Abstract
The quantitative contribution of tropical estuaries to the atmospheric CO2 budget has large uncertainties, both spatially and seasonally. We investigated the seasonal and spatial variations of carbon biogeochemistry downstream of Ho Chi Minh City (Southern Vietnam). We sampled four sites distributed from downstream of a highly urbanised watershed through mangroves to the South China Sea coast during the dry and wet seasons. Measured partial pressure of CO2 (pCO2) ranged from 660 to 3000 μatm during the dry season, and from 740 to 5000 μatm during the wet season. High organic load, dissolved oxygen saturation down to 17%, and pCO2 up to 5000 μatm at the freshwater endmember of the estuary reflected the intense human pressure on this ecosystem. We show that releases from mangrove soils affect the water column pCO2 in this large tropical estuary (~600 m wide and 10–20 m deep). This study is among the few to report direct measurements of both water pCO2 and CO2 emissions in a Southeast Asian tropical estuary located in a highly urbanised watershed. It shows that the contribution of such estuaries may have been previously underestimated, with CO2 emissions ranging from 74 to 876 mmol m−2 day−1 at low current velocity (< 0.2 m s−1). Corresponding gas transfer velocities k600, ranging from 1.7 to 11.0 m day−1, were about 2 to 4 times of k600 estimated using published literature equations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Being generally net heterotrophic, estuaries act as sources of carbon dioxide (CO2) to the atmosphere, while continental shelves are highly productive autotrophic ecosystems and act as sinks of CO2 (Cai 2011; Hofmann et al. 2011). In a context of climate change, identifying natural sinks and sources of CO2 to the atmosphere and understanding how they are influenced by anthropogenic pressure is a major concern (Regnier et al. 2013; Lee 2016). Despite high loads of carbon in estuaries originate from inland watersheds, Cai (2011) argued that the large amount of CO2 emitted from coastal waters must be supported by lateral transport of carbon from the surrounding coastal wetlands, such as mangroves, which are highly productive ecosystems (Alongi 2014). Moreover, high loads of dissolved inorganic carbon (DIC) are released from surrounding sediments, especially when they are covered with mangrove forests, and this carbon can be a major input in coastal budgets (Bouillon et al. 2008; Atkins et al. 2013; Rosentreter et al. 2018).
Recently published global estimates of estuarine CO2 fluxes (FCO2) substantially vary, both spatially and seasonally, and the quantitative contribution of estuaries to the atmospheric CO2 budget has large uncertainties (Chen et al. 2013). Such uncertainties are partially due to the method employed to estimate gas exchanges, which was historically based on CO2 partial pressure (pCO2) measurement. Additionally, the latter was often calculated from the carbonate system equilibrium constants (Chen et al. 2013), and compiled using a function of wind speed (Wanninkhof 1992). Uncertainties could be of high importance especially in Southeast Asia, considered as a hotspot of aquatic CO2 emissions to the atmosphere (Regnier et al. 2013) due to high organic carbon concentrations in rivers (Müller et al. 2016) and the presence of megacities with ineffective wastewater treatment systems (Strady et al. 2017).
To address the knowledge gap on CO2 emissions from tropical estuaries, we measured seasonal and spatial changes in carbon biogeochemistry and quantified CO2 emissions in a human impacted and mangrove dominated Southeast Asian tropical estuary (Can Gio, Southern Vietnam). We hypothesised that (1) both DIC and CO2 releases from surrounding mangrove soils could be traced at the scale of a large estuary such as the Saigon–Dong Nai River system, and (2) emissions of CO2 would be particularly high due to both anthropogenic effects and surrounding mangroves. Our dataset is based on two sampling campaigns (dry season in January–February 2015 and wet season in September–October 2015), which include 24 h time series on four sites distributed from the downstream end of Ho Chi Minh City (upstream the mangrove) to the South China Sea coast. This study provides a foundational set of direct measurements for both water pCO2 and FCO2 in a Southeast Asian tropical estuary with a highly urbanised watershed.
Materials and methods
Study area
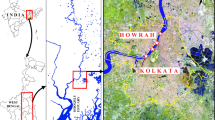
The Saigon–Dong Nai River basin has a total catchment area of 40.6 × 103 km2, approximately 12% of the total terrestrial area of Vietnam, and a main stream length of 628 km (Nippon Koei 1996). Total basin runoff is estimated at 37.4 × 106 m3 year−1 and precipitation averages 2000 mm year−1 (Ringler et al. 2002). The study area is located in a tropical monsoonal environment with a wet season from June to October and a dry season from November to May (Nam et al. 2014). During the wet season, the area receives 90% of its annual precipitation and the river discharge may be up to 30 times higher than lower values measured during the dry season. At the downstream end of Ho Chi Minh City (Southern Vietnam; ~ 13 million inhabitants), the Saigon–Dong Nai River splits and forms a delta that drains the 719.6 km2 of the Can Gio district, designated in 2000 by the UNESCO as the first mangrove biosphere reserve in Vietnam (Tuan and Kuenzer 2012). The north-east border of the biosphere reserve is dedicated to socio-economic development, mostly intensive shrimp farming (24.5% of the total area). Tidal amplitude is variable over time and ranges between 2 to 4 m depending on the season and proximity to the coast line (Tuan and Kuenzer 2012).
We selected the study sites to be at the interface between land uses (Fig. 1): (A) at the downstream end of Ho Chi Minh City (10°39′55″N 106°47′30″E); (B) between shrimp farms and the mangrove forested area (10°34′19″N 106°50′11″E); (C) in the centre of the mangrove protected core (10°31′04″N 106°53′13″E); and (D) between the mangrove forested area and the South China Sea coast (10°29′32″N 106°56′55″E). All sites were located on the main estuarine channel, which has steep eroded banks, a width of about 600 m and a depth of 10–20 m.
Data collection
We monitored water surface carbon biogeochemistry over 24 h time series during the dry (January–February) and monsoon (September–October) periods in 2015. Salinity, pH and water temperature were measured at 5-min intervals using a Yellow Spring Instrument® meter (YSI 6920) immersed 30 cm below water surface and calibrated before each survey. Dissolved oxygen (DO) was monitored similarly with a Hobo® data logger (HOBO U26-001) and also calibrated before each survey. Salinity and pH were not recorded at site C during the dry season due to a sensor malfunction. Salinity was estimated using a linear relationship established on the three other sites between salinity and alkalinity (salinity = 16.1 × alkalinity (mmol C L−1) - 7.1; R2 = 0.99; n = 39). The maximum difference at sites A, B and D during the dry season between calculated and probe measured salinity was 1.8, which was low compared to the amplitude of the salinity gradient (7–25). We used these calculated values for establishing relationships between salinity and POC, %POC, and FCO2 and for associated graphical representations. Current velocity and water depth were measured every hour with a Global Water® FP 101 flow meter handled 30 cm below water surface and a Plastimo® Echotest II depth sounder directed towards the bottom. Air temperature, relative humidity, wind velocity and precipitation were measured using a Hobo® weather station mounted on the sampling boat (4.5 m above water level).
We measured pCO2 at 1-s intervals using a marble-type equilibrator made of a 1.8 L Plexiglas® cylinder (inner diameter 8 cm; height 40 cm) filled with glass marbles to increase gas exchange between the flowing water and the headspace air (Frankignoulle et al. 2001, Yoon et al. 2016). Water was pumped from 30 cm below water surface at a constant rate of 1 L min−1 and injected at the top of the equilibrator, while a similar volume of air connected to a closed air loop was flowing from bottom to the top. The closed air loop was connected to a Li-Cor Biosciences®infrared gas analyser (IRGA) after passing through a silica gel container and a particle filter. We measured pCO2 using an IRGA model LI-8100A at all sites during the dry season and at site A during the wet season, and we used an IRGA model LI 840 at site B, C and D during the wet season. Infrared gas analysers were calibrated before each survey with pure nitrogen (0 μatm CO2) and two CO2 standards (545 ± 11 and 2867 ± 58 μatm CO2) manufactured by Air Liquide®.
We measured FCO2 using a floating chamber anchored to the sampling boat. Every 2 h, five measurements of FCO2 were performed with an incubation period of 5–6 min. The chamber was made of a plastic waste bin (top radius 40 cm; bottom radius 45 cm; height 55 cm) surrounded by an inner tube and weighted with a 5 kg metal chain to increase stability (inner air volume 48.2 L; air–water contact surface 0.152 m2). A closed air loop was connected from the chamber to a Li-Cor Biosciences® IRGA (model LI 820) after passing through a silica gel container and a particle filter. Due to commercial shipping on the estuary and night work it was not practical to freely drift during measurements. We only present measurements from periods when current velocity was under 0.2 m s−1, about 20% of the time. This value of current velocity was considered as critical by Lorke et al. (2015) and our results confirmed such observations (see Electronic supplementary material).
Three replicates of surface water were sampled every 2 h (13 samplings per 24 h time series) using a 10 L plastic bucket. Depending on turbidity, 250 mL to 1.2 L of water was immediately vacuum-filtered through pre-combusted (5 h at 450 °C) and pre-weighted glass fibre filters (Whatman® GF/F 0.7 μm), collecting 10–80 mg of suspended particulate matter (SPM). Filtered water was stored in 40 mL Falcon® tubes for further titration of total alkalinity (TAlk). During the wet season, one sample of unfiltered water was positively filtered through 0.2 μm Sartorius® cellulose acetate syringe filters (to reduce air–water gas exchanges) and stored leaving no air space or bubbles in 23 mL glass vials for further determination of δ13C of dissolved inorganic carbon (δ13CDIC). Filters were preserved at − 25°C until analysis of particulate organic carbon (POC) and water samples were stored at 4 °C until determination of TAlk and δ13CDIC. Results of replicated measurements were averaged before statistical analyses.
Analytical methods and calculations
We calculated the relative dissolved oxygen saturation (% DO) in the water using the ratio of measured oxygen concentration and oxygen solubility at the same salinity and temperature, according to the relations provided by Benson and Krause (1984).
We determined TAlk by Gran electrotitration with 50 μL increments of HCl 0.01 N (accuracy estimated at ± 0.01 mmol L−1). We calculated DIC concentration using pH and TAlk, according to the relations provided by Park (1969) and assuming that TAlk ≈ carbonate alkalinity. Considering the estimated accuracy of TAlk, an uncertainty of 0.2 pH and negligible effects of uncertainties in salinity and temperature, the accuracy of DIC calculations was > 0.30 mmol L−1 at site A during the wet season, and > 0.15 mmol L−1 elsewhere. Dissociation constants of carbonic acid were obtained using the equations provided by Millero et al. (2006) and updated by Millero (2010) for estuarine waters, taking into account salinity and temperature. The carbonate system equilibrium constants (Park 1969) were employed to calculate pCO2 during the wet season, using pH and TAlk.
We analysed δ13CDIC only during the wet season at the stable isotopes laboratory of the Geotop (Géochimie Isotopique) research centre at the Université du Québec in Montréal (Canada). A few mL of sample water was transferred into a 3 mL glass vial using a syringe through a septum. Glass vials were previously filled with 12 drops of 100% phosphoric acid (H3PO4), and purged with helium. Headspace CO2 originating from acidification was analysed using a Micromass MicroGasTM system coupled to an Isoprime 100TM Isotope Ratio Mass Spectrometer (IRMS) in continuous flow mode. Replicate δ13CDIC measurements from similar samples yielded an overall analytical uncertainty < 0.1‰. We constructed a theoretical mixing line between fresh and marine waters as described in Bouillon et al. (2011):
where DIC is the concentration of dissolved inorganic carbon, δ13CDIC is the carbon stable isotope ratio of dissolved inorganic carbon, Sal represents salinity, and the subscripts refer to the marine endmember (M), the freshwater endmember (F), and the sampling point along the salinity gradient (S). Dissolved inorganic isotopic compositions and concentrations at the riverine and marine endmembers were taken at site A at salinity ~ 0 and at site D at salinity ~ 26.
Particulate organic carbon was analysed at the Institut de Recherche pour le Développement in Nouméa (New Caledonia) with a Shimadzu® TOC-L series analyser using a 680 °C combustion catalytic oxidation method. The analyser was combined with a solid sample module (SSM-5000A) for measurements of POC, and a 40% glucose standard was used for calibrations. Repeated measurements of the standard at different concentrations indicated a measurement deviation < 2%.
FCO2 values were determined using the slope of gas partial pressure in the chamber versus time and taking into account air temperature, using the following equation:
where FCO2 is the water–air CO2 flux (μmol m−2 s−1), δpCO2 / δt is the variation of pCO2 versus time (μatm s−1), V is the total volume of the chamber (m3), R is the ideal gas constant (atm m3 K−1 mol−1), T is the absolute air temperature (K), and S is the incubation chamber contact surface (m2).
We determined the gas transfer velocity k using direct measurements of ΔpCO2 and FCO2 and calculation of α (Weiss 1974). The empirical equation established to express CO2 fluxes as a function of salinity, temperature and pCO2 is on the form:
where FCO2 is the water–air CO2 flux (mol m−2 day−1), α is the CO2 solubility coefficient (mol m−3 atm−1) (Weiss 1974), k is the gas transfer velocity (m d−1) and ΔpCO2 is the difference in partial pressure of CO2 between water and the overlying atmosphere (atm). We then normalised k to a Schmidt number of 600 (Sc = 600 for CO2 at 20 °C in freshwater) for further comparisons with published values:
where Sc is the Schmidt number of CO2 at a given temperature and salinity and raised to the power − 0.5 in turbulent systems (Wanninkhof 1992). Constants for the Schmidt number calculation were provided for salinity 0 and 35 by Wanninkhof (1992) and compared to our measured salinities assuming a conservative mixing ratio between fresh and marine waters (Borges et al. 2004). Empirical gas exchange velocities were calculated according to Wanninkhof (1992), using wind velocity at 10 m high (compiled using the Amorocho and DeVries (1980) relationship) and water temperature (velocities named W92 in the following discussion), and Rosentreter et al. (2017), using wind velocity at 10 m high, water depth and current velocity (velocities named R17 in the following discussion).
We considered that site A during the wet season was the freshwater endmember of the estuary, with salinity close to zero showing no mixing with marine waters. On the opposite, site D during both seasons represented the marine endmember. A simple two source mixing of freshwater and seawater could then be used to identifiy possible lateral inputs of DIC to the estuary. We used analysis of covariance (ANCOVA) to test whether a given monitored parameter was affected by the estuarine transit or the season, considering salinity as a quantitative variable and season as a qualitative variable. Residuals distribution was tested for normality and data were log-transformed when necessary. The Fisher statistic (F-test) was reported and subscript numbers indicate variables and residuals degrees of freedom. We used one factor linear regressions to calculate intercept and slope coefficients of the relationships between a given monitored parameter and salinity or SPM. For statistical tests, the criterion for rejecting the null hypothesis (alpha value) was set to 5%. Probe measurements with different logging intervals (salinity, pH, DO, pCO2 and weather parameters) were recalculated on a given timeline using smooth.spline function with R (R Core Team 2017) and setting a time lapse corresponding to that of the water sampling or the chamber flux measurements. For statistical analysis and graphical representations involving water parameters, a step of 2 h was set (13 values per 24 h), except for pCO2 where a step of 20 min was set to increase precision of individual site representations. Statistical analyses and graphical representations were performed using R (R Core Team 2017) and all data used in the manuscript were released as supplementary material (see Electronic supplementary material).
Results
Physico-chemical parameters
During the 24 h time series, daytime air temperature reached ~ 29 °C in January–February (dry season) and ~ 32 °C in September–October (wet season). At night, air temperature dropped to ~ 20 °C during the dry season, and to ~ 24 °C during the wet season. During both seasons, wind speed ranged from 0 to 10 m s−1, with 90% of values under 4.3 m s−1 during the dry season and under 3.6 m s−1 during the wet season. The main difference between seasons was heavy rainfall events generally occurring in the afternoon or early night (wet season) and reaching 50 mm h−1 during short periods of time (1–2 h).
Salinity, temperature, pH and DO saturation in water varied significantly over the 24 h time series and were synchronised with tidal cycles. Salinity along the 40 km of the Can Gio mangrove estuary ranged from 7 to 26 during the dry season, and from 0 to 25 during the wet season (Fig. 2). Water temperature ranged from 26 to 29 °C during the dry season and from 29 to 31 °C during the wet season. Both pH and DO saturation increased linearly with salinity and this relationship was significantly affected by season (ANCOVA; F2,88 = 738.9 and 444.9; psalinity < 0.001 for both, pseason < 0.001 for both; R2 > 0.9 for both). Lower DO saturation and higher pH were measured during the wet season for a given salinity, with a mean difference of - 10.4% in DO saturation and + 0.1 in pH.
Carbon pools
Means and ranges of carbon pools are provided in Table 1. Dissolved inorganic carbon was the dominant carbon pool in the Can Gio mangrove estuary. It increased linearly with salinity and this relationship was significantly affected by season (ANCOVA; F2,88 = 1620; psalinity < 0.001 and pseason < 0.01; R2 = 0.97; Fig. 3a). Higher DIC concentrations were measured during the wet season for a given salinity, with a mean difference of + 0.05 mmol L−1. δ13C isotopic signature of DIC ranged from − 15.4 to − 4.7‰ during the wet season, with lowest values measured at lowest salinity (Fig. 3b). The patterns of the theoretical mixing line of δ13CDIC and measurements matched over the entire salinity gradient (Fig. 3b).
Particulate organic carbon was not significantly affected by salinity but it was affected by season (ANCOVA; F2,94 = 4.27; psalinity = 0.46 and pseason = 0.02; Fig. 4a). Higher POC concentrations were measured during the wet season for a given salinity, with a mean difference of + 0.04 mmol L−1. Suspended particulate matter was not significantly affected either by salinity or season (ANCOVA; F2,101 = 1.72; psalinity = 0.08 and pseason = 0.81) but percentage of POC in SPM decreased significantly with salinity with no effect of season (ANCOVA with log-transformed %POC values; F2,94 = 131.3; psalinity < 0.001 and pseason = 0.16; R2 = 0.74; Fig. 4b). Particulate organic carbon thus increased significantly with SPM concentration and an effect of season was measured (ANCOVA; F2,94 = 336.9; psalinity < 0.001 and pseason < 0.001; Fig. 5). Higher POC concentrations were measured during the wet season for a given SPM concentration, with a mean difference of + 0.04 mmol L−1.
Partial pressure of CO2 ranged from 660 to 3000 μatm during the dry season, and from 740 to 5000 μatm during the wet season (Figs. 6 and 7). It decreased linearly with salinity and higher values were measured during the wet season for a given salinity (ANCOVA; F2,74 = 71.5; psalinity < 0.001 and pseason < 0.001). However, we could not measure pCO2 at site B and C during the dry season due to technical problems and the explanatory power of the linear regression based on wet season values only was very low (R2 = 0.47). Difference in pCO2 between low and high tides during the wet season seemed to depend on the tidal stage rather than on salinity and to be affected by tidal amplitude. It was close to 2000 μatm when the tidal coefficient was under 80 (sites A, C and D) and reached 3000 μatm during a strong spring tide (site B; Table 1 and Fig. 7). Calculated pCO2 notably differed from measured values, especially at site A where values were far above measured pCO2, while effects of tidal-driven stage remained similar (Fig. 7).
Distribution of CO2 partial pressure (µatm) and salinity during the wet season 24 h tidal cycles in the Can Gio mangrove estuary. Continuous data were discretised using a 20 min step (73 values per 24 h tidal cycles). Calculated pCO2 (µatm) was computed using pH and TAlk, according to the carbonate system equilibrium constants (Park 1969). Letters at the upper lefthand corner indicate the sampling site
Water–air CO2 fluxes
Emissions of CO2 at low current velocity (< 0.2 m s−1) ranged from 74 to 792 mmol m−2 day−1 during the dry season, and from 128 to 876 mmol m−2 day−1 during the wet season (Fig. 8a). A linear relationship was measured between FCO2 and salinity, with no effect of season (ANCOVA; F2,20 = 27.7; psalinity < 0.001 and pseason = 0.19; Fig. 8a). Corresponding k600 ranged from 6.4 to 11.0 m day−1 during the dry season and from 1.7 to 10.9 m day−1 during the wet season (Fig. 8b), with significantly higher values obtained during the dry season (Student test; t20 = 2.46; p = 0.02). Both empirical gas exchange velocities (W92 and R17) differed considerably from our experimental values, yielding lower k600 values, always remaining under 7 m day−1 for W92 (on average 3.9 ± 2.4 times lower than measured k600; n = 17) and under 4 m day−1 for R17 (on average 2.2 ± 1.0 times lower than measured k600; n = 17).
Discussion
Physico-chemical functioning of the estuary
The relatively low salinity at the marine endmember of the Can Gio estuary (site D), not exceeding 26, indicates a strong dilution of coastal waters by riverine inputs, most probably due to the close presence of the Mekong delta. Our mangrove estuary is highly impacted by an intense anthropogenic pressure in the higher watershed of the Saigon–Dong Nai Rivers, as observed by Strady et al. (2017). Elevated inputs of labile organic matter enhance oxygen consuming decomposition processes, as confirmed by exceptionnaly low levels of DO saturation (down to 17% at site A during the wet season; Fig. 2b). We expect these processes to contribute to elevated pCO2 levels in the water column and to cause high CO2 emissions at the water–air interface in the estuary.
Carbon distribution and speciation
The distribution of carbon forms in the estuary confirms the contribution of anthropogenic inputs to organic carbon loads. Particulate organic carbon concentrations in the estuary (0.05–0.50 mmol L−1; Table 1) were within the range reported for large Asian rivers by Le et al. (2017), with values usually ranging from 0.01 to 0.75 mmol L−1. Concentrations were higher during the wet season most probably because of increased runoff and soils leaching.
A relationship between POC and SPM (Fig. 5) is a general feature in estuaries and the regression coefficient between both parameters in the present study is similar to that obtained by Le et al. (2017) in Northern Vietnam. As a result, POC concentrations were closely related to SPM concentrations, which were themselves driven by a well-known cycle of suspension, flocculation, settling, deposition, erosion, and resuspension (Verney et al. 2009). In our study, we measured highest SPM values just after the maximum current velocity was reached (see Electronic supplementary material), most probably as a result of resuspension. We thus focused on the evolution of the POC contribution to the SPM pool. Percentage of POC in SPM were higher whatever the season (1.26–5.05%; Fig. 4b) than the average %POC of tropical Asian rivers (1.23%; Huang et al. 2012). Since Can Gio district is located downstream the densely populated Ho Chi Minh City, elevated %POC values at the freshwater endmember of the estuary are probably due to anthropogenic inputs of organic matter (Strady et al. 2017). In benthic sediments of the Saigon–Dong Nai Rivers, Minh et al. (2007) measured the highest %POC in Ho Chi Minh City urban canals, upstream the mangrove area, and values decreased towards the South China Sea, reinforcing the idea that the contribution of POC from the urban area had more effect on %POC than leaching from mangrove soils. The non-linear decrease of %POC along the salinity gradient of the Can Gio mangrove estuary suggests that organic matter is removed from the system during water transit rather than just diluted with marine waters, possibly because of sedimentation or decomposition. The most labile organic fraction is probably rapidly decomposed when entering the estuarine waters, enriched in oxygen compared to the urban waters, inducing the release of CO2 within the water column, which can be further emitted to the atmosphere. Only the most refractory organic matter fraction would thus reach the South China Sea.
Dissolved inorganic carbon concentration at the freshwater endmember of the estuary (0.7–0.8 mmol L−1; Fig. 3a) was low compared to other tropical Asian estuaries, usually above 1 mmol L−1 (Huang et al. 2012; Li et al. 2013), and compared to mangrove creeks of the Ca Mau province (Southern Vietnam; Koné and Borges 2008). Dissolved inorganic carbon concentrations in rivers vary widely depending on the catchment geology and weathering rates (Guo et al. 2008; Bouillon et al. 2011). The Saigon–Dong Nai Rivers basin is dominated by igneous rocks likely to have a low carbonate content (Vietnam geological map available here: http://urlz.fr/4hM7). Biogeochemical processes such as biological uptake (photosynthesis) and CO2 degassing to the atmosphere (Guo et al. 2008) also lower DIC concentration.
The carbon isotopic signature of DIC was down to − 15.4‰ at the freshwater endmember (site A at salinity ~ 0; Fig. 3b), suggesting that organic carbon respiration is the dominant source of DIC in the Can Gio mangrove estuary. The δ13CDIC mostly depends on a combination of weathering rate of mineral carbonates with δ13C ~ 0, organic carbon respiration producing DIC with similar δ13C to that of the organic carbon source, and removal of CO2 with δ13C values 7 to 10‰ lower than that of HCO3− (Finlay 2003; Miyajima et al. 2009). In a simplified form, inputs of highly δ13C-depleted CO2 from heterotrophic respiration (− 30 to − 25‰ in river waters and sediments; Miyajima et al. 2009) would decrease δ13CDIC, and CO2 emissions would increase δ13CDIC because of preferential release of 13C depleted CO2. In our study, DIC entering the estuary had δ13C values closer to those obtained by heterotrophic respiration than to those due to mineral carbonate weathering.
Measurements of DIC and δ13CDIC showed nearly conservative patterns along the salinity gradient of the Can Gio mangrove estuary (Fig. 3), thus rejecting our hypothesis that DIC inputs from the mangrove ecosystem could be traced at the scale of this large estuary. Lateral inputs from mangrove soils are generally richer in DIC with more depleted δ13C values than nearby estuarine waters (Miyajima et al. 2009; Abril et al. 2013; Maher et al. 2013). We expected such inputs to have maintained the δ13CDIC below the theoretical mixing line during the water transit, as observed by Miyajima et al. (2009) in the Khura mangrove dominated estuary in Thailand. In the present study, DIC releases from surrounding mangrove soils appeared to have contributed little to the inorganic carbon enrichment of the water column.
Both direct measurements and calculated values of pCO2 substantially varied during tidal cycles along the salinity gradient of the Can Gio mangrove estuary (Fig. 7). In acidic, organic-rich freshwaters (site A), calculation using pH and TAlk probably overestimated pCO2 (Abril et al. 2015). At the sites located in the mangrove area (B, C and D), the comparison of both approaches suggests that CO2 was added to the water column during ebb, perhaps from lateral and groundwater inputs, whether from aquaculturally altered floodplain or mangrove sediments (Borges and Abril 2011; Atkins et al. 2013; Leopold et al. 2017). Then, CO2 may be emitted to the atmosphere (Atkins et al. 2013; Müller et al. 2016; Leopold et al. 2017) or used by autotrophic organisms (Borges and Abril 2011; Li et al. 2013). Autotrophic production would, however, induce day/night variations in DO saturation, which we did not observe in the estuary, suggesting that CO2 losses are mostly due to water–air emissions. During ebb, measured pCO2 was usually greater than the calculated values of pCO2 from carbonate system equilibrium. During the flood it tended to be below, especially at site B and D that were monitored during strong spring tides (Fig. 7 and Table 1). We purposely chose to monitor the four sites under different tidal conditions, which may have affected the tidal pumping and the magnitude of inputs from the mangrove ecosystem (Call et al. 2015). At the middle of ebb tide, the measured pCO2 rose more slowly, indicating that CO2 inputs were lower or losses were higher during this stage. This phenomenon was particularly pronounced at site B, during a strong spring tide (tidal coefficient 115; Table 1), and when the maximum current velocity reached 1.3 m s−1 (see Electronic supplementary material). We did not expect inputs to be lower at the middle of the ebb tide compared to the beginning and the end of this period. However, current velocity was maximum at this moment and water turbulence created by current velocity may increase CO2 emissions in estuaries (Borges et al. 2004; Ho et al. 2016).
Nevertheless, dissolved CO2 constituted only a small fraction of DIC, ranging from 19 to 133 µmol L−1 after conversion using Henry’s law ([CO2 (aq)] = K0 × pCO2; Weiss 1974). It represented on average 10–1% of DIC, respectively at the riverine and marine endmembers of the estuary and thus even though tidal variations of pCO2 were evident, their effect on DIC was low.
CO2 emissions at the air–water interface
The distribution of FCO2 along the salinity gradient of the estuary reflected the values of pCO2 during the transition from fresh to ocean waters. High quantities of dissolved CO2 are brought at the freshwater endmember of the estuary as well as organic matter, thus leading to high emission rates, while the system tends towards equilibrium with atmospheric pCO2 by the mouth of the estuary.
Emissions of CO2 in the Can Gio mangrove estuary were about 8 times higher than average values reported for Northern hemisphere low latitude estuaries (on average 306.3 vs. 38.9 mmol m−2 day−1 in the review of Chen et al. (2013); Fig. 8a). They were about 7 times higher than the revised global CO2 flux rate from mangrove waters (56.8 ± 8.9 mmol m−2 day−1) calculated by Rosentreter et al. (2018). Similar FCO2 to those measured in our study were observed in two macrotidal estuaries in Western Sarawak (Malaysia), where CO2 fluxes were determined with a floating chamber, and estimated to range from 38.4 to 734.2 mmol m−2 day−1 (Müller et al. 2016). In the Hugli estuary (India), relatively similar to the Can Gio mangrove estuary in terms of size and also highly impacted by anthropogenic pressure, Akhand et al. (2016) calculated an average FCO2 of 88.8 mmol m−2 day−1 using the equation of Ho et al. (2011). The use of floating chambers has been a matter of debate since it may create artificial turbulence at the water–air interface, increasing FCO2 (Matthews et al. 2003; Lorke et al. 2015). Floating chambers are, however, more susceptible to disruption in low turbulence environments compared to high turbulence environments (Vachon et al. 2010). We intended to reduce artificial turbulences by using a large volume floating chamber with a wall extending into the water and weighted to increase stability. In addition, we only included FCO2 measured at low current velocity, reducing the disruptions induced by chamber anchorage (Lorke et al. 2015). We thus feel confident about the validity of our measurements.
Most CO2 emission measurements available have been calculated using pCO2 and the gas transfer velocity k (Chen et al. 2013). Published values of k600 are, however, based on an empirical calculation, using equations established for temperate systems (Raymond and Cole 2001; Borges et al. 2004) or for the ocean-air interface (Wanninkhof 1992). The estimation of k has been of major interest over the last decades and various authors have sought for variables to constrain the predictions of FCO2. The gas transfer velocity is affected by velocity of wind and current, water depth and turbidity (Wanninkhof 1992; Abril et al. 2009; Ho et al. 2011; Rosentreter et al. 2017). In the present study, we could not correctly predict k using only those parameters, suggesting that other factors may also affect the gas transfer velocity, such as organic carbon load, tidal stage, day/night cycle or cargo traffic. Wind velocity is usually considered a crucial parameter influencing water–air exchanges, especially in low turbulence environments (Wanninkhof 1992). It was not especially high in the Can Gio mangrove estuary and may not strongly affect gas exchange rates compared to other parameters affecting turbulence. In addition, monitoring the four sites under different tidal intensity could also have affected CO2 emissions and a larger dataset would be necessary to partition the effect of each factor. Nevertheless, our values of k600 (Fig. 8b) remained on average 2 to 4 times higher than empirical gas exchange velocities (W92 and R17), suggesting that CO2 emissions would have been underestimated using available relationships. In tropical estuaries, high temperatures, in addition to decreased CO2 solubility in the water (Müller et al. 2016), may induce enhanced organic matter decomposition and thus CO2 production, which can partially explain the high FCO2. Moreover, the watershed of Can Gio mangrove estuary is strongly impacted by anthropogenic pressure and the elevated inputs of labile organic carbon may have increased CO2 exchanges. We thus show that the use of floating chambers could help to refine the carbon budget of tropical coastal ecosystems with high organic matter load.
Conclusions
Low DO saturation and high pCO2 in the Can Gio mangrove estuary reflect the anthropogenic pressure upstream of the estuary. Contrary to our hypothesis, DIC releases from surrounding mangrove soils contributed little to the inorganic carbon enrichment of the water column. However, our study highlights that during ebb tide CO2 inputs from adjacent wetlands substantially contributed to the water column pCO2 enrichment and moved the carbonate system towards disequilibrium relative to pCO2 calculated from TAlk and pH. Although the errors in the calculation of pCO2 could be relatively large due to the method employed to obtain TAlk and pH, there is no reason to think that this error increases or decreases according to the tidal stage. We thus believe that the difference between measured and calculated values of pCO2 reflect changes in the carbonate system equilibrium. Our study shows that inputs from mangrove soils affect the water column pCO2 at the scale of a large tropical estuary (~ 600 m wide and 10–20 m deep), as previously observed in smaller mangrove creeks (Abril et al. 2013; Atkins et al. 2013; Call et al. 2015). In addition, we showed that pCO2 has to be studied using direct measurements in such highly dynamic and anthropogenically influenced environments.
The Can Gio mangrove estuary is a source of CO2 to the atmosphere, with highest emissions near the inflowing river. These high CO2 emissions might be due to both the high water temperature of our estuary (26 to 31 °C) and the high organic load (up to 5% of POC in SPM). We showed that emissions under low current velocity (< 0.2 m s−1) would have been strongly underestimated (2 to 4 times) using available published equations to estimate the gas transfer velocity k600. Our results lead us to conclude that CO2 emissions from large tropical estuaries, especially those highly affected by human activities, have been underestimated. The use of floating chambers may help to refine carbon budgets, allowing direct measurements of water–air CO2 emissions.
References
Abril G, Commarieu M-V, Sottolichio A et al (2009) Turbidity limits gas exchange in a large macrotidal estuary. Estuar Coast Shelf Sci 83:342–348. https://doi.org/10.1016/j.ecss.2009.03.006
Abril G, Deborde J, Savoye N et al (2013) Export of 13C-depleted dissolved inorganic carbon from a tidal forest bordering the Amazon estuary. Estuar Coast Shelf Sci 129:23–27. https://doi.org/10.1016/j.ecss.2013.06.020
Abril G, Bouillon S, Darchambeau F et al (2015) Technical Note: Large overestimation of pCO2 calculated from pH and alkalinity in acidic, organic-rich freshwaters. Biogeosciences 12:67–78. https://doi.org/10.5194/bg-12-67-2015
Akhand A, Chanda A, Manna S et al (2016) A comparison of CO2 dynamics and air–water fluxes in a river-dominated estuary and a mangrove-dominated marine estuary. Geophys Res Lett. https://doi.org/10.1002/2016GL070716
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Ann Rev Mar Sci 6:195–219. https://doi.org/10.1146/annurev-marine-010213-135020
Amorocho J, DeVries JJ (1980) A new evaluation of the wind stress coefficient over water surfaces. J Geophys Res Ocean 85:433–442. https://doi.org/10.1029/JC085iC01p00433
Atkins ML, Santos IR, Ruiz-Halpern S, Maher DT (2013) Carbon dioxide dynamics driven by groundwater discharge in a coastal floodplain creek. J Hydrol 493:30–42. https://doi.org/10.1016/j.jhydrol.2013.04.008
Benson BB, Krause D (1984) The concentration and isotopic fractionation of oxygen dissolved in freshwater and seawater in equilibrium with the atmosphere. Limnol Oceanogr 29:620–632
Borges AV, Abril G (2011) 5.04-Carbon dioxide and methane dynamics in estuaries. In: Eric W, Donald M (eds) Treatise on estuarine and coastal science. Academic Press, Amsterdam, pp 119–161
Borges AV, Vanderborght J-P, Schiettecatte L-S et al (2004) Variability of the gas transfer velocity of CO2 in a macrotidal estuary (the Scheldt). Estuaries 27:593–603. https://doi.org/10.1007/BF02907647
Bouillon S, Borges AV, Castañeda-Moya E et al (2008) Mangrove production and carbon sinks: a revision of global budget estimates. Global Biogeochem Cycles. https://doi.org/10.1029/2007GB003052
Bouillon S, Connolly RM, Gillikin DP (2011) Use of stable isotopes to understand food webs and ecosystem functioning in estuaries. In: Wolanski E, McLusky DS (eds) Treatise on estuarine and coastal science. Elsevier, Waltham, pp 143–173
Cai W-J (2011) Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Ann Rev Mar Sci 3:123–145. https://doi.org/10.1146/annurev-marine-120709-142723
Call M, Maher DT, Santos IR et al (2015) Spatial and temporal variability of carbon dioxide and methane fluxes over semi-diurnal and spring–neap–spring timescales in a mangrove creek. Geochim Cosmochim Acta 150:211–225. https://doi.org/10.1016/j.gca.2014.11.023
Chen C-TA, Huang T-H, Chen Y-C et al (2013) Air–sea exchanges of CO2 in the world’s coastal seas. Biogeosciences 10:6509–6544. https://doi.org/10.5194/bg-10-6509-2013
Finlay JC (2003) Controls of streamwater dissolved inorganic carbon dynamics in a forested watershed. Biogeochemistry 62:231–252
Frankignoulle M, Borges A, Biondo R (2001) A new design of equilibrator to monitor carbon dioxide in highly dynamic and turbid environments. Water Res 35:1344–1347. https://doi.org/10.1016/S0043-1354(00)00369-9
Guo X, Cai W-J, Zhai W et al (2008) Seasonal variations in the inorganic carbon system in the Pearl River (Zhujiang) estuary. Cont Shelf Res 28:1424–1434. https://doi.org/10.1016/j.csr.2007.07.011
Ho DT, Schlosser P, Orton PM (2011) On factors controlling air–water gas exchange in a large tidal river. Estuaries Coasts. https://doi.org/10.1007/s12237-011-9396-4
Ho DT, Coffineau N, Hickman B et al (2016) Influence of current velocity and wind speed on air–water gas exchange in a mangrove estuary: gas Exchange in a Mangrove Estuary. Geophys Res Lett 43:3813–3821. https://doi.org/10.1002/2016GL068727
Hofmann EE, Cahill B, Fennel K et al (2011) Modeling the dynamics of continental shelf carbon. Ann Rev Mar Sci 3:93–122. https://doi.org/10.1146/annurev-marine-120709-142740
Huang T-H, Fu Y-H, Pan P-Y, Chen C-TA (2012) Fluvial carbon fluxes in tropical rivers. Curr Opin Environ Sustain 4:162–169. https://doi.org/10.1016/j.cosust.2012.02.004
Koei N (1996) The master plan study on Dong Nai River and surrounding basins water resources development. Final Report. Vol. 9. Appendix VIII. Flood mitigation and urban drainage. Nippon Koei, Tokyo
Koné YJ-M, Borges AV (2008) Dissolved inorganic carbon dynamics in the waters surrounding forested mangroves of the Ca Mau Province (Vietnam). Estuarine Coastal Shelf Sci. https://doi.org/10.1016/j.ecss.2007.10.001
Le TPQ, Dao VN, Rochelle-Newall E et al (2017) Total organic carbon fluxes of the Red River system (Vietnam): TOC fluxes of the Red River. Earth Surf Proc Land. https://doi.org/10.1002/esp.4107
Lee SY (2016) From blue to black: anthropogenic forcing of carbon and nitrogen influx to mangrove-linedestuaries in the South China Sea. Mar Pollut Bull 109:682–690. https://doi.org/10.1016/j.marpolbul.2016.01.008
Leopold A, Marchand C, Deborde J, Allenbach M (2017) Water biogeochemistry of a mangrove-dominated estuary under a semi-arid climate (New Caledonia). Estuaries Coast 40:773. https://doi.org/10.1007/s12237-016-0179-9
Li S, Lu XX, Bush RT (2013) CO2 partial pressure and CO2 emission in the Lower Mekong River. J Hydrol 504:40–56. https://doi.org/10.1016/j.jhydrol.2013.09.024
Lorke A, Bodmer P, Noss C et al (2015) Technical note: drifting versus anchored flux chambers for measuring greenhouse gas emissions from running waters. Biogeosciences 12:7013–7024. https://doi.org/10.5194/bg-12-7013-2015
Maher DT, Santos IR, Golsby-Smith L et al (2013) Groundwater-derived dissolved inorganic and organic carbon exports from a mangrove tidal creek: the missing mangrove carbon sink? Limnol Oceanogr 58:475–488. https://doi.org/10.4319/lo.2013.58.2.0475
Matthews CJD, St.Louis VL, Hesslein RH (2003) Comparison of three techniques used to measure diffusive gas exchange from sheltered aquatic surfaces. Environ Sci Technol 37:772–780. https://doi.org/10.1021/es0205838
Millero FJ (2010) Carbonate constants for estuarine waters. Mar Freshw Res 61:139. https://doi.org/10.1071/MF09254
Millero FJ, Graham TB, Huang F et al (2006) Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Mar Chem 100:80–94. https://doi.org/10.1016/j.marchem.2005.12.001
Minh NH, Minh TB, Iwata H et al (2007) Persistent organic pollutants in sediments from SaiGon–Dong Nai River basin, vietnam: levels and temporal trends. Arch Environ Contam Toxicol 52:458–465. https://doi.org/10.1007/s00244-006-0157-5
Miyajima T, Tsuboi Y, Tanaka Y, Koike I (2009) Export of inorganic carbon from two Southeast Asian mangrove forests to adjacent estuaries as estimated by the stable isotope composition of dissolved inorganic carbon. J Geophys Res. https://doi.org/10.1029/2008JG000861
Müller D, Warneke T, Rixen T et al (2016) Fate of terrestrial organic carbon and associated CO2 and CO emissions from two Southeast Asian estuaries. Biogeosciences 13:691–705. https://doi.org/10.5194/bg-13-691-2016
Nam VN, Sinh LV, Miyagi T et al (2014) An overview of Can Gio district and mangrove biosphere reserve. In: Miyagi T (ed) Studies in Can Gio mangrove biosphere reserve. Tohoku Gakuin University, Ho Chi Minh City
Park PK (1969) Oceanic CO2 system: an evaluation of ten methods of investigation. Limnol Oceanogr 14:179–186. https://doi.org/10.4319/lo.1969.14.2.0179
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.URL https://www.R-project.org/
Raymond PA, Cole JJ (2001) Gas exchange in rivers and estuaries: choosing a gas transfer velocity. Estuaries Coast 24:312–317. https://doi.org/10.2307/1352954
Regnier P, Friedlingstein P, Ciais P et al (2013) Anthropogenic perturbation of the carbon fluxes from land to ocean. Nat Geosci 6:597–607. https://doi.org/10.1038/ngeo1830
Ringler C, Cong NC, Huy NV (2002) Water allocation and use in the Dong Nai River basin in the context of water institution strengthening. In: Integrated water-resources management in a River-basin context: institutional strategies for improving the productivity of agricultural water management, p 215
Rosentreter JA, Maher DT, Ho DT, Call M, Barr JG, Eyre BD (2017) Spatial and temporal variability of CO2 and CH4 gas transfer velocities and quantification of the CH4 microbubble flux in mangrove dominated estuaries: gas transfers in estuaries. Limnol Oceanogr 62:561–578. https://doi.org/10.1002/lno.10444
Rosentreter JA, Maher DT, Erler DV et al (2018) Seasonal and temporal CO2 dynamics in three tropical mangrove creeks: a revision of global mangrove CO2 emissions. Geochim Cosmochim Acta. https://doi.org/10.1016/j.gca.2017.11.026
Strady E, Dang VBH, Némery J et al (2017) Baseline seasonal investigation of nutrients and trace metals in surface waters and sediments along the Saigon River basin impacted by the megacity of Ho Chi Minh (Vietnam). Environ Sci Pollut R 24:3226–3243. https://doi.org/10.1007/s11356-016-7660-7
Tuan VQ, Kuenzer C (2012) Can Gio mangrove biosphere reserve evaluation, 2012: current status, dynamics, and ecosystem services. IUCN Viet Nam Country Office, Hanoi
Vachon D, Prairie YT, Cole JJ (2010) The relationship between near-surface turbulence and gas transfer velocity in freshwater systems and its implications for floating chamber measurements of gas exchange. Limnol Oceanogr 55:1723–1732. https://doi.org/10.4319/lo.2010.55.4.1723
Verney R, Lafite R, Brun-Cottan J-C (2009) Flocculation potential of estuarine particles: the importance of environmental factors and of the spatial and seasonal variability of suspended particulate matter. Estuaries Coasts 32:678–693. https://doi.org/10.1007/s12237-009-9160-1
Wanninkhof R (1992) Relationship between wind speed and gas exchange over the ocean. J Geophys Res Oceans 97:7373–7382. https://doi.org/10.1029/92JC00188
Weiss RF (1974) Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Mar Chem 2:203–215. https://doi.org/10.1016/0304-4203(74)90015-2
Yoon TK, Jin H, Oh N-H, Park J-H (2016) Technical note: assessing gas equilibration systems for continuous pCO2 measurements in inland waters. Biogeosciences 13:3915–3930. https://doi.org/10.5194/bg-13-3915-2016
Acknowledgements
The authors would like to thank the Air Liquide Foundation for providing financial support for gas analysers and samplings field trips. The support of the PEPS CNRS-IRD “Mangrove” is also gratefully acknowledged. The salary of F. David was funded by a grant from the Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation. Vietnamese students and the Can Gio mangrove management board are gratefully thanked for their help during the field trips. The anonymous reviewers and the associate editor, Stephen D. Sebestyen, greatly improved this ms., and they are gratefully thanked.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stephen D. Sebestyen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
David, F., Meziane, T., Tran-Thi, NT. et al. Carbon biogeochemistry and CO2 emissions in a human impacted and mangrove dominated tropical estuary (Can Gio, Vietnam). Biogeochemistry 138, 261–275 (2018). https://doi.org/10.1007/s10533-018-0444-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-018-0444-z