Abstract
Through the use of an enrichment technique, we isolated from the agricultural soils of Morelos in central México a strain of Burkholderia zhejiangensis identified as CEIB S4-3, it’s could use the pesticide methyl parathion (MP) as the only source of carbon and degrade completely p-nitrophenol (PNP). For more efficient MP and PNP degradation by the CEIB S4-3 strain, the absence of an extra carbon source, a large inoculum and an MP concentration up to 50 mg/l are required. Sequence and annotation analysis of the draft genome, showed presence of mpd functional gene, which was expressed and its activity on the MP was confirmed. Additionally, the genes coding for enzymes in the benzoquinone pathway (conducted by Gram-negative bacteria) and the benzenotriol pathway (conducted by Gram-positive bacteria) were found, which was corroborated by identification of intermediary metabolites by HPLC. Thus, we propose that B. zhejiangensis CEIB S4-3 uses both degradation pathways.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methyl parathion (O,O-dimethyl O-4 nitrophenyl phosphorothioate), an organophosphate pesticide, is an extremely toxic compound that is widely used in agriculture worldwide, and it is frequently found in terrestrial and aquatic environments (Papadakis et al. 2015). MP has low persistence in the environment, with reported field half-lives of 1 to 30 days in soils. However, the continuous and excessive application in addition to accidental spills allows further spread. In the environment, there are various abiotic factors that can hydrolyse MP, such as soil pH, temperature and sunlight exposure, but further degradation occurs via microbiota (Wauchope et al. 1992; Singh and Walker 2006). The physicochemical properties of MP make it susceptible to leaching and subsequent contamination of water bodies. On the other hand, because MP is metabolized by both plants and animals, it is not expected to persist or bioconcentrate (Bosco de Salles et al. 2015). However, studies have reported that Girardinichthys multiradiatus captured at the Ignacio Ramirez Dam, México, accumulated methyl parathion of more than 13,461 times in relation to water levels of this compound (De La Vega Salazar et al. 1997).
Because of its toxicity to non-target organisms and because degradation by native organisms is difficult, MP was classified as a persistent organic pollutant and an endocrine disruptor (Liu et al. 2007). The insecticidal activity of MP is the result of irreversible phosphorylation of the acetylcholinesterase enzyme (AChE), which regulates the transmission of nerve impulses. This irreversible phosphorylation of AChE results in the accumulation of the neurotransmitter acetylcholine, which leads to the overstimulation of cholinergic receptors and death (Edwards and Tchounwou 2005; Bigley and Raushel 2013).
Several microorganisms capable of hydrolysing MP have been isolated, such as a Flavobacterium sp. (Sethunathann and Yoshida 1973), Pseudomonas diminuta (Serdar et al. 1982) and Burkholderia cepacia (Ekkhunnatham et al. 2012). They possess the opd and/or mpd genes responsible for the MP hydrolysis. However, reports of bacteria that completely degrade MP are scarce (Liu et al. 2005). This is mainly due to PNP, which is the main product of the MP hydrolysis. Although PNP is less toxic in higher animals, it shows greater toxicity in soil microbiota (Errampalli et al. 1999; Yamamoto et al. 2011).
PNP is a nitroaromatic phenolic compound that is widely used in the synthesis of pesticides, medicines and dyes. In terms of the quantities used and its potential involvement in environmental pollution, it is probably the most important mononitrophenol (Subashchandrabose et al. 2012), and the Environmental Protection Agency (EPA) has classified it as one of the 129 priority pollutants (http://water.epa.gov/scitech/methods/cwa/pollutants.cfm).
Several studies indicate that there are two main routes for degradation of PNP: the benzenetriol (BT) pathway, which is carried out preferably by Gram-positive bacteria, such as Rhodococcus sp. strain PN1 (Takeo et al. 2008), and the hydroquinone (HQ) pathway, which is carried out by Gram-negative bacteria such as Stenotrophomonas sp. SMSP-1 (Shen et al. 2010). Burkholderia species are widely distributed in the environment and in several species such as Burkholderia sp. SJ98 (India), B. jiangsuensis (China) and Burkholderia sp. YI23 (South Korea). A single cluster pnpABA’E2E1FDC has been identified, which has genes that are involved in the PNP degradation pathway (Vikram et al. 2012; Lim et al. 2012; Liu et al. 2014). Although there are numerous reports about the genes and enzymes involved in this process, an analysis of these genes within the genus Burkholderia has not yet been performed.
In a previous work, our group reported a good quality draft sequencing and de novo assembly of the CEIB S4-3 strain (Hernández-Mendoza et al. 2014). In the present study, we report the isolation and identification of the Burkholderia zhejiangensis CEIB S4-3 strain, which is capable of degrading MP and PNP simultaneously. Furthermore, this is the first report of a bacterial strain that possesses one gene and two gene clusters (a complete and partial), which are involved in the MP hydrolysis and degradation of PNP, respectively. Additionally, the characterization and genetic analysis of the distribution of genes involved in MP hydrolysis and PNP degradation by the strain are discussed.
Materials and methods
Reagents and culture medium
Pesticides (analytical grade) were obtained from CHEM SERVICE, with 99.5% purity for MP and 99% for PNP. A stock solution of MP was prepared with methanol (HPLC grade) at 10,000 mg/l and MP was added to the culture media at the indicated concentrations.
To obtain biomass for the inoculum and for the growth of the strain, tryptone soy broth (TSB, Bioxon) and tryptone soy agar (TSA, Bioxon) were used. For degradation testing, mineral salts medium (MSM) was used with the following composition (g/l): 2.92 KH2PO4, 2.74 K2HPO4, 0.20 MgSO4·7H2O, 2.0 KNO3, and 0.99 (NH4)2SO4. Additionally, two ml/l of a trace element solution was added, whose composition is as follows (g/l) 2.8 H3BO3, 2.55 MnSO4·H2O, 0.20 CuSO4·5H2O, 2.43 CoCl2·6H2O, and 0.25 ZnSO4·7H2O at pH 7 (Chino-Flores et al. 2012).
Isolation of bacteria using an enrichment technique
Agricultural soils from the state of Morelos, in central Mexico, were sampled (18°58′26.0″N 99°07′05.0″W). The soils were enriched with commercial MP (50 mg/l/week) to a concentration of 200 mg/l to increase the probability of finding the pesticide-degrading microorganisms. Subsequently, one gram of these soils was added to Erlenmeyer flasks containing 50 ml of MSM with MP as a sole carbon source at a final concentration of 50 mg/l. The flasks were incubated for 7 days at 30 °C at 120 rpm. After 7 days, 500 µl of the culture was used to inoculate 50 ml of fresh MSM enriched with 50 mg/l of MP, which was then incubated under the same conditions. After three consecutive transfers in the indicated medium (3 weeks), serial dilutions were performed and were plated on TSA supplemented with MP (50 mg/l). The plates were incubated at 30 °C for 24–48 h. The well-defined colonies were selected five times to ensure purity. The purified strains were inoculated into MSM containing 50 mg/l of MP. The isolates that turned the culture medium yellow, indicating the presence of PNP, were selected for further investigation.
Degradation tests
The CEIB S4-3 strain was grown in 50 ml of TSB at 30 °C and 120 rpm, until an absorbance of 0.5 at 600 nm. The biomass was recovered by centrifugation at 5000 rpm for 20 min at 4 °C and washed 3 times with phosphate buffer (10 mM, pH 7.0). This was used as the inoculum. At various time intervals, aliquots were taken to determine the pesticide concentration and microbial growth. Uninoculated flasks were used as a control in all experiments. All treatments were performed in triplicate.
Optimization of methyl parathion degradation by the strain CEIB S4-3
To study the effect of various abiotic factors on methyl parathion degradation, appropriate modifications to the incubation systems were made. The parameters that were adjusted for analysis were: pH (4–9), temperature (25–70 °C), MP concentration (10–150 mg/l), additional carbon source (0.05 and 0.1% of glucose) and inoculum size (0.5, 1 and 2%). The pH was adjusted using citric acid-Na2HPO4, 40.0 mM, and glycine-NaOH buffer, 40 mM, for pH between 4.0 and 7.0 and 8.0 and 9.0, respectively. The conditions set for all experiments were previously reported in Wang et al. (2012). In the case of pH and temperature, Escherichia coli DH5α was used as a negative control.
Briefly, the cells were pre-incubated for 24 h at 4 °C, and the reaction mixture consisted of 945 µl of glycine-NaOH buffer (40 mM, pH 9.5), 50 µl of cells and 5 µl of MP (10,000 mg/l) as substrate. A final cell concentration of 2.36 × 1011 CFU/ml was used. The reaction was stopped after 10 min with 200 µl of 10% HNO3. Subsequently, 500 µl of the reaction was added to 5 ml of 0.5 M glycine-NaOH buffer, pH 10, to reveal the yellow colour of PNP, which was measured by spectrophotometry as described later. The relative hydrolytic activity of the cells was considered to be 100%.
Analytical methods
The MP and PNP concentration was measured using HPLC. A 1-ml aliquot was taken at different time points and centrifuged for 10 min at 10,000 rpm to remove the biomass. Subsequently, the supernatants were injected into an HPLC using a Waters 2,996,600 ultraviolet (UV) detector, a photodiode array absorbance detector and an EC 250/4 Nucleosil 120-5 C18 HPLC column. The mobile phase was methanol/water (70/30, v/v), and a flow rate of 0.8 ml/min was used. In addition, PNP was also measured by optical density (OD) on a spectrophotometer (BioMate 3, Thermo Scientific) at a wavelength of 410 nm. The analysis of intermediates in MP degradation was performed by HPLC studies. The mobile phase was methanol/water acidifies (acetic acid 1%) pH 3 (40/60) at a low rate of 1 ml/min. Compounds were identified by comparison of HPLC retention times with its standards.
Cloning and expression of the mpd gene
According to the genome sequence of the CEIB S4-3 strain (Hernández-Mendoza et al. 2014), the mpdFw 5′-GCAACTCCATATGCCCACCCACAAGGAAATCAC-3′ and mpdRev 5′-TGAATTCATCACTTGGGGTTGACGACCGAG-3′ primers were designed. In those primers, the NdeI and EcoRI restriction sites are underlined. The mpd functional gene was amplified via PCR under the following protocol: predenaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing at 62 °C for 45 seg, extension at 72 °C for 1 min, and then post-extension at 72 °C for 10 min. The amplified product has approximately 1.0 kb in length and was ligated into the vector pJET1.2 (Novagen) according to the manufacturer’s specifications and transformed into E. coli DH5α competent cells.
The plasmidic DNA was extracted from the positive clone, digested with the corresponding restriction enzymes and subcloned into the vector pET-22a(+) (Novagen), which encodes a C-terminal hexahistidine tag to give the pET28/mpd vector. The recombinant vector was transformed into E. coli BL21 (DE3) pLysS, the fragment was confirmed via PCR. A positive clone was grown in LB broth with 100 µg/ml ampicillin at 37 °C until OD600 of 0.5 was reached and was induced with 1 mM IPTG for 3 h before being harvested via centrifugation at 4000 rpm for 10 min at 4 °C. The expression of methyl parathion hydrolase (MPH) was visualized using 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE).
Enzyme assay
Enzyme activity was defined as the amount of enzyme required to release 1 µmol of PNP per minute at 30 °C. The cells were grown as described above. Packed cells were harvested, resuspended in 2 ml of 10 mM pH 7.0 phosphate buffer and lysed in a Branson Sonifier 450 sonicator for 90 s (3 s pulse and 9 s of rest), and then centrifuged at 10,000 rmp for 10 min.
The enzyme activity assay was performed according to the methodology described by Chino-Flores et al. (2012). Briefly, MP hydrolysis was measured by monitoring the production of PNP (ɛmax = 17,000 M−1 cm−1) for 15 min using MP as a substrate via a UV spectrophotometer BioMate3 Thermo Scientific. The mixture consisted of a Tris 10 mM at pH 9 buffer and 50 ppm of MP. The reaction was started via protein extract addition, which was obtained as was indicated above. Protein concentration was determined via the Bradford method using bovine serum albumin as the standard. The data are reported as specific activity (µM/min/mg protein).
Identification of intermediate metabolites
The inoculum of CEIB S4-3 strain was prepared as described above. The flasks with MSM were inoculated with the CEIB S4-3 strain until OD 0.5 at 600 nm. The medium was enriched with MP at 50 mg/L as the sole carbon source. The samples were collected at different time intervals and frozen with liquid nitrogen. Subsequently, the cells were lysed for 150 s (5 pulses of 30 s each) in an ultrasonicator (SONIC Vibra Cells). The cell free extracts were subjected to neutral, acidic and alkaline extraction with ethyl acetate, and modified from Chauhan et al. (2010). The compounds were identified by comparison with HPLC retention times of specific metabolites of MP degradation of the standards. This procedure was performed in triplicate.
Statistical analysis
An analysis of variance (ANOVA) was calculated. The mean value of each treatment was analysed by multiple comparisons of means, using Tukey’s test. The data were analysed using the SAS 9.0 (1999) statistical package (α = 0.05).
Genome sequencing and assembly
The CEIB S4-3 strain was grown in 50-ml flasks with 10 ml of TSB and allowed to incubate overnight at 30 °C, 120 rpm. The pellet was recovered via centrifugation at 13,000 rpm for 30 s. Genomic DNA extraction was performed according to the specifications of the Ultra Clean Microbial DNA Isolation Kit (MO BIO Laboratories, Inc.) and stored at −20 °C.
The CEIB S4-3 strain genome was sequenced by running Paired-End type sequencing, using the system Genome Analyzer IIx (GAIIx) (Illumina) at the University Massive DNA Sequencing Unit, Institute of Biotechnology, UNAM. Genome sequencing was achieved by generating a random data set of 34,641,784 reads that were 72 bases long. The bases with poor quality readings were eliminated using the Perl script DynamicTrim (SolexaQA ++). The genome of the strain CEIB S4-3 was de novo assembled using SPAdes (version 3.1.1) (Bankevich et al. 2012). The draft genome was lined with 41 complete genomes (chromosomes, chromids and plasmids) of the genus Burkholderia that are deposited in the GenBank database (ftp://ftp.ncbi.nlm.nih.gov/genbank/genomes/Bacteria/) using the NUCmer program (Kurtz et al. 2004).
PFGE analyses and identification of specific position of contigs
The amount and length of replicons of the Burkholderia sp. S4-3 genome was calculated using the Pulsed-Field Gel Electrophoresis (PFGE) technique with the following conditions: IS: 900″, FS: 900″, volts: 2.2, angle: 106°, buffer: TAE 1X, time: 70 h, agarose: 0.85%, and temperature: 14 °C. The specific position of contigs in the replicons of the Burkholderia sp. CEIB S4-3 draft genome were performed by aligning with the nucleotide collection (nr/nt) database using the Blastn program (Altschul et al.1990) with the following criteria: percentage of coverage >50% and percentage of identity >70%.
Genome annotation and phylogenetic analysis
The annotation of the draft genome of CEIB S4-3 was performed via the automatic prediction of open reading frames (ORFs) using the RAST server (version 2.0) (http://rast.nmpdr.org/) (Overbeek et al. 2014). The prediction of rRNA genes was performed in the draft genome using the server RNAmmer (version 1.2) (http://www.cbs.dtu.dk/services/RNAmmer/) (Lagesen et al. 2007). The 16S rRNA sequence of the CEIB S4-3 strain was ranked with 101 selected sequences of species with a wide variety of the genus Burkholderia and two sequences of the genus Ralstonia (outgroup) using MUSCLE (version 3.7) (Edgar 2004). The phylogenetic tree of 16S rRNA sequences was obtained using MEGA (version 6.0) (Tamura et al. 2013) via the Neighbor-Joining method with 1000 bootstrap replicates.
Detection and mapping of the MP and PNP degrading genes in the genome of the CEIB S4-3 strain
The protein sequences that are reported to hydrolyse MP (MpdB) via B. cepacia (GenBank Accession No. AAY18224.1) and to degrade PNP (PnpABE1E2FDC) via Burkholderia sp. SJ98 (GenBank accession numbers: EKS70311.1, EKS70312.1, AFR33817.1, AFR33818.1, EKS70671.1, WP_008348776.1 and EKS70306.1) (Vikram et al. 2012; Vikram et al. 2013; Min et al. 2014), were used as molecular probes to detect the number of copies and the position of the pnp genes in the contigs of the draft genome of CEIB S4-3 using tBlastn (Altschul et al. 1990). Catabolic genes were mapped in the draft genome of the CEIB S4-3 strain. This includes the detection of ORFs that are located 5 Kb upstream and downstream of catabolic genes. Based on these results, the complete MP and PNP degradation pathway is proposed in the draft genome of the CEIB S4-3 strain. The CDS obtained from the draft genome annotation of B. zhejiangensis CEIB S4-3 were analysed and grouped based on the functional characteristics using the RAST server and GenBank, Clusters of Orthologous Groups (COG) of proteins, KEGG Orthology (KO) and PFAM databases. The following keywords were used to detect possible catabolic proteins in the server and databases mentioned above: parathion, nitrophenol, xenobiotic, pesticide, insecticide, organophosphate, monooxygenase and dioxygenase.
The protein sequences, which were used as molecular probes to detect the catabolic genes found in the CEIB S4-3 strain and 35 complete genomes (CG) and 32 draft genomes (DG) by genus Burkholderia, are reported.
Results
The isolation and characterization of the CEIB S4-3 strain
Through the enrichment technique, nine isolates were obtained. From these nine isolates, one strain, designated CEIB S4-3, had the ability to turn the MSM yellow revealing the presence of PNP and therefore, was selected to conduct further studies. Later, this strain was able to remove the yellow colour from the culture medium, indicating possible MP degradation. According to various biochemical tests, the CEIB S4-3 strain is a Gram-negative rod, catalase positive, capable of metabolizing citrate, does not ferment glucose and is motionless, which corresponds to the main characteristics of the Burkholderia genus.
Genome sequencing and assembly
The CEIB S4-3 genome was assembled de novo using SPAdes software (version 3.1.1) in 154 contigs, 7,666,843 bp in length, an N50 of 156,081 bp and a GC content of 62.77% (Hernández-Mendoza et al. 2014). The draft genome of the CEIB S4-3 strain was aligned with 41 complete genomes (chromosomes and plasmids) of the genus Burkholderia using NUCmer. An identity of 86.95 and 87.6% and an alignment coverage of 4.57 Mb and 5.31 Mb were obtained from the genomes of Burkholderia sp. RPE64, which is formed by one chromosome, two chromids, two plasmids and is 6,964,487 bp in length (Shibata et al. 2013) and Burkholderia sp. YI23, which is formed by one chromosome, two chromids, three plasmids and is 8,896,411 bp in length (Lim et al. 2012), respectively.
PFGE analyses and identification of the specific position of contigs
The amount and length of the replicons of Burkholderia sp. CEIB S4-3 genome was calculated via the PFGE technique (Online Resource, Fig. 1S). The resulting gel of PFGE showed that the Burkholderia sp. CEIB S4-3 genome has four replicons of 2.871, 1.509, 0.9 and 0.8 Mb in length. Thus, the total length proposal for the Burkholderia sp. CEIB S4-3 genome is ~6.08 Mb. Detailed observation of the gel of PFGD suggest that there are two different replicons with ~1.5 Mb in length (Online Resource 1S). Based on this observation, we propose that the Burkholderia sp. CEIB S4-3 genome has five replicons with ~7.58 Mb of total length, which is similar to the total length reported for the draft genome (7.67 Mb) assembly with the SPAdes program.
The specific position of the 154 contigs in the five replicons of the draft genome of Burkholderia sp. CEIB S4-3 was performed by aligning with nucleotide collection (nr/nt) database using the Blastn program. Table 1 shows that replicon 1 corresponds to the bacterial chromosome, consists of 36 contigs and has a total length of 2,846,530 bp; both replicons 2 and 2′ correspond to the chromid 1, consist of 32 contigs (3 and 29 contigs, respectively) and have a total length of 1,703,814 bp; the replicon 3 corresponds to the chromid 2, consists of six contigs and has a total length of 865,659 bp; the replicon 4 corresponds to the plasmid 1, consists of ten contigs and has a total length of 1,369,131 bp; and the replicon 5 corresponds to the plasmid 2, consists of 40 contigs and has a total length of 447,128 bp. Moreover, the specific position of 30 contigs was not identified in the five replicons of the draft genome of Burkholderia sp. S4-3 (Table 1). The bacterial chromosome has a GC content of 62.79765%; the chromids 1 and 2 have a GC content of 61.95026 and 62.57911%, respectively, which is similar to the bacterial chromosome (GC content of <1%); and plasmids 1 and 2 have a GC content of 58.17284 and 59.81155%, respectively, which is different from the bacterial chromosome (GC content greater than 1%) (Table 1). Therefore, the Burkholderia sp. CEIB S4-3 has one chromosome, two chromids and two plasmids, which is similar to the complete genomes of Burkholderia sp. RPE64 (one chromosome, two chromids and two plasmids) and Burkholderia sp. YI23 (one chromosome, two chromids and three plasmids).
Genome annotation and phylogenetic analysis
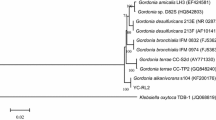
Annotation of the draft genome of CEIB S4-3 was performed using the RAST server (version 2.0), and for which 7278 potential ORFs and 7228 possible CDS (protein coding sequences) were identified. The presence of the RNAr 16S gene (position: 408,788–410,307), 23S gene (position: 410,863–413,743) and 5S gene (position: 413,902–414,013) in contig 3 of the draft genome of CEIB S4-3 were identified using the RNAmmer sever (version 1.2). Phylogenetic analysis showed that CEIB S4-3 grouped with species of the genus B. zhejiangensis (Fig. 1). The draft genome was deposited in the GenBank database with the accession number JSBM00000000.
Phylogenetic relationships of the 16S rRNA sequence of Burkholderia zhejiangensis CEIB S4-3 (bold) with 47sequences of genus Burkholderia and two sequences of genus Ralstonia as outgroup. The phylogenetic tree was obtained by MEGA program (version 6.0) using the Neighbor-Joining method with 1000 bootstrap repetitions. Bootstrap values are displayed in the nodes
MP and PNP degradation by CEIB S4-3
Degradation studies showed a rapid decrease in the concentration of MP and the immediate formation of its major metabolite (Online Resource, Fig. 2S), which was identified as PNP by comparison with a standard. Both compounds were measured using HPLC immediately after taking the samples. When analysing the concentration of MP at time zero, approximately 40% of the pesticide was recovered. This suggests that the reaction rate is very high because in the time between sample preparation and injection into the HPLC, MP decreased by 60%. Within the first 3 h, MP is hydrolysed almost completely. While PNP was approaching its maximum concentration, the bacterial growth was decreasing, probably because of toxicity of PNP for some microorganisms. However, while PNP was being degraded, the growth increased, suggesting that the CEIB S4-3 strain can use it as a sole carbon source. No abiotic degradation was observed in uninoculated controls (data not shown).
Expression of MPH and enzymatic activity
The mpd gene coding for MPH was cloned into the vector and transformed into E. coli BL21 (DE3) pLysS. By analysing the SDS-PAGE gel from the induced crude protein extract of pET28/mpd cells (Online Resource, Fig. 3S, line 3), a band with a weight of approximately 32 kDa was observed, which corresponds to the expected value according to the previous analysis of the amino acid sequence. When evaluating the enzymatic activity of crude protein extracts, the induced vector pET28/mpd showed a specific activity that is 1.4 times greater than the native strain CEIB S4-3 on MP (22.32 and 16.12 µM/min/mg protein, respectively). The vacuum pET28a(+), which was used as a negative control, showed no activity. The hydrolysis activity on other organophosphorus pesticides was also evaluated. MPH showed enzymatic activity on ethyl parathion, diazinon and chlorpyrifos (data not shown).
Identification of intermediate metabolites
To identify the metabolites that result from the MP degradation by the CEIB S4-3 strain, kinetics was performed in which aliquots were taken at different time intervals. Compounds were identified by comparison with their respective standards. The HPLC analysis showed the appearance of benzoquinone (BQ) and hydroquinone (HQ) after 15 min of culture (retention time of 2.71 and 2.06 min, respectively) (Online Resource, Fig. 4Sa, b). Both compounds are the main metabolites in the degradation pathway that are typically followed by Gram-negative bacteria. On the other hand, p-nitrocatechol (p-NC) and benzenetriol (BT) compounds were detected after 30 min of culture (retention time of 4.54 and 1.82 min, respectively) (Online Resource, Fig. 4Sc, d); these compounds are in the path of the Gram-positive bacteria. These results suggest that the strain CEIB S4-3 strain uses both degradation pathways. The HPLC analysis revealed that after 6 h of bacterial growth, these four previously identified metabolites, were not found in the culture medium, which suggest a complete and rapid degradation of MP by the strain.
Effect of MP concentration on its degradation
To study the effect of the initial concentration of MP on the growth of the CEIB S4-3 strain and on pesticide degradation, concentrations ranging between 10 and 150 mg/l were tested. The hydrolysis of MP following the production and degradation of PNP was monitored by spectrometric techniques. In all cases, the growth of the strain was affected by the accumulation of PNP, but at MP concentrations higher than 50 mg/l the strain did not grow. It was observed that PNP generation was caused only by the activity of the inoculum that was added at the beginning of the experiment (Fig. 2a). When using MP concentrations above 50 mg/l, the bacterial growth was diminished, probably because of the accumulation of PNP in the system. According to Tukey analysis, treatment with 50 mg/l is the only one with a significant growth at the end of experiment. The degradation time increases as the concentration of MP increase (Fig. 2b). At concentrations less than 50 mg/l of MP, the generated PNP was completely degraded in approximately 16 h, but at concentrations above 100 mg/l of the pesticide, this metabolite remained in the flask.
Effect of an additional carbon source on MP degradation
Using glucose as an additional source of carbon, bacterial growth was significantly increased during the first 12 h. However, this behaviour was not consistent with the hydrolysis of MP. The highest PNP concentration was reached at 4 h in each case with significant differences according to the Tukey analysis (α = 0.05). As shown in Fig. 5Sa (Online Resource), the growth after 12 h with 0.05 and 0.1% of glucose was almost two times higher than that of the glucose-free culture. However, after 24 h the culture with MP as the sole carbon source showed a significant difference in bacterial growth compared with the culture that contained glucose (Tukey). Although the culture with more glucose had greater growth, the amount of hydrolysed MP is 1.4 times less than in the culture without glucose. This show an inverse relationship between the glucose concentration and the amount of hydrolysed MP. Nevertheless, PNP is completely degraded after 16 h in all cases (Online Resource, Fig. 5Sb).
Effect of the inoculum size on MP degradation
The degradation of MP under different initial concentrations of inoculum was evaluated. As expected, the time required for pesticide hydrolysis decreased significantly when the inoculum was increased (P = 0.001, α = 0.05). In the culture with a 2% inoculum, the highest PNP concentration was reached after 4 h, but a decrease in the growth was observed, probably due to the PNP toxicity to the bacteria. However, after four hours of treatment, an exponential bacterial growth was observed simultaneously with the degradation of PNP. A similar behaviour was observed with the 1% inoculum. The bacterial growth shows a slight decrease during the first 8 h, at which time PNP reaches its highest concentration. After this time, PNP is significantly degraded, and growth starts its exponential phase. Nevertheless, according to the ANOVA analysis, we only found significant difference in the PNP concentration at 8 and 12 h. This means that although an inoculum of 2% is not significantly different in the maximum achieved concentration of PNP, it is different in the cumulative concentration of PNP during its degradation. This indicates that 2% inoculum is more efficient in completely degrading PNP in less time. With the 0.5% inoculum, MP was hydrolysed within the first eight hours. However, PNP accumulated during the 24 h of the experiment, and no bacterial growth was observed. Therefore, PNP cannot be completely degraded (Online Resource, Fig. 6Sa, b).
Effect of pH and temperature on MP degradation
The effect of pH on the residual MP hydrolytic activity of the CEIB S4-3 strain was measured according to Wang et al. (2012), and the obtained results are shown in Fig. 7S (Online Resource). Most residual activity was obtained at pH 6 and 7 with 84.4 ± 3.4 and 94.4 ± 1.5%, respectively (Tukey, α = 0.05). Furthermore, at alkaline pH (8 and 9), activity rapidly decreased. Nevertheless, the activity is maintained above 50% at all pH ranges tested.
The effect of temperature on the hydrolysis of MP by the strain was also assessed. As shown in Fig. 8S (Online Resource), the optimal temperatures for enzyme activity on MP degradation were 35, 40 and 50 °C (Tukey, α = 0.05). The enzyme activity was stable up to 50 °C, but declined rapidly at higher temperatures, possibly due to enzyme denaturation. A negative control (E. coli DH5α) showed no MP hydrolysis activity.
Detection and mapping of Burkholderia zhejiangensis CEIB S4-3 genes that are involved in the hydrolysis of MP and PNP degradation
The reported sequence of a protein that hydrolyses MP (MpdB) in B. cepacia (access number AAY18224.1 in the GenBank database) was used as molecular probe for detecting catabolic genes in the B. zhejiangensis CEIB S4-3 genome. The mpd gene was detected (Fig. 3); it has a length of 867 bp and is located in contig 68. The MPH protein of B. zhejiangensis CEIB S4-3 and the protein of B. cepacia have an identity and similarity percentage of 99% (Hernández-Mendoza et al. 2014), and a value of Expectancy E (E-value) of 0.0.
The mpd gene mapping (gray arrow) in the contig 68 of Burkholderia zhejiangensis CEIB S4-3 genome. ORF 4309, mobile element protein; ORF 4310, PhnB protein; ORF 4311, glyoxalase protein family; ORF 4312, flow antibiotics protein of the major facilitator superfamily (MFS); ORF 4313, hypothetical protein; ORF 4314, sigma 70 factor of the RNA polymerase; ORF 4316, hypothetical protein; 4317 ORF protein B of the general secretory pathway; ORF 4319, ATP-binding-cassette(ABC) transport system type; ORF 4320, ABC transport protein ATP-binding; ORF 4321, ABC transport system type; ORF 4322, likely lipoprotein; and ORF 4323, small chain dehydrogenase
The sequences of proteins that degrade PNP in Burkholderia sp. SJ98 (Vikram et al. 2012, 2013) were used as molecular probes to detect the number of copies of pnp genes in CEIB S4-3. In the B. zhejiangensis CEIB S4-3 genome, the pnpABA’E1E2FDC and pnpE1E2FDC gene clusters were detected (Hernández-Mendoza et al. 2014) in contig 33 and contig 4, respectively (Fig. 4). The alignment between the two PNP proteins of B. Zhejiangensis CEIB S4-3 and Burkholderia sp. SJ98 showed an identity of 67 and 100%, respectively; a similarity of 79 and 100%, respectively; and an E-value of 3e−71 and 0.0, respectively. These gene groups code for enzymes that catalyse the reactions that degrade PNP.
Schematic representation of two genes groups involved in PNP degradation in draft genome of B. zhejiangensis CEIB S4-3. a pnpE1E2FDCG genes cluster found in the negative strand of contig 4. ORF 2024, ABC transporter; ORF 2025, ABC transporter; ORF 2026, ABC transporter; ORF 2027, transcriptional regulator of arabinose operon; ORF 2028, hypothetical protein; ORF 2029, hypothetical membrane anchored protein; ORF 2036, hypothetical protein; ORF 2037, cytochrome C; ORF 2038, flavoprotein with fumarate reductase/succinate dehydrogenase domain; ORF 2039, hypothetical protein; ORF 2041, transcriptional regulator of the AraC family; and ORF 2042, transcriptional regulator of the LysR family. b pnpABA’E1E2FDCG genes cluster found in the negative strand of contig 33. ORF 2881, transcriptional regulator of the IcIR family; ORF 2882, transporter permease; ORF 2883, hypothetical protein; ORF 2891, hypothetical protein; ORF 2892, mobile element protein; ORF 2893, mobile element protein; ORF 2894, mobile element protein; and ORF 2895, hypothetical protein
When we searched the cluster of genes involved in PNP degradation within 35 CG and 32 DG of the Burkholderia genus, 15 CG and 17 DG containing the gene cluster pnpE1E2FDCG were identified (Table 2). This gene cluster was located in 14 chromids and one chromosome (Burkholderia fungorum ATCC BAA-463). Moreover, the gene cluster pnpABA’E1E2FDCG was found in one CG (Burkholderia sp. YI23) and 3 DG of Burkholderia: Burkholderia sp SJ98, Burkholderia jiangsuensis MP-1 and B. zhejiangensis OP-1.
In pnpE1E2FDCG and pnpABA’E1E2FDCG clusters, we found the pnpE1E2 genes that have a strong identity with hydroquinone 1,2 dioxygenase subunits, as reported by Vikram et al. (2012). This enzyme is responsible for cleavage of the aromatic ring in the HQ pathway of PNP degradation and is mostly used by gram-negative bacteria. The pnpC gene used by Gram-positive bacteria that encode for hydroxyphenol 1, 2 dioxygenase and is responsible for ring cleavage in the BT pathway was also found. Therefore, we propose that the B. zhejiangensis strain CEIB S4-3 uses both PNP degradation pathways. We are currently undertaking the necessary experiments to confirm this proposal.
We also propose that the pnpG gene can play a similar role as p-nitrophenol (PNP) monooxygenase II in the BT pathway for PNP degradation. In the genome of B. zhejiangensis CEIB S4-3, we found 25 monooxygenases and 50 dioxygenases that can participate in the degradation of PNP.
ORFs that are located 5 kb upstream and downstream of mpd gene in contig 18, and pnpE1E2FDC and pnpABA’E1E2FDC gene clusters in contigs 4 and 33, respectively (Fig. 4) were mapped in the draft genome of B. zhejiangensis CEIB S4-3. We found the presence of a gene named pnpG downstream of two PNP degradation gene clusters that encodes for a protein with a possible functional ferredoxin 4-nitrocatechol monooxygenase subunit. The pnpG gene has a length of 387 and 321 bp and is located in contigs 4 and 33, respectively. The two PnpG proteins have a percent identity of 55% and a similarity of 72%. We propose that the PnpG protein can have an activity similar to monooxygenase PNP II in the BT pathway for PNP degradation.
The proteins obtained from the annotation of the B. zhejiangensis CEIB S4-3 draft genome were analysed and grouped based on their functional characteristics when utilizing the Rapid Annotation using Subsystem Technology (RAST) server and the GenBank, Closter of Orthologous Groups (COG), KEGG Orthology (KO) and Protein Families (PFAM) databases. To detect possible catabolic genes in the genome of B. zhejiangensis CEIB S4-3, we searched the RAST server and GenBank, COG and PFAM KO databases using the following keywords: esterase, parathion, nitrophenol, xenobiotic, pesticide, insecticide, organophosphate, monooxygenase and dioxygenase. The COG, KO and PFAM databases had no significant results from searching for catabolic genes with these keywords. We detected 59 esterases, 25 monooxygenases and 50 dioxygenases in the RAST server. In addition, 51 esterases, 26 monooxygenases and 62 dioxygenases were found in the GenBank database. These results suggest that other genes can be involved in the hydrolysis of MP (e.g. esterases) and PNP degradation (e.g. dioxygenases and monooxygenases).
Proposal for the MP degradation pathway in Burkholderia zhejiangensis CEIB S4-3
Catabolic genes that are involved in MP hydrolysis and PNP degradation via the homology search of amino acid sequences and keywords were proposed. The mpd gene was cloned and expressed and the MPH enzyme had activity on the MP, which indicates that this enzyme is probably responsible for MP hydrolysis by the strain CEIB S4-3. Figure 5 shows the proposed metabolic pathway for MP hydrolysis and PNP degradation in B. zhejiangensis CEIB S4-3. BQ, HQ, p-NC and BT metabolites were also identified using the HPLC analysis, which supports the proposed hypothetical pathway.
Proposed metabolic pathway used by Burkholderia zhejiangensis CEIB S4-3 for MP hydrolysis and PNP degradation. In figure we show completely information about pathways; enzymes involved in each reaction; the gene responsible for the enzyme expression (found through bioinformatic analysis); and the enzyme type that could hypothetically be involved in the chemical reaction indicated in the figure (Adapted from information reported by Zhang et al. 2012; Vikram et al. 2013). However, according to this figure, we were able to identify four of the compounds involved which are: Benzoquinone, Hydroquinone, p-Nitrocatechol and 1,2,4-Benzenetriol
The mpd gene that carries out MP hydrolysis, and two groups of genes involved in PNP degradation were identified. The complete cluster, pnpABA’E1E2FDC, was located in contig 33 and possibly belong to a plasmid; and another partial cluster, pnpE1E2FDC, located in the contig 4, possibly belong to a chromid.
We propose that the pnpG gene can have a similar activity to the monooxygenase II PNP protein in the BT pathway for PNP degradation. The B. zhejiangensis CEIB S4-3 genome contains 59 esterases with promiscuous activity that can hydrolyse MP, as well as 25 monooxygenases and 50 dioxygenases that can participate in PNP degradation.
Discussion
The isolation of bacterial strains through enrichment techniques has been extensively used for pesticide biodegradation (Goda et al. 2010; Pakala et al. 2007). When the microbes are exposed to selective pressures such as xenobiotic compounds, microbial populations with a great degradation potential can be obtained. In this work we were able to isolate a bacterium, designated CEIB S4-3, which is capable of hydrolysing MP and degrading PNP and is the first strain reported in México.
Phylogenetic analysis showed that the CEIB S4-3 strain grouped with B. zhejiangensis (Fig. 1). This species was first reported in Lu et al. (2012) and shares certain characteristics with our strain, such as being Gram-negative, non-spore-forming and non-mobile. The same analysis showed that Burkholderia sp. SJ98 and Burkholderia sp. YI23 is also grouped with this species, which suggest that it belongs to the B. zhejiangensis species and evolved from a common ancestor (Fig. 1). Both Burkholderia sp. SJ98 and Burkholderia sp. Y123 have also been reported as degrading organophosphate pesticides or their intermediaries. Some strains such as B. caryophilli PG2982, Burkholderia. sp. NF100, Burkholderia sp. FDS-1, B. jiangsuensis and B. cepacia PCL3 have the potential to degrade pesticides (Dotson et al. 1996; Hayatsu et al. 2000; Zhang et al. 2006; Liu et al. 2014; Plangklang and Reungsang 2011). However, the B. cepacia complex of organisms have presented health problems because they are pathogenic to humans.
Generally, the first step in the metabolism of organophosphates is the hydrolytic cleavage of the P–O, P–F, P–S or P–C bonds found in a wide variety of organophosphate pesticides (Singh and Walker 2006). The B. zhejiangensis strain CEIB S4-3 carried out MP hydrolysis for the first 3 h, although its growth is affected by PNP accumulation. There are several reports that provide information about the toxicity of PNP in various microorganisms. The toxicity of aromatic nitro compound is associated with the products formed during the nitro group reduction, such as aromatic hydroxylamine derivatives, that can react with biomolecules, such as DNA, and cause toxic and mutagenic effects (Gómez 2010). The CEIB S4-3 strain has the molecular mechanisms to degrade PNP, which makes this strain a promising tool for bioremediation.
It is very important to know the mechanism and the environmental conditions for bacterial MP degradation because it helps to optimize operating conditions to make the process more efficient. Some bacteria, such as Enterobacter sp. Cons002, are capable of hydrolysing MP only in the presence of glucose (Chino-Flores et al. 2012). However, we found that the CEIB S4-3 strain does not require an alternative carbon source (like glucose) to improve the process. In fact, MP hydrolysis decreases in the presence of glucose. Similar behaviour was observed when Qiu et al. (2006) evaluated PNP degradation by Ochrobactrum sp. B2; they report that glucose promotes growth without affecting degradation.
Through bioinformatic analysis, mpd gene that encodes MPH was identified. This gene has a length of 1021 pb and was cloned into the expression vector pET28a (+). Using the SDS-PAGE gel, we found that MPH has an approximate weight of 32 kDa. Due to its high specific activity, MPH can be responsible for MP hydrolysis by the strain CEIB S4-3. Homologous genes of Burkholderia sp. YI23 and contig 73 of B. zhejiangensis OP-1were identified with a 99% identity in plasmid 2 (356.263 bp). This observation suggests that the mpd gene of B. zhejiangensis CEIB S4-3 can be located on a plasmid.
On the other hand, when evaluating the initial size of the inoculum, it was found to be closely related to the rate of MP hydrolysis. When the residual activity of complete cells of the CEIB S4-3 strain was evaluated, we found that the strain had better MP hydrolysis activity at pH 6 and 7 and in a wide range of temperatures (35–50 °C), showing good thermal and pH stability. When we compared our results with other organophosphate hydrolases reports from different microorganisms, we found that hydrolases enzyme activities may occur in a wide range of temperatures and pH. For example, P. pseudoalcaligenes has an optimum activity for hydrolysing MP at 65 °C and pH 9.0 (Wu et al. 2004) and S. cerevisiae has an optimal activity at pH 7 and a temperature of 20 °C (Takayama et al. 2006).
The toxicity of nitroaromatic compounds at high concentrations limits their degradation by microorganisms, which was observed when the concentration of MP in the culture medium increased. The CEIB S4-3 strain retained its hydrolysis ability, but did not show the same ability to degrade PNP, suggesting that PNP is toxic to the cells. This may be due to the difficulty of PNP biodegradation because of the nitro group in the aromatic ring; therefore, the PNP and MP degradation is mainly studied at lower concentrations (Hanne et al. 1993).
In various microorganisms, such as in Pseudomonas sp. WBC-3 strain (Zhang et al. 2012) and B. jiangsuensis sp. MP-1 (Liu et al. 2014), a cluster has been reported that has the genes involved in the degradation of PNP. The pnpABA’E1E2FDC gene cluster found in contig 33 of the draft genome of B. zhejiangensis CEIB S4-3 contained a duplication of a gene encoding PNP monooxygenase-I. If these two genes have activity, this can explain the rapid PNP degradation by the CEIB S4-3 strain. In contig 4, the pnpE1E2FDC gene cluster was detected, which is a partial gene because neither the pnpA nor the pnpB genes that encode reductase benzoquinone were detected, making this the first report of a bacterium that has two clusters.
The PnpABA’E1E2FDCG group genes are located in the contig 33 (JSBM01000076.1) of the B. zhejiangensis S4-3 CEIB genome, which has a length of 71.21 bp, a GC content of 54.93%, and it is located on plasmid 1. This group of genes was also identified in Burkholderia sp. YI23 (plasmid 2), Burkholderia sp. SJ98 (contig 12) and B. zhejiangensis OP-1 (contig 38). These results suggest that these genes may be preferably located in plasmids and are conserved in the species of Burkholderia. Furthermore, the pnpE1E2FDCG genes group is located in the contig 4 of B. zhejiangensis S4-3 CEIB, which has a length of 312.41 bp, a GC content of 62.83% and is located in the chromid 2. It was also identified in Burkholderia sp. in RPE64 and Burkholderia sp. YI23 (chromids 2) and Burkholderia sp. SJ98 (contig 11), in B. zhejiangensis OP-1 (contig 1) and in 28 genomes of Burkholderia genus. These observations suggest that this group of genes can be preferably located in chromids and be conserved in the genus Burkholderia.
Using the HPLC studies, it was possible to identify four metabolites that are involved in the degradation pathways of the PNP: BQ, HQ, p-NC and BT. While B. zhejiangensis CEIB S4-3 is a Gram-negative bacteria, in this work, we found gene clusters that suggest that bacteria use Gram-negative and Gram-positive pathways for PNP degradation. Such is the case of the pnpE1E2 gene, which has a strong identity with the hydroquinone 1,2 dioxygenase subunits that are responsible for the cleavage of aromatic ring in the HQ pathway. Additionally, the pnpC gene that encodes for hydroxyphenol 1,2 dioxygenase, which is responsible for cleavage of the ring in the BT pathway, was also found. These findings explain the efficiency of MP degradation by this strain. The time in which the intermediates appeared, as well as the concentrations, suggest that the CEIB S4-3 strain preferentially uses the Gram-negative degradation pathway but is able to use the Gram-positive pathway at the same time. As we said before, we are currently carrying out work to verify this proposal.
The sequence and annotation analysis of the fragments indicates that the organization of the pnp cluster in B. zhejiangensis CEIB S4-3 is very similar to the cluster in Burkholderia sp. SJ98. However, this strain does not have any genes that are responsible for MP hydrolysis. The MPH enzyme of CEIB S4-3 showed activity on various organophosphorus pesticides, such as ethyl parathion, diazinon and chlorpyrifos, thus, the CEIB S4-3 strain can be used as a tool for biodegradation of these pesticides.
This work is the first report of a bacterium whose genome comprises genes involved in both, MP hydrolysis and PNP degradation. This bacterium was isolated from agricultural soils in México and was identified as B. zhejiangensis CEIB S4-3. It has a similarity with the genomes of Burkholderia sp. SJ98 (isolated from agricultural fields in Assam, India, that degrade PNP and 2C4NP); Burkholderia sp. YI23 (which degrades the organophosphate insecticide Fenitrothion); Burkholderia sp. RPE64 and B. zhejiangensis OP-1 (isolated from a system of wastewater treatment in the community Zhejiang, China that has the ability to hydrolyse MP).
A thorough genome and degradation processes analysis can eventually allow the optimization of CEIB S4-3′s physiological state for bioremediation processes, as well as for new and more efficient methods for the degradation of pollutants.
Conclusion
A strain isolated from the agricultural soils of the Morelos State, México, was identified as B. zhejiangensis CEIB S4-3 and was able to completely degrade MP using it as the only source of carbon. This strain has a higher residual activity in neutral or slightly acidic pH, and a high tolerance for temperatures up to 50 °C. Bioinformatics analysis showed that the B. zhejiangensis strain CEIB S4-3 has the genes involved in the PNP degradation pathway from both Gram-positive and Gram-negative microorganisms. In addition, the identified metabolites suggest that the strain uses both pathways.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. doi:10.1016/S0022-2836(05)80360-2
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 9(5):455–477. doi:10.1089/cmb.2012.0021
Bigley AN, Raushel FM (2013) Catalytic mechanisms for phosphotriesterases. Biochem Biophys Acta 1834:443–453. doi:10.1016/j.bbapap.2012.04.004
Bosco de Salles J, Matos Lopes R, de Salles CMC, Cassano VPF, de Oliveira MM, Cunha Bastos VLF, Bastos JC (2015) Bioconcentration and acute intoxication of Brazilian freshwater fishes by the methyl parathion organophosphate pesticide. BioMed Res Int. doi:10.1155/2015/197196
Chauhan A, Pandey G, Sharma NK, Paul D, Pandey J, Jain RK (2010) p-Nitrophenol degradation via 4-nitrocatechol in Burkholderia sp. SJ98 and cloning of some of the lower pathway genes. Environ Sci Technol 44(9):3435–3441
Chino-Flores C, Dantán-González E, Vázquez-Ramos A, Tinoco-Valencia R, Díaz-Méndez R, Sánchez-Salinas E, Castrejón-Godínez ML, Ramos-Quintana F, Ortiz-Hernández ML (2012) Isolation of the opdE gene that encodes for a new hydrolase of Enterobacter sp. capable of degrading organophosphorus pesticides. Biodegradation 23(3):387–397. doi:10.1007/s10532-011-9517-6
De la Vega Salazar MY, Martínez Tabche L, Macías García C (1997) Bioaccumulation of methyl parathion and its toxicology in several species of the freshwater community in Ignacio Ramirez Dam in Mexico. Ecotoxicol Environ Saf 38(1):53–62. doi:10.1006/eesa.1997.1551
Dotson SB, Smith CE, Ling CS, Barry GF, Kishore GM (1996) Identification, characterization, and cloning of a phosphonate monoester hydrolase from Burkholderia caryophilli PG2982. J Biol Chem 271(42):25754–25761
Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform 5:113. doi:10.1186/1471-2105-5-113
Edwards FL, Tchounwou PB (2005) Environmental toxicology and health effects associated with methyl parathion exposure—a scientific review. Int J Environ Res Public Health 2(3):430–441
Ekkhunnatham A, Jongsareejit B, Yamkunthong W, Wichitwechkarn J (2012) Purification and characterization of methyl parathion hydrolase from Burkholderia cepacia capable of degrading organophosphate insecticides. World J Microbiol Biotechnol 28:1739–1746. doi:10.1007/s11274-011-0985-y
Errampalli D, Tresse O, Lee H, Trevors JT (1999) Bacterial survival and mineralization of p-nitrophenol in soil by green fluorescent protein-marked Moraxella sp. G21 encapsulated cells. FEMS Microbiol Ecol 30:229–236
Goda SK, Elsayed IE, Khodair TA, El-Sayed W, Mohamed ME (2010) Screening for and isolation and identification of malathion-degrading bacteria: cloning and sequencing a gene that potentially encodes the malathion-degrading enzyme, carboxylestrase in soil bacteria. Biodegradation 21(6):903–913. doi:10.1007/s10532-010-9350-3
Gómez R (2010) Degradación de compuestos nitroaromáticos por Rhodobacter: purificación de la nitrorreductasa Npr. Tesis de Doctorado, Universidad de Córdoba
Hanne LF, Kirk LL, Appel SM, Narayan AD, Bains KK (1993) Degradation and induction specificity in actinomycetes that degrade p-nitrophenol. Appl Environ Microbiol 59(10):3505–3508
Hayatsu M, Hirano M, Tokuda S (2000) Involvement of two plasmids in fenitrothion degradation by Burkholderia sp. Strain NF100. Appl Environ Microbiol 66:1737–1740
Hernández-Mendoza A, Martínez-Ocampo F, Lozano-Aguirre Beltrán LF, Popoca-Ursino EC, Ortiz-Hernández L, Sánchez-Salinas E, Dantán-González E (2014) Draft genome sequence of the organophosphorus compound-degrading Burkholderia zhejiangensis strain CEIB S4-3. Genome Announc 2(6):e01323-14. doi:10.1128/genomeA.01323-14
Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL (2004) Versatile and open software for comparing large genomes. Genome Biol 5(2):R12. doi:10.1186/gb-2004-5-2-r12
Lagesen K, Hallin P, Rødland EA, Staerfeldt HH, Rognes T, Ussery DW (2007) RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res 35(9):3100–3108. doi:10.1093/nar/gkm160
Lim JS, Choi BS, Choi AY, Kim KD, Kim DI, Choi IY, Ka JO (2012) Complete genome sequence of the fenitrothion-degrading Burkholderia sp. strain YI23. Genome Announc. 194(4):896. doi:10.1128/JB.06479-11
Liu H, Zhang JJ, Wang SJ, Zhang XE, Zhou NY (2005) Plasmid-borne catabolism of methyl parathion and p-nitrophenol in Pseudomonas sp. strain WBC-3. Biochem Biophys Res Commun 334:1107–1114
Liu FY, Hong MZ, Liu DM, Li YW, Shou PS, Yan H, Shi GQ (2007) Biodegradation of methyl parathion by Acinetobacter radioresistens USTB-04. J Environ Sci 19:1257–1260
Liu XY, Li CX, Luo XJ, Lai QL, Xu JH (2014) Burkholderia jiangsuensis sp. nov., a methyl parathion degrading bacterium, isolated from methyl parathion contaminated soil. Int J Syst Evol Microbiol 64(9):3247–3253. doi:10.1099/ijs.0.064444-0
Lu P, Zheng LQ, Sun JJ, Liu HM, Li SP, Hong Q, Li WJ (2012) Burkholderia zhejiangensis sp. nov., a methyl-parathion-degrading bacterium isolated from a wastewater-treatment system. Int J Syst Evol Microbiol 62(Pt 6):1337–1341. doi:10.1099/ijs.0.035428-0
Min J, Zhang JJ, Zhou NY (2014) The gene cluster for para-nitrophenol catabolism is responsible for 2-chloro-4-nitrophenol degradation in Burkholderia sp. strain SJ98. Appl Environ Microbiol 80(19):6212–6222. doi:10.1128/AEM.02093-14
Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R (2014) The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42(Database issue):D206–D214. doi:10.1093/nar/gkt1226
Pakala SB, Gorla P, Pinjari AB, Krovidi RK, Baru R, Yanamandra M, Merrick M, Siddavattam D (2007) Biodegradation of methyl parathion and p-nitrophenol: evidence for the presence of a p-nitrophenol 2-hydroxylase in a Gram-negative Serratia sp. strain DS001. Appl Microbiol Biotechnol 73(6):1452–1462
Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Makris KC, Papadopoulou-Mourkidou E (2015) A pesticide monitoring survey in rivers and lakes of northern Greece and its human and ecotoxicological risk assessment. Ecotoxicol Environ Saf 116:1–9. doi:10.1016/j.ecoenv.2015.02.033
Plangklang P, Reungsang A (2011) lux-Marking and application of carbofuran degrader Burkholderia cepacia PCL3. N Biotechnol 28(6):798–805. doi:10.1016/j.nbt.2011.04.005
Qiu X, Zhong Q, Li M, Bai W, Li B (2006) Biodegradation of p-nitrophenol by methyl parathion-degrading Ochrobactrum sp. B2. Int Biodeterior Biodegradation 59:297–301
Serdar CM, Gibson DT, Munnecke DM, Lancaster JH (1982) Plasmid Involvement in Parathion Hydrolysis by Pseudomonas diminuta. Appl Environ Microbiol 44(1):246–249
Sethunathann N, Yoshida T (1973) A Flavobacterium sp. that degrades diazinon and parathion. Can J Microbiol 19:873–875
Shen W, Liu W, Zhang J, Tao J, Deng H, Cao H, Cui Z (2010) Cloning and characterization of a gene cluster involved in the catabolism of p-nitrophenol from Pseudomonas putida DLL-E4. Bioresour Technol 101(19):7516–7522. doi:10.1016/j.biortech.2010.04.052
Shibata TF, Maeda T, Nikoh N, Yamaguchi K, Oshima K, Hattori M, Nishiyama T, Hasebe M, Fukatsu T, Kikuchi Y, Shigenobu S (2013) Complete genome sequence of Burkholderia sp. strain RPE64, bacterial symbiont of the bean bug Riptortus pedestris. Genome Announc 1(4):e00441-13. doi:10.1128/genomeA.00441-13
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30(3):428–471. doi:10.1111/j.1574-6976.2006.00018.x
Subashchandrabose SR, Megharaj M, Venkateswarlu K, Naidu R (2012) p-Nitrophenol toxicity to and its removal by three select soil isolates of microalgae: the role of antioxidants. Environ Toxicol Chem 31(9):1980–1988. doi:10.1002/etc.1931
Takayama K, Suye S, Kuroda K, Ueda M, Kitaguchi T, Tsuchiyama K, Fukuda T, Chen W, Mulchandani A (2006) Surface display of organophosphorus hydrolase on Saccharomyces cerevisiae. Biotechnol Prog 22:939–943. doi:10.1021/bp060107b
Takeo M, Murakami M, Niihara S, Yamamoto K, Nishimura M, Kato D, Negoro S (2008) Mechanism of 4-nitrophenol oxidation in Rhodococcus sp. Strain PN1: characterization of the two-component 4-nitrophenol hydroxylase and regulation of its expression. J Bacteriol 190(22):7367–7374. doi:10.1128/JB.00742-08
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Vikram S, Pandey J, Bhalla N, Pandey G, Ghosh A, Khan F, Jain RK, Raghava GP (2012) Branching of the p-nitrophenol (PNP) degradation pathway in Burkholderia sp. strain SJ98: evidences from genetic characterization of PNP gene cluster. AMB Express 2(1):30. doi:10.1186/2191-0855-2-30
Vikram S, Pandey J, Kumar S, Raghava GP (2013) Genes involved in degradation of para-nitrophenol are differentially arranged in form of non-contiguous gene clusters in Burkholderia sp. strain SJ98. PLoS ONE 8(12):e84766. doi:10.1371/journal.pone.0084766
Wang XX, Chi Z, Ru SG, Chi ZM (2012) Genetic surface-display of methyl parathion hydrolase on Yarrowia lipolytica for removal of methyl parathion in water. Biodegradation 23(5):763–774. doi:10.1007/s10532-012-9551-z
Wauchope RD, Buttler TM, Hornsby AG, Augustijn-Beckers PW, Burt JP (1992) The SCS/ARS/CES pesticide properties database for environmental decision-making. Rev Environ Contam Toxicol 123:1–155
Wu NF, Deng MJ, Liang GY, Chu XY, Yao B, Fan YL (2004) Cloning and expression of ophc2, a new organophosphorus hydrolase gene. Chin Sci Bull 49:1245–1249
Yamamoto K, Nishimura M, Kato D, Takeo M, Negoro S (2011) Identification and characterization of another 4-nitrophenol degradation gene cluster, nps, in Rhodococcus sp. strain PN1. J Biosci Bioeng 111(6):687–694. doi:10.1016/j.jbiosc.2011.01.016
Zhang Z, Hong Q, Xu J, Zhang X, Li S (2006) Isolation of fenitrothion-degrading strain Burkholderia sp. FDS-1 and cloning of mpd gene. Biodegradation 17(3):275–283. doi:10.1007/s10532-005-7130-2
Zhang S, Sun W, Xu L, Zheng X, Chu X, Tian J, Fan Y (2012) Identification of the para-nitrophenol catabolic pathway, and characterization of three enzymes involved in the hydroquinone pathway, in Pseudomonas sp. 1–7. BMC Microbiol 12(1):4–11
Acknowledgments
This work was funded for the National Council for Science and Technology (CONACYT for its acronym in Spanish), founds CB2014-240414, FM-S-293552 and ECP-T-351,686. To Janette Onofre Lemus, for her support in analyzing the B. zhejiangensis CEIB S4-3 genome through the Pulsed-Field Gel Electrophoresis (PFGE) technique. To Luis Fernando Lozano Aguirre Beltrán, for his support in the assembly and annotation of the B. zhejiangensis CEIB S4-3 genome. Also, for the support in the phylogenetic analysis of 16S rRNA sequences of the genus Burkholderia.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Popoca-Ursino, E.C., Martínez-Ocampo, F., Dantán-González, E. et al. Characterization of methyl parathion degradation by a Burkholderia zhejiangensis strain, CEIB S4-3, isolated from agricultural soils. Biodegradation 28, 351–367 (2017). https://doi.org/10.1007/s10532-017-9801-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-017-9801-1