Abstract
Biological nitrogen fixation (BNF), a key reaction of the nitrogen cycle, is catalyzed by the enzyme nitrogenase. The best studied isoform of this metalloenzyme requires molybdenum (Mo) at its active center to reduce atmospheric dinitrogen (N2) into bioavailable ammonium. The Mo-dependent nitrogenase is found in all diazotrophs and is the only nitrogenase reported in diazotrophs that form N2-fixing symbioses with higher plants. In addition to the canonical Mo nitrogenase, two alternative nitrogenases, which use either vanadium (V) or iron (Fe) instead of Mo are known to fix nitrogen. They have been identified in ecologically important groups including free-living bacteria in soils and freshwaters and as symbionts of certain cryptogamic covers. Despite the discovery of these alternative isoforms more than 40 years ago, BNF is still believed to primarily rely on Mo. Here, we review existing studies on alternative nitrogenases in terrestrial settings, spanning inland forests to coastal ecosystems. These studies show frequent Mo limitation of BNF, ubiquitous distribution of alternative nitrogenase genes and significant contributions of alternative nitrogenases to N2 fixation in ecosystems ranging from the tropics to the subarctic. The effect of temperature on nitrogenase isoform activity and regulation is also discussed. We present recently developed methods for measuring alternative nitrogenase activity in the field and discuss the associated analytical challenges. Finally, we discuss how the enzymatic diversity of nitrogenase forces a re-examination of existing knowledge gaps and our understanding of BNF in nature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is a main limiting nutrient for primary production in unmanaged terrestrial ecosystems (Wang et al. 2010). In addition, reduced N bioavailability to primary producers strongly shapes the response of ecosystems to climate change (Luo et al. 2004; Reich et al. 2006; Sigurdsson et al. 2013). Biological nitrogen fixation (BNF), which converts the abundant but inert dinitrogen (N2) molecule into ammonia, is the primary route for entry of new reactive, biologically available N (or ‘fixed’ N) in the absence of human activities (Galloway et al. 2004; Vitousek et al. 2013). The energetically demanding and oxygen (O2) sensitive reaction is catalysed by the metalloenzyme nitrogenase which is found in prokaryotes living in symbiotic or mutualistic associations (e.g., with higher plants and lichens), in association with bryophytes or living freely (e.g., in soils) (Gaby and Buckley 2011). The nitrogenase enzyme occurs in three different isoforms; the molybdenum (Mo)- nitrogenase found in all diazotrophs and the only one known in diazotrophs forming symbioses with higher plants, and two alternative nitrogenases, the vanadium (V) and iron-only (Fe) nitrogenases, found in free-living bacteria and cyanobacteria. While all nitrogenases require large amount of Fe, the three isoforms differ chiefly by the metal (Mo, V or Fe) at a critical active site position (see section “The nitrogenase isoforms”). BNF is usually considered to rely primarily on the Mo isoform of the enzyme (Mo-Nase). Until recently, the importance and contribution of the other two nitrogenase isoforms, the V and Fe-only nitrogenases (V-Nase and Fe-Nase), to N2 fixation in natural ecosystems (Bishop et al. 1980; Chisnell et al. 1988; Robson et al. 1986) had been little studied. Yet over the last decade, several findings suggest that alternative N fixation (i.e., nitrogen fixation by the V- and Fe-Nases) is an important ecological feature of BNF in terrestrial ecosystems and can contribute up to 70% of total BNF in some diazotrophs (e.g. cyanolichens) (Darnajoux et al. 2017, 2019; McRose et al. 2017b; Zhang et al. 2016).
This article provides a comprehensive overview of recent developments regarding the environmental significance of alternative N fixation and how it impacts our understanding of terrestrial BNF. First, we briefly present the Mo, V and Fe-only isoforms of the nitrogenase enzyme. Then we discuss and compare methods currently available for detecting the presence and activity of the alternative V and Fe-only nitrogenases in the field, including some of the limitations inherent to each method. We also provide a review of existing studies, most of which have been published in the last 5 years, on the characterization and quantification of alternative nitrogenase activity in tropical, temperate and boreal ecosystems, including forests and coastal environments. Finally, we discuss how alternative nitrogenases affect our understanding of BNF and highlight the necessity of filling existing knowledge gaps on their role, importance, and activity in nature.
The nitrogenase isoforms
Three isoforms of the prokaryotic enzyme nitrogenase have been identified in Archaea and Bacteria, and across many bacterial phyla, including Cyanobacteria, Proteobacteria, Firmicutes (Bishop et al. 1982; Boison et al. 2006; Chien et al. 2000; Chisnell et al. 1988; Eady 1996; Fallik et al. 1991; Hales 1990; Loveless and Bishop 1999; McRose et al. 2017b; Thiel 1993). These three nitrogenase isoforms, encoded by separate genes (Boyd et al. 2011; Chien et al. 2000; Dixon and Kahn 2004; Eady 1996; Hamilton et al. 2011; Raina et al. 1988; Schuddekopf et al. 1993; Thiel 1993; Werner Klipp 2004; Wolfinger and Bishop 1991; Zinoni et al. 1993, see section “Nitrogenase genes” for details), are structurally and phylogenetically related and are mainly distinguished by their metal cofactor; the molybdenum nitrogenase (Mo-Nase) with its FeMo cofactor, the vanadium nitrogenase (V-Nase) with its FeV cofactor and the iron nitrogenase (Fe-Nase) with its Fe-only cofactor (Bishop and Premakumar 1992; Joerger and Bishop 1988) (Fig. 1a).
Overview of the nitrogenase isoforms. a Stoichiometry of N fixation by the three Nase isoforms in vitro (Eady 1996; Wall 2004). b Structural differences in Mo-Fe, V-Fe, and Fe–Fe proteins. Electrons travel from the [4Fe-4S] cluster in the Fe protein (not shown) to the P-cluster, located between the α- and β-subunits (green and blue, respectively), to the FeMo/FeV/FeFe-cofactor in the α subunit, where N2 reduction occurs. The role of γ-subunit (purple) in alternative nitrogenases is not well understood. *The structure for the FeFe protein is a model based on the FeV protein adapted from (Mus et al. 2018). c Summary of maximum likelihood phylogenetic relationships between concatenated HDK protein sequences for nif/vnf/anf and uncharacterized forms of nitrogenase calculated by Boyd et al. (2011) and adapted from Mus (2018), Boyd et al. (2011), Mus et al. (2018). Filled circles at nodes indicate bootstrap values > 80. Adapted from (Mus et al. 2018). d Distribution of alternative nitrogenases in sequenced genomes of organisms across diverse phyla and locations of isolation. Based on (McRose et al. 2017b)
In the process of reducing N2 to ammonium (NH4+), the nitrogenase enzyme also reacts with H+ to produce H2. H2 production, which consumes both electrons and ATP, seems to be tightly coupled to N2 reduction at the enzyme active site (Harris et al. 2019). One H2 is produced for every reduced N2 by the Mo-nitrogenase, but the H2 to N2 ratio appears to be much higher for the V- and the Fe-only nitrogenase, at least in vitro (Fig. 1a). The nitrogenase enzyme also reacts with a suite of doubly and triply bonded small molecules such acetylene, cyanide, nitrous oxide, and others (Burgess and Lowe 1996). Reaction kinetics and substrate specificity are usually isozyme-dependent and have been extensively used to gain insight into enzyme mechanisms. For example, unlike Mo-nitrogenase, the V-nitrogenase can reduce carbon monoxide into small hydrocarbons (Lee et al. 2010) and both Fe- and V-nitrogenase have been found to reduce carbon dioxide into methane (Zheng et al. 2018). The stable isotopic composition of certain nitrogenase products can also distinguish different isoforms (Zhang et al. 2014, 2016; Luxem et al. 2020a).
The evolution of the three nitrogenase isozymes over geological times, and which isozyme may have evolved first, have been extensively discussed (Anbar and Knoll 2002; Boyd et al. 2011; Peters and Boyd 2015). The canonical Mo-Nase is found in all N2-fixing bacteria and archaea (diazotrophs) and is the only nitrogenase reported in diazotrophs that form N2-fixing symbioses with higher plants (de Bruijn 2015; Dilworth and Loneragan 1991; Fallik et al. 1991). The so-called “alternative” nitrogenases (i.e., V-Nase and Fe-only Nase) are found in a limited, but diverse, number of diazotroph species, including ecologically important groups such as free-living soil bacteria and archaea (Bishop et al. 1980, 1982; Bishop and Premakumar 1992; Chien et al. 2000; Chisnell et al. 1988; Fallik et al. 1991; Hales 1990; Joerger and Bishop 1988; Joerger et al. 1989; McRose et al. 2017b; Pau et al. 1993; Robson et al. 1986), cyanobacteria (Boison et al. 2006; Kentemich et al. 1988; Masukawa et al. 2009; Ni et al. 1990; Thiel 1993; Thiel and Pratte 2013), and cyanolichens (Darnajoux et al. 2017; Gagunashvili and Andresson 2018; Hodkinson et al. 2014; Zhang et al. 2016) (Fig. 1b).
Biological nitrogen fixation (BNF) has long been considered to rely primarily on the Mo isoform for several reasons. N2-fixing symbionts with higher plants (Rhizobium and Frankia), believed to be the major contributors to BNF, do not possess alternative nitrogenase genes. In addition, at room temperature, the Mo-Nase isoform fixes N2 more efficiently than the alternative nitrogenases: the Mo-Nase requires “only” 16 mol of ATP per mole of N2, vs. 24 and 40 mol of ATP per mole of N2 for the V- and Fe-only Nases, respectively, as assessed with in vitro assays (Fig. 1a). In the laboratory, diazotrophs that possess more than one nitrogenase isoform preferentially express the Mo-nitrogenase when conditions, such as adequate environmental Mo, allow the synthesis of a functional enzyme (Boyd et al. 2011; Jacobitz and Bishop 1992; Jacobson et al. 1986; Maynard et al. 1994; Oda et al. 2005; Peters and Boyd 2015). Consequently, alternative nitrogenases appear to be "back up" enzymes for maintaining the N anabolism of diazotrophs (see section “Where have alternative nitrogenases been found so far?”).
Nonetheless, there is mounting evidence that alternative nitrogenases are significant contributors to BNF on a global scale. First, several recent reports highlight the importance of non-symbiotic N2 fixation, epiphytic N2 fixation associated with bryophytes and symbiotic N2 fixation with fungi for global N input, especially in high latitude ecosystems, to which alternative nitrogenases could contribute (Chapin et al. 1990; DeLuca et al. 2002; Elbert et al. 2012; Michelsen et al. 2012; Rousk et al. 2013). The widespread occurrence of diazotrophs harboring alternative nitrogenase genes across many different environments (Betancourt et al. 2008; McRose et al. 2017b) suggests that these genes provide a significant competitive advantage to their hosts and possibly play important ecological and biogeochemical roles. These findings force a reassessment of the relative contribution of symbiotic N fixation in higher plants to terrestrial BNF and concomitantly, of the predominance of the Mo-isoform in the same process (Reed et al. 2011).
Experimental methods for the detection of alternative nitrogenases in nature
Nitrogenase genes
The synthesis of a functional nitrogenase enzyme is a complex process involving as many as 21 genes for regulation, electron transfer, chaperone and stability functions, protein maturation, synthesis of metal cofactors, and of course protein synthesis (Rubio and Ludden 2005). The inventory of genes required for N fixation varies amongst different diazotrophs, although a minimal core of 6 structural and cofactor biosynthetic genes is conserved (Dos Santos et al. 2012). The nitrogenase enzyme is made of two proteins: the MoFe protein, a heterodimer containing the active site of the enzyme, and the Fe protein, a homodimer sometimes referred to as the nitrogenase reductase, which transfers electrons to the active site for reduction of N2 to NH3 (Peters et al. 1995). The MoFe protein is encoded by the nifDK gene while the Fe protein is encoded by the nifH gene in the Mo-nitrogenase (Seefeldt et al. 2009). Alternative nitrogenases are encoded by similar sets of genes, referred to as vnfDK and vnfH (for the V-nitrogenase) and anfDK and anfH (for the Fe-only nitrogenase). Alternative nitrogenases require an additional gene, vnfG (or anfG), which is necessary for processing aponitrogenase to functional nitrogenase (Chatterjee et al. 1997). Unlike nifH, vnfH and anfH genes, which are the most widely used molecular markers to identify diazotrophs from natural environments (Zehr et al. 2003), nifD, vnfD and anfD sequences cluster according to the nitrogenase isozyme.
A few studies have taken advantage of this robust clustering to study the presence of alternative nitrogenase genes in various natural environments, including soils, coastal sediments, rice fields, and lichens (Betancourt et al. 2008; Boison et al. 2006; Hodkinson et al. 2014; McRose et al. 2017b). Next generation molecular biology tools, such as meta-transcriptomics, have been used to investigate the expression of alternative nitrogenases in pure culture, soil microcosms and sediment mesocosms (Bellenger et al. 2014; Bowen et al. 2014; Hamilton et al. 2011). To the best of our knowledge, it exists no report of alternative nitrogenase transcripts from environmental samples. However, while these approaches provide valuable genomic and transcriptomic information, reliable estimates of the relative contributions of alternative nitrogenases to BNF rates require direct measurement of nitrogenase activity.
Quantification of alternative nitrogenase rates in environmental samples
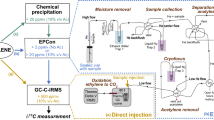
There are currently four primary methods available for the quantification of alternative BNF. Three of them (R ratio, ethane production, ISARA) are based on the reduction of acetylene by nitrogenase, a well-known reaction that has been used to assay nitrogenase activity since 1968 (Hardy et al. 1968). The fourth one is based on nitrogen stable isotope fractionation during N fixation by nitrogenase (15N fractionation). A fifth method, which has yet to be tested in the field and is thus not described in detail here, is based on the large hydrogen isotope fractionation during CO2 reduction to methane by alternative nitrogenases (Luxem et al. 2020a).
R ratio
In addition to reducing N2 into ammonium, the nitrogenase enzyme can reduce acetylene to ethylene (Hardy et al. 1968) and the Acetylene Reduction Assay (ARA), which measures the rate of acetylene reduction (AR) as a proxy for nitrogenase activity, has been commonly used in the laboratory and in the field for the last 50 years. ARA is non-destructive, sensitive and requires only low cost equipment (a gas chromatograph equipped with a flame ionization detector). The conversion of acetylene reduction rates into N2 fixation rates requires correction by a conversion ratio R, defined as R = (acetylene reduction rate)/(N2 fixation rate). The R ratio has a theoretical value (R = 3–4) based on the respective number of electrons needed to reduce acetylene and N2 by the Mo-Nase (Hardy et al. 1973). This value was also validated in pure cultures (Eady and Robson 1984). Due to the extra production of H2 by alternative nitrogenases, these isoforms have distinctive R ratios: ~ 0.5 and 1 for the Fe- and V-Nase respectively (Bellenger et al. 2014). Thus the R ratio can potentially be used to detect the presence of active alternative nitrogenases. The R ratio can be measured experimentally by performing incubations with either acetylene or a 15N2 atmosphere, simultaneously on duplicate samples or consecutively on the same sample, and measuring both the acetylene reduction rate and the rate of 15N incorporation into the sample (Hardy et al. 1973). Precision on R ratio measurements is thus negatively affected by both the error and bias from the ARA and 15N incubation methods, which could be particularly important for low-activity samples. In addition, because of the inhibition of acetylene on 15N2 reduction, both methods cannot be undertaken on the same samples at the same time, which also increases the uncertainty of the R ratio determination. Measurements of R ratios also suffer from potential artifacts such as the presence of easily absorbed forms of 15N in commercial 15N2 stocks (Dabundo et al. 2014), or/and the slow dissolution kinetics of 15N2 gas (Mohr et al. 2010). Several factors can further complicate the interpretation of R ratio measurements in environmental samples. In soils, heterogeneity in the natural abundance of 15N in a given sample can result in significant errors on the rates of 15N incorporation. In addition, processes such as BNF-independent ethylene cycling by microbes and plants in soils can affect the value of the R ratio, complicating interpretation of such data (Bleecker and Kende 2000; Nagahama et al. 1994). Finally, the R ratio has been shown to be variable in marine sediments and in cyanobacteria, a highly relevant group of diazotrophs worldwide, with values ranging from 1.5 to as high as 100 (for review, see Bellenger et al. 2014; Mohr et al. 2010; Seitzinger and Garber 1987). Some of these low values may reflect the ability of some cyanobacteria to release a substantial fraction of their fixed nitrogen in the extracellular media (Mulholland and Bernhardt 2005; Mulholland et al. 2004). Excretion of newly fixed nitrogen results in artificially low rates of N2 fixation, since these rates are measured on the basis of 15N incorporation into biomass. Thus, while measurements of the R ratio can sometimes be useful (Bellenger et al. 2014), additional methods are required for the reliable detection of alternative nitrogen fixation activity.
Ethane production during ARA
The alternative V-Nase and Fe-Nase produce a significant amount of ethane (C2H6) in addition to ethylene (C2H4) during acetylene reduction (typically 3–8% of ethane relative to ethylene (Dilworth et al. 1987; Dilworth et al. 1988)). In contrast, the Mo-Nase produces very small amount of ethane that have only been measured at high temperatures (above 40 °C) (Dilworth et al. 1993). Accordingly, the production of ethane has been used as a reliable proxy for alternative nitrogenase activity in many laboratory studies (Attridge and Rowell 1997; Chakraborty and Samaddar 1995; Davis et al. 1996; Fallik et al. 1991; Schneider et al. 1991). This method is difficult to use in the field, however, as acetylene reduction rates, which reflect a mixture of Mo-Nase and alternative nitrogenase activity, are typically an order of magnitude lower than in pure cultures, resulting in ethane yields that are near or below the detection limit of standard gas chromatographs. For this reason, the detection of ethane in environmental samples may require the pre-concentration of the sample headspace before measurement by gas chromatography. The cycling of ethane by microbes in processes unrelated to BNF (Chen et al. 2019; Fukuda et al. 1984; Singh et al. 2017), the temperature dependency of the ethane to ethylene electron flux ratio in V-Nase (Dilworth et al. 1988), and laboratory studies of nitrogenase mechanism which show that mutant Mo-Nases can also produce ethane (Scott et al. 1992) are additional limitations on interpreting ethane signals as indicators of alternative BNF. Nonetheless, our studies have shown that measurements of ethane production as a proxy for alternative nitrogenase activity can be highly successful in systems with relatively low biological complexity and high BNF activity (e.g. ethane production in the ppm range) such as cyanolichens (Darnajoux et al. 2019).
Isotopic acetylene reduction assay (ISARA)
This new method uses the 13C isotope fractionation of acetylene reduction to ethylene to estimate the contribution of alternative nitrogenases to activity within the acetylene reduction assay. The three nitrogenase isoforms are characterized by distinct 13C fractionations (13εAR = δ13Cacetylene − δ13Cethylene) during acetylene reduction to ethylene: ~ 13–15% for Mo-Nase, ~ 7–9% for V-Nase, and ~ 6–7% for Fe-Nase (Zhang et al. 2016). In this assay, the δ13C of the source acetylene and the produced ethylene are measured using a gas chromatograph-combustion-isotope ratio mass spectrometer. The total rate of acetylene reduction is also measured. The contribution of alternative Nases versus Mo-Nase to acetylene reduction can then be estimated by comparing the 13C fractionation of the sample versus isoform-specific values: faltARA = (13εAR MoNase − 13εAR sample)/(13εAR MoNase − 13εAR altNase). The use of Fe-Nase 13εAR (= 5.8–6.5‰, Zhang et al. 2016) for the value of 13εAR altNase in the above calculation produces the most conservative estimates of the fractional contribution of alternative nitrogenase activity to acetylene reduction. Independent evidence for V-Nase or Fe-Nases (e.g., DNA or RNA sequences, ethane/ethylene ratios from ARA and/or metal content) can be used to choose the appropriate value of 13εARaltNase for calculations. Finally, total and isoform specific N2 reduction rates can be estimated by correcting acetylene reduction rates with the relevant R ratios for different isoforms as determined by ISARA (i.e., total N2 fixation rate = AR rate × (1 − faltARA)/RMoNase + AR rate × faltARA/RaltNase). Although this method is still in its infancy and requires further testing in the laboratory and in the field to establish its full potential, it is to date the most reliable way to evaluate the contribution of alternative nitrogenases to environmental N2 fixation rates.
15N fractionation
As shown in Zhang et al. (2014), the three nitrogenase isozymes when reducing N2 also produce distinct organism-scale 15N isotope fractionations: 15εfix = ~ 2‰ for Mo-Nase, ~ 6 ‰ for V-Nase and ε 8‰ for Fe-Nase, where 15εfix = δ15Ndissolved N2—δ 15N Nfixer biomass and δ 15N = [(15N/14Nsample/15N/14Nair) − 1] × 1000 in per mil (‰) units. Thus, the 15N/14N isotope ratios of biomass, typically expressed as δ15N values, can in theory be used to evaluate the contribution of alternative nitrogenases to N input in environmental samples with N2-fixing activity. This approach has been successfully used to demonstrate the role of alternative nitrogenases in past ocean anoxia (Zhang et al. 2014). The presence of multiple N sources with varying and often unknown δ15N along with the existence of complex and variable N cycle processes (e.g., ammonium uptake, nitrification, denitrification) that impart their own δ 15N signal to the bulk biomass, makes the interpretation of field samples from modern environments inherently challenging. To properly constrain alternative BNF based on biomass δ15N, we recommend focusing on samples with biomass containing primarily newly fixed N from BNF, along with the use of other independent measurements of alternative nitrogenase activity (see above).
In summary, several methods currently exist to assess the contribution of alternative nitrogenases to BNF with their specific strengths and weaknesses (Table 1). Cross-calibration of the four methods on the same samples (i.e. cyanolichens) shows the most robust correlations between ISARA and Ethane production data (Fig. 2a). 15N fractionation data measured for samples primarily comprised of new N from BNF also show good correlation with ethane production (Fig. 2b). Not surprisingly perhaps, the R ratio method appears to be the least reliable (Fig. 2c). The high detection limits of these methods are currently one of the major hurdles for the assessment of alternative nitrogenase activity in natural samples. The 15N fractionation approach requires BNF to be the primary driver of sample 15N/14N, limiting the application of this method to samples primarily comprised of newly fixed N. Measurements of ethane production for the detection of alternative nitrogenases can only be achieved on samples with high acetylene reduction rates because of two compounding factors: the production of ethane is only a few % of the production of ethylene and the limit of detection of most GC methods used to measure C2H6 production is in the high ppbv range. ISARA analyses require a minimum of ~ 300 ppmv ethylene for accurate detection of nitrogenase isoform activity. In both cases (ethane and ISARA), sensitivity can be improved with a pre-concentration step, which requires extra handling and increases costs.
Comparison of current methods for the assessment of alternative nitrogenase activity. Correlation between the 13ε(ethylene) as measured by ISARA and: a ethane production, b15N fractionation, and c the R ratio. Each data point corresponds to the same sample analyzed with two different methods. All data were collected in Peltigera cyanolichens
We note that these high detection limits, which often require several-hour incubations to accumulate measurable amount of analyte in the incubation chambers, currently preclude kinetic studies on short time scales (i.e., a few minutes) to understand how diazotrophs modulate the expression and use of nitrogenase isoforms in response to environmental conditions (e.g., metal availability, temperature, energy resources).
Each method requires different levels of expertise and equipment. The acetylene reduction method (ethylene and ethane production) is the most user friendly (Table 1). It only requires simple and affordable GC equipment, available in many research facilities with limited training. Methods based on δ15N measurements (R ratio and 15N fractionation) require more specialized equipment and expertise. ISARA is a more difficult method to implement as most fee-for-service stable isotope facilities have yet to offer the option of 13C measurements of acetylene and ethylene (Table 1). With regard to analytical cost, methods requiring the analysis of stable isotope fractionation (13C for ISARA and 15N for 15N fractionation and R-ratio) are more expensive than methods solely relying on GC analysis (C2H6 production), even though numerous facilities now provide cost effective stable isotopes analyses (Table 1).
In the absence of a one-fits-all method, the use of complementary approaches to estimate the contribution of alternative nitrogenase to BNF is recommended. In the future, new methods, such as ISARA and 15N fractionation, need to be implemented in a systematic manner to: (i) better evaluate the contribution of alternative nitrogenases in a wide range of ecosystems and biomes, and (ii) refine data collected using more classical approaches (i.e., ethane production and R ratio).
Where have alternative nitrogenases been found so far?
Because some of the methods for the detection of alternative nitrogenases are still relatively new, and the data on alternative nitrogenase activity are still sparse, we have included in this section habitats that have been shown to have low Mo content, or where Mo limitation of N fixation has been demonstrated, as places likely to harbor alternative nitrogenases activity.
Terrestrial environments—soils
Soils are a particularly favorable environments to look for alternative nitrogenases because Mo is one of the least abundant biometals in the earth crust, soils and plants (Kabata-Pendias 2010; Wedepohl 1995). Accordingly, Mo limitation of BNF has been reported in both agrosystems (Gupta 1997; Hafner et al. 1992; Srivastava et al. 1998; Vieira et al. 1998a, b) and in unmanaged ecosystems (see below and Fig. 3). In soils, the first evidence of Mo limitation was provided by Silvester and coworkers in tree litter, decaying wood and cyanolichen (Lobaria sp.) within Pacific northwestern forests (Silvester 1989; Horstmann et al. 1982). Other studies on the effects of trace metal and phosphorus (P) additions in Hawaiian soils indicated that Mo additions could promote higher BNF rates (Crews et al. 2000; Vitousek 1999; Vitousek and Hobbie 2000). Two other studies in the tropical forests of Panama reported Mo limitation of asymbiotic N2 fixation in leaf litter (Barron et al. 2009; Wurzburger et al. 2012). A study in the Belize rainforest showed Mo limitation of BNF in volcanic soils, but not in limestone soils (Winbourne et al 2017). Mo limitation of BNF in leaf litter has also been reported in cold, temperate latitudes (Jean et al. 2013). Other studies reported no effect of Mo additions on BNF in soils and litter (Dynarsky and Houlton 2018), showing that Mo limitation is common but not ubiquitous. More details on the results of nutrient addition (N, P and Mo) on free-living nitrogen fixation in leaf litter and soils can be found in a recent meta-analysis by Dynarski and Houlton (2018). Mo limitation of BNF in soils has thus been observed over a wide range of latitudes, ranging from tropical to cold temperate environments.
Global map of alternative BNF distribution. The map indicates locations where Mo limitation of BNF (red dots), alternative nitrogenase genes (green dots), and activity of alternative BNF (blue dots) have been reported in environmental samples (Barron et al. 2009; Betancourt et al. 2008; Gagunashvili and Andresson 2018; Hodkinson et al. 2014; Jean et al. 2013; Ma et al. 2019; McRose et al. 2017b; Reed et al. 2013; Rousk et al. 2017; Silvester 1989; Winbourne et al. 2017; Wurzburger et al. 2012; Zhang et al. 2016)
In sharp contrast to Mo, Fe is the most abundant biometal in the earth crust and total V concentrations are on average 2 to 200 times higher than Mo concentrations in soils and leaf litter (Kabata-Pendias 2010), suggesting that V and/or Fe could be used to relieve Mo limitation in soils. The first studies on alternative nitrogenase genes in the environment showed that they were present in a variety of terrestrial environments such as soils, wood chips, coastal sediments, and rice fields (Betancourt et al. 2008; Boison et al. 2006; Loveless et al. 1999) (Fig. 3). More recently, we demonstrated the contribution of alternative nitrogenases to BNF in the rhizosphere and soil habitats, using R ratio values reported in the literature for tropical, temperate and boreal forests (Bellenger et al. 2014). In spite of a few exceptions (Sellstedt et al. 1986; Batterman et al 2018), this analysis showed that R ratios reported in the literature for plant root nodules, known to own only the Mo-Nase, were usually within the theoretical 3–4 range with little variability. R ratios reported for free-living bacteria in soils were highly variable and ranged from less than 1 to more than 6 (Graham et al. 1980; Liengen 1999; Martensson and Ljunggren 1984; Minchin et al. 1983; Moisander et al. 1996; Nohrstedt 1983; Schwintzer and Tjepkema 1994; Wilson et al. 2012). This variability in soil R ratios has often been attributed to the difficulty of measuring 15 N incorporation in environmental samples with low BNF rates. Nonetheless, a careful analysis of the soil data revealed two distinct peaks in R ratio distribution: one centered on R ratios of ~ 3–4, values consistent with the Mo-isoform, and a second peak centered on R ratios of ~ 1–2, values characteristic of alternative nitrogenases (Bellenger et al. 2014). Variability in R ratios reported in soils thus reflects, at least in part, the contribution of different nitrogenase isoforms to BNF. In the same study (Bellenger et al. 2014), the variability of the R ratio was used successfully to evaluate the contribution of alternative nitrogenases to BNF in temperate forest soil microcosms. Nonetheless, as detailed in section “Quantification of alternative nitrogenase rates in environmental samples”, the R ratio has proven to be the least reliable method to characterize alternative BNF.
Cyanolichens and mosses in boreal and subarctic regions.
Mo limitation of BNF has been observed in cryptogamic species, such as mosses and cyanolichens (Fig. 2), which are major contributors to N cycling in high latitude ecosystems. The first evidence of Mo limitation in cryptogams was published by Silvester and coworkers in Lobaria spp. cyanolichens in Oregon, US (Horstmann et al. 1982). However, a more recent study on Lobaria and Usnea cyanolichens from the same region (Oregon) reported no effect of Mo addition on BNF (Marks et al. 2015b). High spatiotemporal variability in Mo limitation, which has been reported in leaf litter and cryptogams (Jean et al. 2013; Rousk et al. 2017; Wurzburger et al. 2012), may explain some of the discrepancies between these two studies. More recently, Mo limitation of BNF has been demonstrated in cryptogams (bryophytes and cyanolichens) from high latitude and altitude regions (Eastern Canada, Northern Europe and Chile) (Darnajoux et al. 2014, 2017; Perez et al. 2017). Interestingly, studies on the cyanolichen Peltigera aphthosa showed that V levels are 2–5 times higher than Mo in cyanolichens with high rates of N2 fixation, indicating that V may be used to relieve Mo limitation.
The presence of the V-Nase gene was recently reported in the Peltigera genera, which is ubiquitous in boreal and subarctic regions (Hodkinson et al. 2014). Two additional studies with boreal Peltigera cyanolichens collected in Sweden and Eastern Canada demonstrated the presence and activity of alternative nitrogenases using ISARA measurements and natural abundance 15N isotope signatures (Darnajoux et al. 2017; Zhang et al. 2016). The contribution of alternative nitrogenases to BNF in these cyanolichens ranged from 20 to 60% in laboratory incubations at 10 °C. The contribution of alternative BNF to N2 fixation in Peltigera cyanolichens was ultimately confirmed in a more recent, ecosystem scale study across a 600 km latitudinal transect in Eastern Canada (Darnajoux et al. 2019). In this study, where the authors conducted a wide-ranging assessment across 10 species of Peltigera cyanolichens, V-Nase BNF was found to contribute between 0% of BNF at Mo-rich southern sites and ~ 50% of BNF at Mo-limited northern sites (Darnajoux et al. 2019). This study identified Mo availability as the dominant control for spatial and temporal variations in V-Nase activity across all sites and over the growing season. Mo limitation was more prominent at higher latitudes along the transect. This was most likely due to lower atmospheric deposition of both N and Mo (Darnajoux et al. 2015), resulting in a higher need for BNF to sustain biological N demand concomitant with Mo scarcity. Because Mo and V are contaminants in fossil fuels (Vouk and Piver 1983), atmospheric deposition might also play an important, but underexplored, role in controlling environmental metal distributions and alleviating Mo limitation in some areas.
Mo limitation of BNF by cyanobacteria associated with feather mosses in boreal forests in Canada and subarctic tundra in Sweden has been recently reported (Rousk et al. 2017). Other studies reported no effect of Mo addition on moss BNF (Scott et al. 2018; Smith 1984). The extent and spatiotemporal variability of Mo limitation remains difficult to predict for moss BNF due to the scarcity of data and the lack of direct evidence of the presence and activity of alternative nitrogenases in feather mosses. Nonetheless, the report of Mo limitation of moss BNF is of particular significance considering the critical role of cryptogamic species like mosses for BNF in boreal and subarctic ecosystems (DeLuca et al. 2002) and calls for further research.
Coastal environments
In contrast to terrestrial ecosystems, Mo is one of the most abundant micronutrient in marine waters, with concentrations around 100 nM (Collier 1985a), while V is found at concentrations ranging from 35 to 45 nM (Collier 1985b) and Fe is hardly detectable in dissolved inorganic form (Rijkenberg et al. 2014). Unsurprisingly, alternative nitrogenases have yet to be found in the open oceans (McRose et al. 2017b). However, under sulfidic conditions (> 11 μM H2S(aq)), such as those sometimes found in marine and coastal sediments, Mo can react with sulfides to form thiomolybdates (MoOxS(4−x)2−, with 1 < x < 4) (Erickson and Helz 2000; Helz et al. 1996). Thiomolybdates readily react with particles such as Fe phases, Mn-oxides, and organic matter (Wagner et al. 2017), potentially reducing Mo availability to N2-fixers. This chemistry may account for recent findings of alternative nitrogenase genes (McRose et al. 2017b) and alternative nitrogenase enzyme activity (Zhang et al. 2016) in salt marsh microbial mats and sediments (Cape Cod, MA) (Fig. 3). Estimated using ISARA, the contribution of alternative nitrogenases to BNF in these sulfide-rich samples ranged from 20 to 55% (Zhang et al. 2016). In a similar study examining BNF in a brackish mangrove environment within the Florida Everglades, alternative nitrogenases were found to contribute over 24% of total BNF in leaf litter samples (McRose et al. 2017b). These findings are consistent with previous reports on alternative nitrogenase genes in N2-fixing isolates from mangrove sediments in Puerto Rico and North Carolina creek sediment (Betancourt et al. 2008). The presence of alternative nitrogenase genes was also recently reported in anoxic coastal waters off the coast of Peru (Christiansen and Loscher 2019), again suggesting that alternative nitrogen fixation in marine and coastal ecosystems may be more prevalent than usually recognized.
What factors favor the expression and activity of alternative nitrogenases over the canonical Mo-nitrogenase?
Low Mo availability
Molybdenum is known to prevent the synthesis of functional alternative nitrogenases in the model N2 fixing bacteria, Azotobacter vinelandii and Rhodobacter capsulatus (Jacobitz and Bishop 1992; Jacobson et al. 1986; Kutsche et al. 1996; Masepohl et al. 2002) and in the freshwater heterocystous cyanobacterium Anabaena variabilis (Pratte et al. 2013; Thiel and Pratte 2013, 2014). In terrestrial environments, low Mo availability likely favors alternative nitrogenases use by diazotrophs. But alternative nitrogenases may also play a role when Mo appears to be sufficient. Abundant Mo does not automatically preclude alternative nitrogenase expression as shown in A. vinelandii grown at low temperatures (Walmsley and Kennedy 1991), in solid medium (Maynard et al. 1994) and in a strain of the N2-fixing, alpha-proteobacterium Rhodopseudomonas palustris (Oda et al. 2005) (also see section “How robust are current BNF estimates?”). In addition, the use of the V- or Fe-only nitrogenases to relieve Mo limitation must be understood in the context of the strategies and costs of acquiring Mo, V, and Fe in diazotrophs.
The acquisition of Mo, V and Fe in the natural environment is the result of complex and potentially expensive processes. Alternative nitrogenases are a way to relieve Mo limitation of BNF without requiring Mo uptake, thereby providing a competitive advantage to their hosts. In the top soil layer, Mo and V are found mainly as the oxoanions molybdate and vanadate, complexed by organic matter (phenolic and tannin-like compounds) and/or adsorbed on oxide surfaces (Goldberg et al. 1996; Marks et al. 2015a; Reddy and Gloss 1993; Wichard et al. 2009b). As expected, the chemical speciation of Mo in the soil is influenced by vegetative cover and organic matter decomposition (Wichard et al. 2009b). While organic matter binding prevents the leaching of metals away from the soil, it also reduces their bioavailability (Reddy and Gloss 1993; Wichard et al. 2009b). At the same time, Fe availability in oxic environments is low due to the insolubility of Fe oxides. Many microorganisms have evolved efficient strategies to recruit metals from their surroundings by producing small organic ligands, known as metallophores. Metallophores bind metals in the extracellular environment, allowing a better control of metal speciation and thus of metal uptake by the metallophore-producing microorganisms (Kim et al. 2005; Kraepiel et al. 2009; Neilands 1995). Specific metallophore-based uptake systems have been characterized for Mo and V in diazotrophs (Bellenger et al. 2008b; Kraepiel et al. 2009; Liermann et al. 2005; Wichard et al. 2008, 2009a). Metallophore-mediated acquisition of metal is expensive, as shown in a recent study of the free-living soil N2-fixer A. vinelandii which invests up to 35% of its newly fixed N into metallophore production to support metal acquisition (McRose et al. 2017a). In most laboratory experiments in which Mo and V are provided in readily available forms (molybdate and vanadate salts), V is acquired and the V-Nase is activated only after total depletion of Mo in the medium (Bellenger et al. 2011). Jouogo Noumsi et al. showed that the presence of tannic acids (used here as analogs of natural organic matter) significantly affected A. vinelandii metal acquisition and nitrogenase use strategy; metallophore production significantly increased to compete with tannic acids for Mo and V. Mo and V were taken up simultaneously and the V-Nase was activated at lower cell densities than in control cultures without tannic acid (Jouogo Noumsi et al. 2016). This suggests that in the presence of tannic acid, it was more beneficial for the cells to reduce metallophore production and use the V isoform of the nitrogenase than to increase metallophore production to fulfill their nitrogen requirements exclusively with the Mo-nitrogenase. Alternative nitrogenases may thus provide diazotrophs with additional flexibility in the management of their intracellular metal quotas in the natural environment.
Low temperatures
Low temperatures are known to alter nitrogenase isoform expression and activity. In at least one model organism (Azotobacter vinelandii), the alternative nitrogenase genes are not repressed by Mo at temperatures below 14–20 °C (Walmsley and Kennedy 1991). In addition, while the efficiencies of all nitrogenases decrease with decreasing temperatures, the efficiency of the V-Nase declines less than its Mo counterpart. This has been shown by in vitro studies on purified enzymes almost three decades ago (Miller and Eady 1988) and it has been confirmed more recently in vivo using the model organism Anabaena variabilis (Darnajoux et al. unpublished). At temperatures below 10–12 °C, the Mo and the V isoforms of the nitrogenase showed comparable acetylene reduction activity and A. variabilis cultures grown in Mo-only and V-only media achieved similar growth rates at temperature below 20 °C. Thus, there may not be a growth advantage for diazotrophs using the Mo-Nase over the V-Nase at low temperatures. This has important, and yet still understudied, consequences for BNF in natural and managed ecosystems since Mo limitation appears to be widespread (see sections “Where have alternative nitrogenases been found so far?” and “Low Mo availability”) and the Earth annual average temperature is ~ + 14 °C.
To what extent does nitrogenase diversity affect our understanding of biological nitrogen fixation?
The active contribution of alternative nitrogenase to BNF across a wide range of contrasting ecosystems raises several important questions regarding our current understanding of BNF.
Changes in acetylene reduction rates: do they reflect changes in N2 fixation rates or a switch to a different nitrogenase isozyme?
As described above, Mo limitation of BNF has been demonstrated across a wide range of ecosystems from low to high latitudes (Barron et al. 2009; Jean et al. 2013; Rousk et al. 2017; Silvester 1989; Wurzburger et al. 2012). Most, if not all, of these studies used the acetylene reduction assay, ARA, as a proxy for N2 fixation and documented Mo limitation as an increase of AR rates in response to Mo additions. It is conceivable that in these Mo-limited habitats, alternative nitrogenases are responsible for the bulk of N2 fixation prior to Mo-addition. Because the Mo-Nase is more efficient at reducing acetylene than the alternative nitrogenases (Bellenger et al. 2014), the observed increase in acetylene reduction rates may be driven, at least partially, by an increased contribution of the Mo-Nase in response to Mo additions. Diazotrophs can switch from one nitrogenase to another in a matter of few hours (Bellenger et al. 2011) and a switch from alternative to canonical (i.e. Mo-nitrogenase) N2 fixation is certainly possible in field experiments where samples are usually pre-incubated for 12–18 h before being assayed for acetylene reduction. The increase in N2 fixation rates in response to Mo addition could thus be vastly overestimated.
The increased contribution of Mo-Nase after Mo addition was recently illustrated in a study on the effect of Mo addition to feather moss BNF in the arctic tundra (Rousk et al. 2017). In this study BNF by mosses was measured at field temperatures using both ARA and 15N incorporation. Control mosses were characterized by variable R ratios (ARA/N2), suggesting the contribution of more than one nitrogenase isoform to BNF. In contrast, after 24 h incubations, the Mo-treated mosses yielded R ratio values (3–4) characteristic of the Mo-isoform.
In addition, the high seasonal and spatial variability in acetylene reduction rates often measured in field samples may partially reflect variable contributions of the different nitrogenase isoforms to BNF. In the future, particular attention to the contribution of alternative nitrogenases to N fixation in Mo-limited systems is warranted. In addition to the use of the R ratio, as illustrated by Rousk et al. (2017), a direct measurement of alternative nitrogenase activity using the ethane method or ISARA may also be required to understand ARA spatial and temporal variability. In these systems, N fixation rates must be measured using the 15N incorporation method rather than ARA due to uncertainties in the value of the R ratio. Independently of the method, careful quantification of alternative nitrogenase activity is critical for our understanding of ARA spatial and temporal variability and thus for improving N2 fixation estimates in the future.
How robust are current BNF estimates?
There is currently renewed interest in N2 fixation in locales where surface-normalized rates are low (such as forest soils, tree canopies and moss-covered tundra), following the recognition that low rates over large areas may be significant for N inputs on local and global scales (DeLuca et al. 2002; Elbert et al. 2012; Lagerstrom et al. 2007; Lindo and Whiteley 2011; Menge and Hedin 2009; Sullivan et al. 2014). Our results, along with others (Betancourt et al. 2008; Boison et al. 2006; Darnajoux et al. 2014, 2017,2019; Rousk et al. 2017; Zhang et al. 2016), show that alternative nitrogenases contribute to BNF in some of these important N2 fixing habitats, including cryptogamic covers that contribute up to half of BNF on land (Elbert et al. 2012).
This finding raises questions regarding the reliability of some existing BNF estimates. ARA is often the preferred method to assess BNF in environmental samples. The conversion of AR rates into N2 fixation rates requires using the R ratio, which is dependent on the type of nitrogenase. Unfortunately, the R ratio is not always measured in field studies, partly because accurate measurements of both ARA and 15N incorporation in samples with low BNF activity can be challenging. Even in field studies where the R ratio is measured, measurements are often carried out under laboratory conditions that can be poorly representative of the field (particularly for temperature (DeLuca et al. 2002)). Overall, studies that performed both ARA and 15N incubations on the same samples in the field (Bellenger et al. 2014; Hedin et al. 2009; Vile et al. 2014) suggest that variations in the R ratio due to nitrogenase isozyme diversity have been underestimated.
Poorly calibrated ARA (and thus potentially overestimated R ratios) in systems where alternative nitrogenases are important may result in inaccurate estimates of N2 fixation rates. For instance, in a study on cyanolichens, Darnajoux et al. reported that improper consideration of alternative nitrogenase activity in environmental samples leads to severe underestimation (up to ~ 50%) of BNF activity (Darnajoux et al. 2017). This is in agreement with the large underestimates of BNF (by ~ 30%) measured for microbial mats, leaf litter and sulfidic sediments (Zhang et al 2016; McRose et al 2017b).
Quantification of alternative BNF and a systematic revision of N2 fixation rates based on ARA is required to refine N input estimates in habitats where alternative nitrogenases are present and active. We cannot stress enough the need to calibrate ARA with 15N incorporation on a large number of samples (not only a small subset as often reported) and under environmentally relevant conditions for the accurate determination of N2 fixation rates based on acetylene reduction rates.
High latitude ecosystems, which are currently experiencing rapid warming, and where cryptogamic covers significantly contribute to BNF, are of particular interest. In these low N input ecosystems, primary production and its response to global climate change are strongly constrained by N (Lebauer and Treseder 2008; Wang et al. 2010; Heimann and Reichstein 2008; Sigurdsson et al. 2013).
Perspectives and future research directions
Nitrogenase metal co-factor biogeochemistry
The use and activity of the various nitrogenase isoforms in terrestrial ecosystems is intertwined with the biogeochemistry of Mo, V and Fe. We highlight below important knowledge gaps that need to be addressed.
Inclusion of trace metals in organic matter analyses
Litter decomposition is a critical process that controls nutrient cycling at the forest scale. Nutrient dynamics during litter decomposition was recently proposed to play an important role in the emergence of P and Mo limitation of BNF in cold temperate forests (Pourhassan et al. 2016). Reed et al. reported that a leaf litter matrix with a Mo content < 200 ng g−1 was prone to Mo limitation of BNF in tropical forests (Reed et al. 2013). A similar Mo threshold was recently reported in high-latitude cyanolichens, where alternative nitrogenases significantly contributed to BNF in samples with a Mo content below 250 ng g−1 (Darnajoux et al. 2019). These new findings open the possibility to screen ecosystems worldwide for Mo limitation by mining literature data on the elemental composition of soil, litter and cryptogams. Unfortunately, trace metals are not routinely included in soil and litter analyses, which often focus on C and N. When trace metals are reported, Mo and especially V are rarely considered, except in studies on contaminated soils (Johnson and Hale 2004; Lawrey 1978). In addition, datasets on trace metal concentrations in terrestrial samples must be examined carefully, as sample preparation for elemental analysis must avoid metal-based equipment, such as steel Wiley mills and roller grinders, which can be a source of metal contamination (Marks et al. 2015b). Mo is particularly sensitive to contamination during sample preparation due to its low environmental concentrations and its presence is numerous metal alloys.
The importance of including Mo and V in soil analyses is further illustrated by studies on symbiotic N2-fixation in plants. Hungate et al. reported a long-term decline in N2 fixation by the leguminous vine Galactia elliottii under elevated atmospheric CO2. The CO2-induced decline was attributed to a decrease in Mo availability to the plant due to either a decrease in pH or an increase in soil organic matter content (Hungate et al. 2004). The effect, if any, of these changes in soil Mo availability on heterotrophic N2 fixers was not tested. In a more recent study, Perakis et al. reported coupled soil accumulation of C, P and Mo in forests shaped by legacies of symbiotic N2-fixing trees. This nutrient accumulation was proposed as a mean to alleviate nutrient limitation for heterotrophic N2-fixers and promote soil BNF (Perakis et al. 2017).
The ongoing and anticipated effects of global climate change on both primary production (litter biomass) and organic matter decomposition are likely to strongly affect phosphorus and metal limitations, as well as alternative nitrogenases activity at the forest scale. A more systematic analysis of trace metals in environmental samples is the first step to build the global database needed to disentangle the complex interactions between major nutrients and trace metal dynamics, and BNF.
Proper assessment of Mo bioavailability to diazotrophs
A better evaluation of Mo bioavailability to diazotrophs is needed to refine the Mo threshold reported by Reed et al. (2013) and Darnajoux et al. (2019), because Mo limitation of BNF (and associated alternative nitrogenase expression) may be more directly dependent on Mo bioavailability than on total Mo content. Current methods to characterize Mo bioavailability were originally designed for higher plants using soft extraction approaches (e.g. resin binds, ammonia oxalate) (Lang and Kaupenjohann 1999; Liu et al. 1996; Poledniok and Buhl 2003). The extent to which these methods measure Mo availability to free living diazotrophs remains to be validated. Diazotrophs possess specialised high affinity uptake systems for Mo and V (e.g., metallophores) allowing them to dynamically affect metal speciation in their microenvironment to support their growth. New extraction methods mimicking metallophore efficiency for metal retrieval (i.e., Fe, Mo, V) from different environmental sources (e.g., organic matter, oxides) may provide better estimates for nitrogenase metal cofactor bioavailability to diazotrophs than the soft extraction methods currently used. The use of synthetized or culture-purified metallophores as metal extractants could be explored.
Consider costs of metal acquisition in studies on trace metal limitation of BNF
There is increasing evidence that metallophores play an important role in nitrogenase metal cofactor acquisition and homeostasis. Research under environmentally relevant conditions, i.e., with organic matter and iron oxides present in the medium, are required to understand how the use of alternative nitrogenases is modulated by the cost of the metallophore-assisted acquisition of nitrogenase metal cofactors.
Physiology of diazotrophs
The constraints imposed by trace metal bioavailability on the use of alternative nitrogenases depend on physiology of diazotrophs, many aspects of which remain poorly understood. We describe below a few areas that require further study in this context.
Characterize metal acquisition and homeostasis in a greater diversity of diazotrophs
Our conceptual understanding of how diazotrophs manage cellular metal budgets for N2 fixation is largely based on studies of the soil-dwelling, aerobic heterotroph Azotobacter vinelandii (Bellenger et al. 2008a, 2011; Bishop et al. 1986; Jacobitz and Bishop 1992; Pau et al. 1993) and the freshwater heterocystous cyanobacterium Anabaena variabilis (Darnajoux et al. 2014; Thiel et al. 2002; Thiel and Pratte 2013; Zahalak et al. 2004). While these organisms have provided mechanistic insights on important metal thresholds and acquisition strategies, they represent only a dismal fraction of the environmental and physiological diversity of terrestrial diazotrophs, which span soil, freshwater, and coastal habitats as well as aerobic and anaerobic metabolisms. Interestingly, in the facultative anaerobe Rhodopseudomonas palustris, the expression of alternative nitrogenases is not repressed by Mo (Oda et al. 2005) as transcription of alternative nitrogenase genes was observed in Mo-replete medium by a diazotrophic strain unable to synthesize active Mo nitrogenase. Thus, alternative nitrogenase expression may not always be controlled by Mo availability as is usually assumed. In addition, beyond R. palustris, the strategies used by anaerobic diazotrophs to manage their metal quotas remain largely unexplored. More research is required to fully establish the occurrence of Mo regulation over alternative nitrogenase expression in diazotrophs.
Constraints on metabolic cost of canonical vs alternative nitrogenases
The metabolic costs of using different nitrogenase isoforms for BNF has been little explored beyond comparisons of growth and BNF rates. Studies carried out at room temperature (20–30 °C) report that growth based on V-Nase is 20 to 40% slower than Mo-Nase (Bellenger et al. 2011; McRose et al. 2017a; Thiel and Pratte 2013; Zhang et al. 2014). However, a recent study (Luxem et al. 2020b) shows that the type of carbon source utilized by R. palustris grown anaerobically under photoheterotrophic conditions at ~ 20 °C re-orders the relative growth rates of Mo-Nase and V-Nase based growth. Use of a more reduced carbon source like acetate rather than succinate led to slightly faster growth based on V-Nase than on Mo-Nase due to changes in biomass composition. In addition, a much lower H2 production:N2 reduction ratio for V-Nase was measured in vivo than previously observed for this enzyme within in vitro assays (i.e., Fig. 1a). The paradigm of intrinsically slow growth based on alternative nitrogenases has also been challenged by findings that the V-Nase and Mo-Nase -based growth rates of the cyanobacterium Anabaena variabilis are similar at cold temperatures (< 20 °C) (Darnajoux et al. unpublished). Thus changes in cellular metabolism (Luxem et al. 2020b), differences in the temperature dependence of the isoform specific activities (Miller and Eady 1988; Darnajoux et al. unpublished), and complexity of cellular nitrogenase regulation suggest that alternative BNF may be favored in specific environments. We suggest that future work considers the influence of metal management, temperature, energy availability, and metabolic N demands holistically in developing a mechanistic framework for how and why different nitrogenase isoforms are used in nature.
Conclusion
Three main factors may explain why the contribution of alternative nitrogenases to BNF has been overlooked for the last 40 years. (1) They are absent in bacteria that form symbiosis with higher plants, which have long been considered the major contributors to BNF worldwide. (2) The assessment of alternative nitrogenases activity in environmental samples was impeded by methodological constrains. (3) The unwarranted extrapolation of laboratory experiments performed on a limited number of model organisms to the field may have led to misconceptions regarding the uptake and intracellular management of Mo, V and Fe, as well as the regulation of nitrogenase isoforms by diazotrophs. However, an increasing body of evidence points to the importance of alternative nitrogenases to BNF in nature. It is now possible and urgent to investigate nitrogenase diversity in the field and quantify of the contribution of alternative nitrogenases to BNF by updating traditional methods and implementing complementary new methods such as ISARA. Nitrogenase diversity needs to be included in our conceptual models of BNF, particularly because of its potentially impact on BNF estimates. It is also important to improve our understanding of how trace metal dynamics, temperature, and diazotroph physiology influence nitrogenase diversity and BNF in unmanaged ecosystems. Since semantics matter, we suggest that it is now time to stop referring to the V- and Fe-only nitrogenases as “alternative nitrogenases”, as this qualifier unconsciously confines them to a secondary role in terrestrial BNF, and start calling and considering them for what they really are- “complementary nitrogenases”—used by a wide range of prokaryotes to sustain N fixation under challenging environmental conditions.
References
Anbar AD, Knoll AH (2002) Proterozoic ocean chemistry and evolution: a bioinorganic bridge? Science 297:1137–1142
Attridge EM, Rowell P (1997) Growth, heterocyst differentiation and nitrogenase activity in the cyanobacteria Anabaena variabillis and Anabaena cylindrica in response to molybdenum and vanadium. New Phytol 135:517–526
Barron AR, Wurzberger N, Bellenger JP, Wright SJ, Kraepiel AML, Hedin LO (2009) Molybdenum limits nitrogen fixation in tropical forest soils. Nat Geosci 2:42–45
Batterman SA, Hall JS, Turnet BL, Hedin LO, Kimiko Lahaela Walter J, Sheldon P, van Breugel M (2018) Phosphatase activity and nitrogen fixation reflect species differences, not nutrient trading or nutrient balance, across tropical rainforest trees. Ecol Lett 21:1486–1495
Bellenger JP, Wichard T, Kraepiel AML (2008a) Vanadium requirements and uptake kinetics in the dinitrogen-fixing bacterium Azotobacter vinelandii. Appl Environ Microbiol 74:1478–1484
Bellenger JP, Wichard T, Kustka AB, Kraepiel AML (2008b) Nitrogen fixing soil bacterium uses catechol siderophores for molybdenum and vanadium acquisition. Nat Geosci 1:243–246
Bellenger JP, Wichard T, Xu Y, Kraepiel AM (2011) Essential metals for nitrogen fixation in a free-living N(2)-fixing bacterium: chelation, homeostasis and high use efficiency. Environ Microbiol 13:1395–1411
Bellenger JP, Xu Y, Zhang X, Morel FMM, Kraepiel AM (2014) Possible contribution of alternative nitrogenases to nitrogen fixation by asymbiotic N2-fixing bacteria in soils. Soil Biol Biochem 69:413–420
Betancourt DA, Loveless TM, Brown JW, Bishop PE (2008) Characterization of diazotrophs containing Mo-independent nitrogenase, isolated from diverse natural environments. Appl Environ Microbiol 74:3471–3480
Bishop PE, Premakumar R (1992) Alternative nitrogen fixation systems. In: Stacey G, Burris RH, Evans HJ (eds) Biological nitrogen fixation. Chapman & Hall, New York
Bishop PE, Jarlenski DML, Hetherington DR (1980) Evidence for an alternative nitrogen fixation system in Azotoacter vinelandii. Proc Nat Acad Sci USA 77:7342–7346
Bishop PE, Jarlenski DML, Hetherington DR (1982) Expression of an alternative nitrogen fixation system in Azotobacter vinelandii. J Bacteriol 150:1244–1251
Bishop PE, Premakumar R, Dean DR, Jacobson MR, Chnisnell JR, Rizzo TM, Kopczynski J (1986) Nitrogen fixation by Azotobacter vinelandii strains having deletions in structural genes for nitrogenase. Science 232:92–94
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Boison G, Steingen C, Stal LJ, Bothe H (2006) The rice field cyanobacteria Anabaena azotica and Anabaena sp. CH1 express vanadium-dependent nitrogenase. Arch Microbiol 186:367–376
Bowen JL, Babbin AR, Kearns PJ, Ward BB (2014) Connecting the dots: linking nitrogen cycle gene expression to nitrogen fluxes in marine sediment mesocosms. Front Microbiol 5:429
Boyd ES, Hamilton TL, Peters JW (2011) An alternative path for the evolution of biological nitrogen fixation. Front Microbiol 2:205
Burgess BK, Lowe DJ (1996) Mechanism of molybdenum nitrogenase. Chem Rev 96:2983–3012
Chakraborty B, Samaddar KR (1995) Evidence for the occurence of an alternative nitrogenase system in Azospirillum brasilense. FEMS Microbiol Let 127:127–131
Chapin DM, Bliss LC, Bledsoe LJ (1990) Environmental regulation of nitrogen fixation in a high artic lowland ecosystem. Can J Bot 69:2744–2755
Chatterjee R, Allen RM, Ludden PW, Shah VK (1997) In vitro synthesis of the iron-molybdenum cofactor and maturation of the nif-encoded apodinitrogenase. J Biol Chem 272:21604–21608
Chen SC, Musat N, Lechtenfeld OJ, Paschke H, Schmidt M, Said N, Popp D, Calabrese F, Stryhanyuk H, Jaekel U, Zhu YG, Joye SB, Richnow HH, Widdel F, Musat F (2019) Anaerobic oxidation of ethane by archaea from a marine hydrocarbon seep. Nature 568:108–111
Chien YT, Auerbuch V, Brabban AD, Zinder SH (2000) Analysis of genes encoding an alternative nitrogenase in the archaeon Methanosarcina barkeri 227. J Bacteriol 182:3247–3253
Chisnell JR, Premakumar R, Bishop PE (1988) Purification of the second alternative nitrogenase from a nifHDK deletion strain of Azotobacter vinelandii. J Bacteriol 170:27–33
Christiansen CF, Loscher CR (2019) Facets of diazotrophy in the OMZ off Peru revisited- what we couldn’t see from a single marker gene approach. Biology. https://doi.org/10.1101/558072
Collier RW (1985a) Molybdenum in the Northeast Pacific Ocean. Limnol Oceanogr 30:1351–1354
Collier RW (1985b) Particulate and dissolved vanadium in the North Pacific Ocean. Nature 309:441–444
Crews TE, Farrington H, Vitousek PM (2000) Changes in asymbiotic, heterotrophic nitrogen fixation on leaf litter of Metrosideros polymorpha with long-term ecosystem development in Hawaii. Ecosystems 3:386–395
Dabundo R, Lehmann MF, Treibergs L, Tobias CR, Altabet MA, Moisander PH, Granger J (2014) The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE 9:e110335
Darnajoux R, Constantin J, Miadlikowska J, Lutzoni F, Bellenger JP (2014) Is vanadium a biometal for boreal cyanolichens? New Phytol 202:765–771
Darnajoux R, Lutzoni F, Miadlikowska J, Bellenger JP (2015) Determination of elemental baseline using peltigeralean lichens from Northeastern Canada (Quebec): initial data collection for long term monitoring of the impact of global climate change on boreal and subarctic area in Canada. Sci Total Environ 533:1–7
Darnajoux R, Zhang X, McRose DL, Miadlikowska J, Lutzoni F, Kraepiel AM, Bellenger JP (2017) Biological nitrogen fixation by alternative nitrogenases in boreal cyanolichens: importance of molybdenum availability and implications for current biological nitrogen fixation estimates. New Phytol 213:680–689
Darnajoux R, Magain N, Renaudin M, Lutzoni F, Bellenger JP, Zhang X (2019) Molybdenum threshold for ecosystem-scale alternative vanadium nitorgenase activity in boreal forests. Proc Natl Acad Sci USA 116:24682–24688
Davis R, Lehmann L, Petrovich R, Shah VK, Roberts GP, Ludden PW (1996) Purification and characterization of the alternative nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol 178:1445–1450
de Bruijn FJ (ed) (2015) Biological nitrogen fixation. Wiley, Hoboken
DeLuca TH, Zackrison O, Nilsson M-C, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature 419:917–920
Dilworth MJ, Loneragan JF (1991) An alternative nitrogenase is not expressed in molybdenum-deficient legume root nodules. New Phytol 118:303–308
Dilworth MJ, Eady RR, Robson RL, Miller RW (1987) Ethane formation from acetylene as a potential test for vanadium nitrogenase in vivo. Nature 327:167–168
Dilworth MJ, Eady RR, Eldridge ME (1988) The vanadium nitrogenase of Azotobacter chroococcum: Reduction of acetylene and ethylene to ethane. Biochem J 249:745–751
Dilworth MJ, Eldridge ME, Eady RR (1993) The molybdenum and vanadium nitrogenases of Azotobacter chroococcum: effect of elevated temperature on N2 reduction. Biochem J 289:395–400
Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2:621–631
Dos Santos PC, Fang Z, Mason SW, Setubal JC, Dixon R (2012) Distribution of nitrogen fixation and nitrogenase-like sequences amongst microbial genomes. BMC Genomics 13:162
Dynarski KA, Houlton BZ (2018) Nutrient limitation of terrestrial free-living nitrogen fixation. New Phytol 217:1050–1061
Eady RR (1996) Structure-function relationships of alternative nitrogenase. Chem Rev 96:3013–3030
Eady RR, Robson RL (1984) Characteristics of N2 fixation in Mo-limited batch and continuous cultures of azotobacter vinelandii. Biochem J 224:853–862
Elbert W, Weber B, Burrows S, Steinkamp J, Budel B, Andreae MO, Poschl U (2012) Contribution of cryptogamic cover to the global cycles of carbon and nitrogen. Nat Geosci 5:459–462
Erickson BD, Helz GR (2000) Mo (IV) speciation in sulfidic water: stability and lability of thiomolybdates. Geochim Cosmochim Acta 64:1149–1158
Fallik E, Chan YK, Robson RL (1991) Determination of alternative nitrogenases in aerobic gram-negative nitrogen-fixing bacteria. J Bacteriol 173:365–371
Fukuda H, Fujii T, Ogawa T (1984) Microbial production of C2-hydrocarbons, ethane, ethylene and acetylene. Agri Biol Chem 48:1363–1365
Gaby JC, Buckley DH (2011) A global census of nitrogenase diversity. Environ Microbiol 13:1790–1799
Gagunashvili AN, Andresson OS (2018) Distinctive characters of Nostoc genomes in cyanolichens. BMC Genomics 19:434
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vorosmarty CJ (2004) Nitrogen cycles; past, present and future. Biogeochemistry 70:153–226
Goldberg S, Forster HS, Godfrey CL (1996) Molybdenum adsorption on oxydes, clay minerals, and soils. Soil Sci Soc Am J 60:425–432
Graham BM, Hamilton RD, Campbell NER (1980) Comparison of the nitrogen-15 uptake and acetylene reduction methods for estimating the rates of nitrogen fixation by freshwater blue-green algae. Can J Fish Aquat Sci 37:488–493
Gupta UC (1997) Molybdenum in agriculture. Cambridge University Press, Cambridge
Hafner H, Ndunguru BJ, Bationo A, Marschner H (1992) Effect of nitrogen, phosphorus and molybdenum application on growth and synbiotic N2-fixation of groundnut in an acid sandy soil in Niger. Fert Res 31:69–77
Hales BJ (1990) Alternative nitrogenase. Adv Inorg Biochem 8:165–198
Hamilton TL, Ludwig M, Dixon R, Boyd ES, Dos Santos PC, Setubal JC, Bryant DA, Dean DR, Peters JW (2011) Transcriptional profiling of nitrogen fixation in Azotobacter vinelandii. J Bacteriol 193:4477–4486
Hardy RWF, Holsten RD, Jackson RD, Burns RC (1968) Acetylene-ethylene assay for N2 fixation—laboratory and field evaluation. Plant Physiol 43:1185–1207
Hardy RWF, Burns RC, Holsten RD (1973) Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol Biochem 5:47–81
Harris DF, Lukoyanov DA, Kallas H, Trncik C, Yang ZY, Compton P, Kelleher N, Einsle O, Dean DR, Hoffman BM (2019) Mo-, V- and Fe-nitrogenases use a universal eight-electron reduction-elimination mechanism to achieve N2 reduction. Biochemistry 58:3293–3301
Hedin LO, Brookshire ENJ, Menge DNL, Barron AR (2009) The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol Syst 40:613–635
Heimann M, Reichstein M (2008) Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–292
Helz GR, Miller CV, Charnock JM, Mosselmans JFW, Pattrick RAD, Garner CD, Vaugahn DJ (1996) Mechanism of Mo removal from the sea and its concentration in black shales: EXAFS evidence. Geochim Cosmochim Acta 60:3631–3642
Hodkinson BP, Allen JL, Forrest L, Goffinet B, Serusiaux E, Andresson OS, Miao V, Bellenger JP, Lutzoni F (2014) Lichen-symbiotic cyanobacteria associated with Peltigera have an alternative vanadium-dependent nitrogen fixation system. Eur J Phycol 49:11–19
Horstmann JL, Denison WC, Silvester WB (1982) 15N2 fixation and Mo enhancement of acetylene reduction by Lobaria spp. New Phytol 92:235–241
Hungate BA, Stiling PD, Dijkstra P, Johnson DW, Ketterer ME, Hymus GJ, Hinkle CR, Drake BG (2004) CO2 elicits long-term decline in nitrogen fixation. Science 304:1291
Jacobitz S, Bishop PE (1992) Regulation of nitrogenase-2 in Azotobacter vinelandii by ammonium, molybdenum, and vanadium. J Bacteriol 174:3884–3888
Jacobson MR, Premakumar R, Bishop PE (1986) Transcriptional regulation of nitrogen fixation by molybdenum in Azotobacter vinelandii. J Bacteriol 167:480–486
Jean ME, Phalyvong K, Forest Drolet J, Bellenger JP (2013) Molybdenum and phosphorus limitation of asymbiotic nitrogen fixation in forests of Eastern Canada: influence of the vegetative cover and seasonal variability. Soil Biol Biochem 67:140–146
Joerger RD, Bishop PE (1988) Bacterial alternative nitrogen fixation systems. Crit Rev Microbiol 16:1–14
Joerger RD, Jacobson MR, Premakumar R, Wolfinger ED, Bishop PE (1989) Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J Bacteriol 171:1075–1086
Johnson D, Hale B (2004) White birch (Betula papyrifera Marshall) foliar litter decomposition in relation to trace metal atmospheric inputs at metal-contaminated and uncontaminated sites near Sudbury, Ontario and Rouyn-Noranda, Quebec, Canada. Environ Pollut 127:65–72
Jouogo Noumsi C, Pourhassan N, Darnajoux R, Deicke M, Wichard T, Burrus V, Bellenger JP (2016) Effect of organic matter on nitrogenase metal cofactors homeostasis in Azotobacter vinelandii under diazotrophic conditions. Environ Microbiol Rep 8:76–84
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th edn. CRC Press, Taylor & Francis Group, Boca Raton, FL
Kentemich T, Danneberg G, Hundeshagen B, Bothe H (1988) Evidence for the occurrence of the alternative, vanadium-containing nitrogenase in the cyanobacterium Anabaena variabilis. FEMS Microbiol Lett 51:19–24
Kim HJ, Galeva N, Larive CK, Alterman M, Graham DW (2005) Purification and physical–chemical properties of methanobactin: a chalkophore from Methylosinus trichosporium OB3b. Biochemistry 44:5140–5148
Kraepiel AML, Bellenger JP, Wichard T, Morel FMM (2009) Multiples roles of sidrophores in free-living nitrogen-fixing bacteria. Biometals 22:573–581
Kutsche M, Leimkuhler S, Angermuller S, Klipp W (1996) Promoters controlling expression of the alternative nitrogenase and the molybdenum uptake system in Rhodobacter capsulatus are activated by NtrC, independent of sigma54, and repressed by molybdenum. J Bacteriol 178:2010–2017
Lagerstrom A, Nilsson MC, Zackrisson O, Wardle DA (2007) Ecosystem input of nitrogen through biological fixation in feather mosses during ecosystem retrogression. Ecology 21:1027–1033
Lang F, Kaupenjohann M (1999) Molybdenum fractions and mobilization kinetics in acid forest soils. J Plant Nut Soil Sci 162:309–314
Lawrey JD (1978) Trace metal dynamics in decomposing leaf litter in habitats variously influenced by coal strip mining. Can J Bot 56:953–962
LeBauer D, Treseder K (2008) Nitrogen limitation of net primary productivity. Ecology 89:371–379
Lee CC, Hu Y, Ribbe MW (2010) Vanadium nitrogenase reduces CO. Science 329:642
Liengen L (1999) Conversion factor between acetylene reduction and nitrogen fixation in free-living cyanobacteria from high artic habitats. Can J Microbiol 45:223–229
Liermann LJ, Guynn RL, Anbar A, Brantley SL (2005) Production of a molybdophore during metal-targeted dissolution of silicates by soil bacteria. Chem Geol 220:285–302
Lindo Z, Whiteley JA (2011) Old trees contribute bio-available nitrogen through canopy bryophytes. Plant Soil 342:141–148
Liu D, Clark DJ, Crutchfield JD, Sims JL (1996) Effect of pH of ammonium oxalate extracting solutions of prediction of plant-available molybdenum in soil. Commun Soil Sci Plant Anal 27:2511–2541
Loveless TM, Bishop PE (1999) Identification of genes unique to Mo-independent nitrogenase systems in diverse diazotrophs. Can J Microbiol 45:1–6
Loveless TM, Saah JR, Bishop PE (1999) Isolation of nitrogen-fixing bacteria containing molybdenum-independent nitrogenases from natural environments. Appl Environ Microbiol 65:4223–4226
Luo Y, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 54:731–739
Luxem KE, Leavitt WD, Zhang X (2020a) Large hydrogen isotope fractionations distinguish nitrogenase-derived methane from other sources. https://doi.org/10.1101/2020.04.10.036657
Luxem KL, Kraepiel AML, Zhang L, Waldbauer J, Zhang X (2020b) Carbon substrate re-orders relative growth of a bacterium using Mo-, V-, or Fe-nitrogenase for nitrogen fixation. Environ Mirobiol. https://doi.org/10.1111/1462-2920.14955
Ma J, Bei Q, Wang X, Lan P, Liu G, Lin X, Liu Q, Lin Z, Liu B, Zhang Y, Jin H, Hu T, Zhu J, Xie Z (2019) Impacts of Mo application on biological nitrogen fixation and diazotrophic communities in a flooded rice-soil system. Sci Total Environ 649:686–694
Marks JA, Perakis SS, King EK, Pett-Ridge JC (2015a) Soil organic matter regulates molybdenum storage and mobility in forests. Biogeochemistry 125:167–183
Marks JA, Pett-Ridge JC, Parakis SS, Allen JL, McCune B (2015b) Response of the nitrogen-fixing lichen Lobaria pulmonaria to phosphorus, molybdenum and vanadium. Ecosphere 6:1–17
Martensson AM, Ljunggren HD (1984) A comparison between the acetylene reduction method, the isotope dilution method and the total nitrogen difference method for measuring nitrogen fixation in lucerne (Medicago sativa L.). Plant Soil 81:177–184
Masepohl B, Drepper T, Paschen A, Gross S, Pawlowski A, Raabe K, Riedel KU, Klipp W (2002) Regulation of nitrogen fixation in the phototrophic purple bacterium Rhodobacter capsulatus. J Mol Microbiol Biotechnol 4:243–248
Masukawa H, Zhang X, Yamazaki E, Iwata S, Nakamura K, Mochimaru M, Inoue K, Sakurai H (2009) Survey of the distribution of different types of nitrogenases and hydrogenases in heterocyst-forming cyanobacteria. Mar Biotechnol (NY) 11:397–409
Maynard RH, Premakumar R, Bishop PE (1994) Mo-independent nitrogenase 3 is advantageous for diazotrophic growth of Azotobacter vinelandii on solid medium containing molybdenum. J Bacteriol 176:5583–5586
McRose DL, Baars O, Morel FMM, Kraepiel AML (2017a) Siderophore production in Azotobacter vinelandii in response to Fe-, Mo- and V-limitation. Environ Microbiol 19:3595–3605
McRose DL, Zhang X, Kraepiel AM, Morel FM (2017b) Diversity and activity of alternative nitrogenases in sequenced genomes and coastal environments. Front Microbiol 8:267
Menge DN, Hedin LO (2009) Nitrogen fixation in different biogeochemical niches along a 120 000-year chronosequence in New Zealand. Ecology 90:2190–2201
Michelsen A, Rinnan R, Jonasson S (2012) Two decades of experimental manipulations of heaths and forest understory in the subarctic. Ambio 41(Suppl 3):218–230
Miller RW, Eady RR (1988) Molybdenum and vanadium nitrogenase of Azotobacter Chroococcum. Biochem J 256:429–432
Minchin FR, Witty JF, Sheehy JE, Muller M (1983) A major error in the acetylene reduction assay: decreases in nodular nitrogenase activity under assay conditions. J Exp Bot 34:641–649
Mohr W, Grosskopf T, Wallace DW, LaRoche J (2010) Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5:e12583
Moisander P, Lehtumaki J, Sivonen K, Kononen K (1996) Comparison of 15N2 and acetylene reduction methods for the measurement of nitrogen fixation by Baltic Sea cyanobacteria. Phycologia 35:140–146
Mulholland MR, Bernhardt PW (2005) The effect of growth rate, phosphorus concentration, and temperature on N2 fixation, carbon fixation, and nitrogen release in continuous cultures of Trichodesmium IMS101. Limnol Oceanogr 50:839–849
Mulholland MR, Bronk DA, Capone DG (2004) Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aqua Micro Ecol 37:85–94
Mus F, Alleman AB, Pence N, Seefeldt LC, Peters JW (2018) Exploring the alternatives of biological nitrogen fixation. Metallomics 10:523–538
Nagahama K, Yoshino K, Matsuoka M, Sato M, Tanase S, Ogawa T, Fukuda H (1994) Ethylene production by strains of the plant-pathogenic bacterium Pseudomonas syringae depends upon the presence of indigenous plasmids carrying homologous genes for the ethylene-forming enzyme. Microbiology 140:2309–2313
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270:26723–26726
Ni CV, Yakuninin AF, Gogotov IN (1990) Influence of molybdenum, vanadium, and tungsten on growth and nitrogenase synthesis of the free-living cyanobacterium Anabaena azollae. Microbiology 59:395–398
Nohrstedt H (1983) Conversion factor between acetylene reduction and nitrogen fixation in soil: effect of water content and nitrogenase activity. Soil Biol Biochem 15:275–279
Oda Y, Samanta SK, Rey FE, Wu L, Liu X, Yan T, Zhou J, Harwood CS (2005) Functional genomic analysis of three nitrogenase isozymes in the photosynthetic bacterium Rhodopseudomonas palustris. J Bacteriol 187:7784–7794
Pau RN, Eldridge ME, Lowe DJ, Mitchenall LA, Eady RR (1993) Molybdenum-independent nitrogenase of Azotobacter vinelandii: a function species of alternatice nitrogenase-3 isolated from a molybdenum-tolerant strain contains an iron-molybdenum cofactor. Biochem J 293:101–107
Perakis SS, Pett-Ridge JC, Catricala CE (2017) Nutrient feedbacks to soil heterotrophic nitrogen fixation in forests. Biogeochemistry 134:41–55
Perez CA, Silva WA, Aravena JC, Armesto JJ (2017) Limitations and relevance of biological nitrogen fixation during postglacial succession in cordillera Darwin, Tierra del Fuego, Chile. Arct Antarct Alp Res 49:29–42
Peters JW, Boyd ES (2015) Exploring alternative paths for the evolution of biological nitrogen fixation. In: de Bruijn FJ (ed) biological nitrogen fixation. Wiley, Hoboken, NJ
Peters JW, Fisher K, Dean DR (1995) Nitrogenase structure and function: a biochemical-genetic perspective. Annu Rev Microbiol 49:335–366
Poledniok J, Buhl F (2003) Speciation of vanadium in soil. Talanta 59:1–8
Pourhassan N, Bruno S, Davidson Jewell M, Shipley B, Roy S, Bellenger JP (2016) Phosphorus and micronutrient dynamics during gymnosperm and angiosperm litters decomposition in temperate cold forest from Eastern Canada. Geoderma 273:25–31
Pratte BS, Sheridan R, James JA, Thiel T (2013) Regulation of V-nitrogenase genes in Anabaena variabilis by RNA processing and by dual repressors. Mol Microbiol 88:413–424
Raina R, Reddy MA, Ghosal D, Das HK (1988) Characterization of the gene for the Fe-protein of the vanadium dependent alternative nitrogenase of Azotobacter vinelandii and construction of a Tn5 mutant. Mol Gen Genet 214:121–127
Reddy KJ, Gloss SP (1993) Geochemical speciation as related to the mobility of F, Mo and Se in soil leachates. Appl Geochem 2:159–163
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a comtemporary perspective. Annu Rev Ecol Evol Syst 42:489–512
Reed SC, Cleveland CC, Townsend AR (2013) Relationship among phosphorus, molybdenum and free living nitrogen fixation in tropical rain forests: result from observational and experimental analyses. Biogeochemistry 114:135–147
Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JM, Naeem S, Trost J (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Rijkenberg MJA, Middag R, Laan P, Gerringa LJA, van Aken HM, Schoemann V, de Jong JTM, de Baar HJW (2014) The distribution of dissolved iron in the west Atlantic ocean. PLoS ONE 9:e101323
Robson RL, Eady RR, Richardson TH, Miller RW, Hawkins M, Postgate JR (1986) The alternative nitrogenase of Azotobacter chroococcum is a vanadium enzyme. Nature 322:388–390
Rousk K, Jones DL, Deluca TH (2013) Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Front Microbiol 4:150
Rousk K, Degboe J, Michelsen A, Bradley R, Bellenger JP (2017) Molybdenum and phosphorus limitation of moss-associated nitrogen fixation in boreal ecosystems. New Phytol 214:97–107
Rubio LM, Ludden PW (2005) Maturation of nitrogenase: a biochemical puzzle. J Bacteriol 187:405–414
Schneider K, Muller A, Schramm U, Klipp W (1991) Demonstration of a molybdenum- and vanadium-independent nitrogenase in a nifHDK-deletion mutant of Rhodobacter capsulatus. Eur J Biochem 195:653–661
Schuddekopf K, Hennecke S, Liese U, Kutsche M, Klipp W (1993) Characterization of anf genes specific for the alternative nitrogenase and identification of nif genes required for both nitrogenases in Rhodobacter capsulatus. Mol Microbiol 8:673–684
Schwintzer CR, Tjepkema JD (1994) Factors affecting the acetylene to 15N2 conversion ratio in root nodules of Myrica gale 1. Plant Physiol 106:1041–1047
Scott DJ, Dean DR, Newton WE (1992) Nitrogenase-catalyzed ethane production and CO-sensitive hydrogen evolution from MoFe proteins having amino acid substitutions in an alpha-subunit FeMo cofactor-binding domain. J Biol Chem 267:20002–20010
Scott DL, Bradley RL, Bellenger JP, Houle D, Gundale MJ, Rousk K, DeLuca TH (2018) Anthropogenic deposition of heavy metals and phosphorus may reduce biological N2 fixation in boreal forest mosses. Sci Tot Environ 630:203–210
Seefeldt LC, Hoffman BM, Dean DR (2009) Mechanism of Mo-dependent nitrogenase. Annu Rev Biochem 78:701–722
Seitzinger SP, Garber JH (1987) Nitrogen fixation and 15N2 calibration of the acetylene reduction assay in coastal marine sediments. Mar Ecol 37:65–73
Sellstedt A (1986) Acetylene reduction, H2 evolution and 15N2 fixation in the Alnus incana-Frankia symbiosis. Planta 167:382–386
Sigurdsson BD, Medhurst JL, Wallin G, Eggertsson O, Linder S (2013) Growth of mature boreal Norway spruce was not affected by elevated [CO2] and/or air temperature unless nutrient availability was improved. Tree Physiol 33:1192–1205
Silvester WB (1989) Molybdenum limitation of asymbiotic nitrogen fixation in forests of pacific northwest America. Soil Biol Biochem 21:283–289
Singh R, Guzman MS, Bose A (2017) Anaerobic oxidation of ethane, propane, and butane by marine microbes: a mini review. Front Microbiol 8:2056
Smith VR (1984) Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a subantarctic island. Appl Environ Microbiol 48:594–600
Srivastava TK, Ahlawat IPS, Panwar JDS (1998) Effect of phosphorous, molybdenum and biofertilizers on productivity of pea (Pisum sativum L.). Ind J Plant Physiol 3:237–239
Sullivan BW, Smith WK, Townsend AR, Nasto MK, Reed SC, Chazdon RL, Cleveland CC (2014) Spatially robust estimates of biological nitrogen (N) fixation imply substantial human alteration of the tropical N cycle. Proc Natl Acad Sci USA 111:8101–8106
Thiel T (1993) Characterization of genes for an alternative nitrogenase in the cyanobacterium Anabaena variabilis. J Bacteriol 175:6276–6286
Thiel T, Pratte BS (2013) Alternative nitrogenases in Anabaena variabilis: the role of molybdate and vanadate in nitrogenase gene. Adv Microbiol 3:87–95
Thiel T, Pratte BS (2014) Regulation of three nitrogenase gene clusters in the cyanobacterium Anabaena variabilis ATCC 29413. Life 4:944–967
Thiel T, Pratte B, Zahalak M (2002) Transport of molybdate in the cyanobacterium Anabaena variabilis ATCC 29413. Arch Microbiol 179:50–56
Vieira RF, Cardoso EJBN, Vieira C, Casssini STA (1998a) Foliar application of molybdenum in common bean. I. Nitrogenase and reductase activities in a soil high fertility. J Plant Nutr 21:169–180
Vieira RF, Vieira C, Cardoso EJBN, Mosquim PR (1998b) Foliar application of molybdenum in common bean.II. Nitrogenase in a soil of low fertility. J Plant Nutr 21:2141–2151
Vile M, Wieder RK, Zivkovic T, Scott KD, Vitt DH, Hartsock JA, Iosue CL, Quinn CL, Petix M, Fillingim HM, Popma JMA, Dynarski KA, Jackman TR, Albright CM, Wykoff DD (2014) N2-fixation by methanotrophs sustains carbon and nitrogen accumulation in pristine peatlands. Biogeochemistry 121:317–328
Vitousek P (1999) Nutrient limitation to nitrogen fixation in young volcanic sites. Ecosystems 2:505–510
Vitousek PM, Hobbie S (2000) Heterotrophic nitrogen fixation in decomposing litter: patterns and regulation. Ecology 81:2366–2376
Vitousek PM, Menge DN, Reed SC, Cleveland CC (2013) Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philos Trans R Soc Lond B 368:20130119
Vouk VB, Piver WT (1983) Metallic elements in fossil fuel combustion products: amounts and form of emissions and evaluation of carcinogenicity and mutagenicity. Environ Health Perspect 47:201–225
Wagner A, Chappaz A, Lyons TW (2017) Molybdenum speciation and burial pathway in weakly sulfidic environments: insights from XAFS. Geochim Cosmochim Acta 206:18–29
Wall JD (2004) Rain or shine–a phototroph that delivers. Nat Biotechnol 22:40–41
Walmsley J, Kennedy C (1991) Temperature-dependent regulation by molybdenum and vanadium of expression of the structural genes encoding the three nitrogenases in Azotobacter vinelandii. Appl Environ Microbiol 57:622–624
Wang YP, Law RM, Pak B (2010) A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeochemistry 7:2261–2282
Wedepohl KH (1995) The composition of the continental crust. Geochim Cosmochim Acta 59:1217–1232
Werner Klipp BM, Gallon JR, Newton WE (eds) (2004) Genetics and regulation of nitrogen fixation in free-living bacteria. Kluwer Academic Publishers, New-York
Wichard T, Bellenger JP, Loison A, Kraepiel AML (2008) Catechols siderophores control tungsten uptake and toxicity in the nitrogen-fixer bacterium Azotobacter vinelandii. Environ Sci Technol 42:2408–2413
Wichard T, Bellenger JP, Morel FMM, Kraepiel AML (2009a) Role of the pyoverdine siderophore azotobactin in the bacterial acquisition of nitrogenase metal cofactors. Environ Sci Technol 43:7218–7224
Wichard T, Mishra B, Myneni SCB, Bellenger JP, Kraepiel AML (2009b) Storage and bioavailability of molybdenum in soils increased by organic matter complexation. Nat Geosci 2:625–629
Wilson ST, Bottjer D, Church MJ, Karl DM (2012) Comparative assessment of nitrogen fixation methodologies, conducted in the oligotrophic North Pacific Ocean. Appl Environ Microbiol 78:6516–6523
Winbourne JB, Brewer SW, Houlton BZ (2017) Iron controls over di-nitrogen fixation in karst tropical forest. Ecology 98:773–781
Wolfinger ED, Bishop PE (1991) Nucleotide sequence and mutational analysis of the vnfENX region of Azotobacter vinelandii. J Bacteriol 173:7565–7572
Wurzburger N, Bellenger JP, Kraepiel AM, Hedin LO (2012) Molybdenum and phosphorus interact to constrain asymbiotic nitrogen fixation in tropical forests. PLoS ONE 7:e33710
Zahalak M, Pratte B, Werth KJ, Thiel T (2004) Molybdate transport and its effect on nitrogen utilization in the cyanobacterium Anabaena variabilis ATCC 29413. Mol Microbiol 51:539–549
Zehr JP, Jenkins BD, Short SM, Steward GF (2003) Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ Microbiol 5:539–554
Zhang X, Sigman DM, Morel FM, Kraepiel AM (2014) Nitrogen isotope fractionation by alternative nitrogenases and past ocean anoxia. Proc Natl Acad Sci USA 111:4782–4787
Zhang X, McRose DL, Darnajoux R, Bellenger JP, Morel FMM, Kraepiel AM (2016) Alternative nitrogenase activity in the environment and nitrogen cycle implications. Biogeochemistry 127:189–198
Zheng Y, Harris DF, Yu Z, Fu Y, Poudel S, Ledbetter RN, Fixen KR, Yang Z-Y, Boyd ES, Lidstrom ME, Seefeldt LC, Harwood CS (2018) A pathway for biological methane production using bacterial iron-only nitrogenase. Nat Microbiol 3:281–286
Zinoni F, Robson RM, Robson RL (1993) Organization of potential alternative nitrogenase genes from Clostridium pasteurianum. Biochim Biophys Acta 1174:83–86
Funding
Funding was provided by Natural Sciences and Engineering Research Council of Canada (Grant No. CRC-950-230570), the U.S. National Science Foundation (Grant No. EAR-1631814), and a Simons Foundation/Life Science Research Foundation Postdoctoral Fellowship (to R.D.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Steven Perakis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellenger, J.P., Darnajoux, R., Zhang, X. et al. Biological nitrogen fixation by alternative nitrogenases in terrestrial ecosystems: a review. Biogeochemistry 149, 53–73 (2020). https://doi.org/10.1007/s10533-020-00666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00666-7