doi:10.1099/mic.0.2006/004911-0
Yang L et al (2008) In situ growth rates and biofilm development of Pseudomonas aeruginosa populations in chronic lung infections. J Bacteriol 190:2767–2776. doi:10.1128/JB.01581-07
Yoon SS et al (2002) Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev Cell 3:593–603. doi:10.1016/S1534-5807(02)00295-2
Acknowledgements
We acknowledge with appreciation the excellent work of many researchers in this field that for reasons of space could not be cited here. Our research is supported by grants from the Australian National Health and Medical Research Council and the Australian Cystic Fibrosis Trust. AK is a recipient of a New Zealand Bright Futures PhD scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lamont, I.L., Konings, A.F. & Reid, D.W. Iron acquisition by Pseudomonas aeruginosa in the lungs of patients with cystic fibrosis. Biometals 22, 53–60 (2009). https://doi.org/10.1007/s10534-008-9197-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-008-9197-9