Abstract
Studies measuring the damage in degraded environments have increased, and it is necessary to obtain reliable biological and ecological indicators for the recovery of such degraded environments and subsequent preservation. This study aimed to conduct a survey of local insects and evaluate their utility in monitoring forest restoration in a degraded area in the Atlantic Forest biome in Ribeirao Grande, State of São Paulo, Brazil. Collections with Shuey traps were conducted from 2006 to 2011 in four distinct forest fragments characterized by environmental impacts, human actions, and phytophysiognomic profiles: Forest (FO), a preserved area with native plant species; “Capoeira” (CA), an area in the natural regeneration process; Planting (PL), an area reforested with native plants; and Pasture (PA), an area with only shrubs. A total of 2,456 specimens of Calliphoridae (Diptera) were collected. Mesembrinella bellardiana was the most abundant (n = 1,884) and dominant species in all environments sampled. The relative abundance of M. bellardiana in the most preserved environment (60.2 % FO, 24.4 % CA, 8.6 % PL, and 6.7 % PA) and other ecological parameters showed that it could be a bioindicator species, i.e., data on its presence or absence directly reflected the status of local preservation. Information on Neotropical dipterans associated with the forested environment is very scarce in the literature. To our knowledge, this is the first study describing the occurrence of Calliphora lopesi (Calliphoridae) in the State of São Paulo.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Atlantic Rainforest biome, one of the most important in the world, extends over a large part of Brazil and, in a lesser extent, into Paraguay and Argentina. It is a mosaic of diverse ecosystems, with structures and floristic compositions varying according to soil type, topography, and climatic characteristics (IBAMA 2012). It is one of the most threatened tropical forests in the world, especially by human actions associated with agricultural activities and rearing of livestock (Dean 2004). It is highly fragmented and only 7.3 % of the original forest cover remains (IBAMA 2012).
Ecological disequilibrium caused by fragmentation can substantially influence diversity. In environments in competitive balance, the total diversity of species is normally low because certain species that coexist avoid direct competition for limited resources (Huston 1995). As a result, measuring the effect of fragmentation in some communities, particularly in invertebrate communities, is not a simple task (Offerman et al. 1995; Didham 1997).
Many invertebrate taxa, including insects, are biological and ecological indicators of degradation because of their low resistance to environmental imbalances (Margalef 1951; Kremen et al. 1993; Brown and Hutchings 1997). They are excellent for qualitative measures in short-term because they have high reproductive capacity, which ensures rapid reestablishment of original populations (Brown 1997; Holloway et al. 1987), while vertebrates can take decades to restructure population equilibrium (Brown 1997).
Diptera may be used to quantify the degree of human interference on natural environments (Gadelha et al. 2009; Bizzo et al. 2010; Mata et al. 2010) because of their rapid populational response capacity. Some insects are well adapted to live in inhospitable and highly modified environments, which is often evidenced by their proximity to humans and their domestic animals (i.e., synanthropic classification) (Greenberg 1973; Linhares 1981). However, other insects are associated only with undisturbed environments (D’Almeida and Lopes 1983; Mariluis et al. 1990; Doge et al. 2008; Ferraz et al. 2010; Sousa et al. 2011a, b).
Analyses of species richness, faunistic composition, and other ecological parameters associated with different levels of impact on the environment are used to indicate if a given group of organisms could be useful for monitoring local recovery. Thus, this study aimed to survey local insects and to evaluate the utility of this information in monitoring forest restoration in an area of the Atlantic Forest biome degraded by mineral extraction and cement manufacturing.
Materials and methods
Area of study
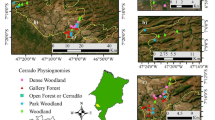
The site, Intermontes Farm, comprised 343 ha and is located near the State Park Intervales in the county of Ribeirão Grande, State of São Paulo, Brazil. It is triangulated among the coordinates 24°0′5.74′′S:48°20′21.81′′W, 24°0′16.43′′S:48°20′23.96′′W, and 24°0′10.34′′S:48°20′29.07′′W. This area is included in the Geomorphological Atlantic Plateau Province, Plateau Guapiara zone, which comprises the elevated region of Paranapiacaba Serra and extends up to the sedimentary cover of the Paraná basin, which includes the effluents of the upper course of the left margin of Paranapanema (Nave 2005).
The collections were conducted in a 215.4 ha area. Four distinct fragments were chosen based on environmental impact, human action, and phytophysiognomic profiles (Nave 2005): Forest (FO), with 74.49 ha, is a well-preserved area with native plant species whose heights range from 12 to 20 m; “Capoeira” (CA), with 51.09 ha, is an area in the process of natural restoration and has predominantly shorter (2–6 m high) forest formations and few tree species. It is generally in the early stages of succession, and has low infestation rates of Brachiaria decumbens; Planting (PL), with 55.43 ha, was reforested with native plants planted from 2001 to 2004; and Pasture (PA), with 34.39 ha, has only shrubs, mainly B. decumbens.
Sampling
Two Shuey traps (Shuey 1997) using fermented sugarcane juice and banana as bait, were placed approximately 50 m apart from each other for 24 h once a month in each fragment in January to April, June, August, November, and December from 2006 to 2011. Insects were removed, killed by transferring to a killing jar, and transferred to appropriately labeled containers. Insects were mounted and identified using dichotomous keys (Guimarães 1977; Carvalho and Ribeiro 2000; Bonatto 2001; Mello 2003) in the Laboratory of Entomology of the Department of Animal Biology, IB, UNICAMP.
Monthly of temperature and precipitation means for the study period were obtained from the meteorological database of the National Institute of Meteorology (INMET) (BDMEP 2012).
Data analysis
Abundance, dominance, diversity, equitability, and similarity of species were compared. The comparison among the abundance of fragments was performed using the t test and values were considered significant at P < 0.05. Analyses of correlation between fragment type and species abundance, and between diversity and climatic conditions were performed using the correlation coefficient nonparametric Spearman.
The Berger–Parker index (d), which expresses the proportional importance of the most abundant species in a given area or sample (Magurran 1988), was used to measure the degree of dominance among the studied areas. The value of this index is obtained by expression d = Nmax/N, where “d” is the degree of dominance, Nmax is the number of individuals of the most abundant species, and N is the total number of individuals sampled in the area. The Shannon-Wiener index (H′) was used to calculate the species diversity. The Shannon’s equitability index (J′) was calculated according to the formula: J′ = H′/Hmax, where H′ is the Shannon-Wiener’s index and Hmax is the logarithm of the total number of species in the sample. Equitability assumes a value between 0 and 1, with 1 being complete evenness. The similarity analysis, calculated using the Jaccard’s coefficient Ij = c/a + b − c, allows evaluation of significant differences in species compositions among different environments. The values range between −1 and 1, with values close to 1 being larger differences (Moreno 2001).
Statistical analyzes were performed using Bioestat™ software (Ayres et al. 2003) and all analyzes of ecological parameters were done with the programs Past™ (Hammer et al. 2001) and Dives™ (Rodrigues 2007).
Results
A total of 2,456 specimens of Calliphoridae (Diptera), distributed in 11 genera and 13 species (Table 1), were collected from 2006 to 2011. The highest abundance was found in the more preserved fragment (FO n = 1482). However, the greatest number of species (S = 11) occurred in the more impacted environment (PA). The lowest values of diversity were found in most preserved environments (FO H′ = 0.31, and CA H′ = 0.23) due to high dominance, as shown by the Berger–Parker index (d) values (Table 1). This was expected because the dominance of one or more species in a given environment tends to reduce local diversity. The lowest values of equitability in fragments CA and FO (Table 1) shows that individuals are not distributed equally among these species, which is also occasioned by dominance.
In all fragments, Mesembrinella bellardiana was the most abundant species (n = 1,884) and represented more than 50 % of all individuals collected. This species also showed significant preference (P < 0.05) for more preserved environments, as indicated by their abundance (FO n = 1140, CA n = 521, PL n = 140, and PA n = 83). Mesembrinella peregrina was the second most abundant species (FO n = 275, CA n = 48, PL n = 24, and PA n = 26) and was more closely associated with more preserved environments (P < 0.05). It was the second most dominant species in all fragments except for PL, where it was co-dominant with Laneella nigripes.
The abundance of all other species was low, though there is a variation in the order of abundance in different fragments. In general, the relative abundance did not exceed 10 % (Table 1), except for L. nigripes (11.3 %) in PL.
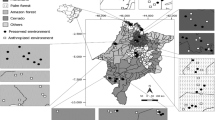
Faunistic composition between FO and CA was more similar than that between PL and PA (Fig. 1) showing that the pairs of fragments shared distinct vegetation and therefore provide diversified niches. Although M. bellardiana, M. peregrina, L. nigripes and Lucilia eximia were present in all fragments, the abundances were slightly higher when considering the sum of the number of individuals in the pairs FO and CA, except for Cochliomyia macellaria, where the abundance was higher in pairs PL and PA.
All fragments contained equal numbers of species considered rare (n < 2) varying only the faunistic composition (Table 1). Hemilucilia segmentaria is found only at PL and PA and Eumesembrinella quadrilineata is found at FO and CA. Chrysomya megacephala, species classified as exotic or adapted to anthropogenic environments (Linhares 1981), is present in a single habitat (PA), the most degraded environment.
Average annual temperatures ranged from 19.4 to 23.1 °C during the study period and were the lowest and highest temperatures in 2006 and 2010, respectively (Fig. 2). The highest levels of precipitation, 113.4 and 148.4 mm, were recorded during 2008 and 2009, while the lowest level, ~101 mm, was recorded between 2006 and 2007 (Fig. 2). Thus, the climate can be classified as humid temperate without a dry season, according to Köeppen (1948). The values obtained from Spearman’s test showed a significant positive correlation between temperature and the annual diversity indices (Fig. 3) (r = 0.84; P < 0.05), but there was no significant correlation with rainfall (r = 0.35; P > 0.05).
Discussion
Sampling was originally proposed for Lepidoptera. However, given the considerable abundance of dipterans (Calliphoridae) collected, we decided to analyze some ecological parameters that could be useful to assess the impacts of different levels of disturbance on biological diversity in the area of our study and to determine the possible use of these insects as bioindicators of local diversity.
A factor that may be useful in validating the use of our data is the long period of collection (~36 months), which increases the species inventory and the probability of detecting rare species (Summerville et al. 2003). Most notable, we recorded the first occurrence of Calliphora lopesi in the State of São Paulo, which had only been reported in Southern Brazil and the highlands of the state of Rio de Janeiro.
The biology and ecology of blowflies is widely documented in the literature because of their importance in the medical, veterinary, and forensic fields (Linhares and Thyssen 2007). In large part, this importance is due to the synanthropic behavior exhibited by most of the species (Greenberg 1971). However, with regard to species belonging to the subfamily Mesembrinellinae, mostly from wildlife, the habits of adults and the niche exploited by immatures are almost unknown (Guimarães 1977), which makes the necessity of studies on these native dipterans even more relevant.
The highest abundance of Mesembrinellinae species has been associated with the most preserved areas of the Amazon rainforest (Esposito and Carvalho 2006) and Atlantic Rainforest (Gadelha et al. 2009; Ferraz et al. 2010). In our study, a progressive increase in abundance of these species, in particular of M. bellardiana, as the area become less impacted, corroborates the existence of adaptations of these flies for this type of environment and ecosystem. Mata et al. (2008) obtained satisfactory results in a study of Drosophilidae (Diptera) in intact and disturbed areas of “cerrado”, the Brazilian savanna-like biome, and demonstrated that native species may become promising monitoring tools in these environments.
In vegetation recovery projects, given the monitoring conditions, is notorious observe that as they occur the rescue of diversity and the direction to the equilibrium environmental, insects respond in terms of density and diversity (Mcgeoch 1998). In our study, we observed that density (or abundance) seemed to follow this path, but not diversity, probably due to the dominance of M. bellardiana. Since there are no studies on the biology of this species in the literature we don’t know whether it might have a greater preference for bait used in our traps, when compared to the other species.
Because of its abundance, M. peregrina also seems to fulfill the requirements as characteristic species of preserved environments, represented in our case by fragment FO. As expected, the synanthropic species are not characteristic of preserved environments, although there were few records of Chrysomya spp. rarely associated with the forest environment (D’Almeida and Lopes 1983; Leandro and D’Almeida 2005). Regarding to the other species of Calliphoridae found, the lack of more data on biology and ecology affect the understanding of how they could be appropriately used for monitoring and preservation of natural environments.
According to Ferreira and Lacerda (1993), the distribution of Calliphoridae is strongly influenced by variations in climatic conditions and each species may respond with different seasonal patterns. Even in this unpretentious study, we observed that diversity was influenced by temperature.
From the present study, we concluded that: (1) the trap Shuey described for Lepidoptera collected a representative sample of muscomorpha fauna in both preserved and less preserved areas, especially in the most preserved areas; (2) the area in environmental recuperation (PL) in Intermontes Farm has a high diversity of Calliphoridae, but with marked dominance of few species; (3) planting native seedlings for restoration of original conditions has not had a significant effect on increasing local Calliphoridae diversity; and (4) the presence of Calliphora lopesi suggests that the studied area is part of an important ecological corridor of the Atlantic Forest biome and that there may be other species of calliphorids not yet recorded or known at the State of São Paulo or even the rest of the country.
References
Ayres M, Ayres-Jr M, Ayres DL, Santos AAS (2003) BioEstat 3.0—Aplicações Estatísticas nas Áreas das Ciências Bio-Médicas. Sociedade Civil Mamirauá, Belém, p 291
BDMEP (2012) Banco de Dados Metereológicos para Ensino e Pesquisa. http://www.inmet.gov.br/projetos/rede/pesquisa. Acessed 12 Sept 2012
Bizzo L, Gottschalk MS, De Toni DC, Hofmann PRP (2010) Seasonal dynamics of a drosophilid (Diptera) assemblage and its potencial as bioindicator in open environments. Iheringia Zool 100:185–191
Bonatto SR (2001) Revisão e análise cladística de Mesembrinellinae stat. restaur. (Diptera, Caliphoridae). PhD Thesis, Universidade Federal do Paraná
Brown KS (1997) Diversity, disturbance, and sustainable use of Neotropical forests: insects as indicators for conservation monitoring. J Insect Conserv 1:25–42
Brown KS, Hutchings RW (1997) Disturbance, fragmentation, and the dynamics of diversity in Amazonian forest butterflies. In: Laurance WF, Bierregaard-Jr RO (eds) Tropical forest remnants: ecology, management, and conservation of fragmented communities. University of Chicago Press, Chicago, p 632
Carvalho CJB, Ribeiro PB (2000) Chave de identificação das espécies de Calliphoridae (Diptera) do Sul do Brasil. Rev Bras Parasitol V 9:169–173
D’Almeida JM, Lopes HS (1983) Sinantropia de Dípteros Muscóides (Calliphoridae) no Estado do Rio de Janeiro. Arq Univ Fed Rural 6:39–48
Dean W (2004) A Ferro e Fogo: A História e a Devastação da Mata Atlântica Brasileira. Companhia das Letras, São Paulo, p 484
Didham RK (1997) An overview of invertebrate responses to forest fragmentation. In: Watt AD, Stork NE, Hunter MD (eds) Forests and Insects. Chapman and Hall, London, pp 301–318, 424
Doge JS, Valente VLS, Hofmann PRP (2008) Drosophilids (Diptera) from an Atlantic Forest Area in Santa Catarina, Southern Brazil. Rev Bras Entomol 52:615–624
Esposito MC, Carvalho FS (2006) Composição e abundância de califorídeos e mesembrinelídeos (Insecta, Diptera) nas clareiras e matas da base de extração petrolífera, Bacia do Rio Urucu, Coari, Amazonas. http://projetos.inpa.gov.br/ctpetro/workshop_site/Resumos_pt1/pdf/04CALIFORIDEOS_CRISTINA_REVISADO.pdf. Accessed 11 oct 2012
Ferraz ACP, Gadelha BQ, Aguiar-Coelho VM (2010) Influência Climática e Antrópica na Abundância e Riqueza de Calliphoridae (Diptera) em Fragmento Florestal da Reserva Biológica do Tinguá, RJ. Neotrop Entomol 39:476–485
Ferreira MJM, Lacerda PV (1993) Muscóides sinantrópicos associados ao lixo urbano em Goiânia, Goiás. Rev Bras Zool 10:185–195
Gadelha BQ, Ferraz ACP, Aguiar-Coelho VM (2009) A importância dos mesembrinelíneos (Diptera: Calliphoridae) e seu potencial como indicadores de preservação ambiental. Oecol Bras 13:661–665
Greenberg B (1971) Flies and diseases. Ecology, classification and biotic association, 1st edn. Princeton University, Princeton, p 856
Greenberg B (1973) Flies and diseases. Biology and disease transmission, 1st edn. Princeton University, Princeton, p 447
Guimarães JH (1977) A systematic revision of the Mesembrinellidae, stat. nov. (Diptera, Cyclorrhapha). Arq Zool 29:1–109
Hammer O, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and analysis. Paleontol Elet 4:1–9
Holloway JD, Bradley JD, Carter JD (1987) Guides to insects of importance to man. Lepidoptera, 1. C.A.B. International, Wallingford, p 62
Huston AH (1995) Biological diversity: the coexistence of species on changing landscapes. Cambridge University, Cambridge, p 681
IBAMA (2012) Ecossistemas brasileiros—Mata Atlântica. http://www.ibama.gov.br/ecossistemas/mata_atlantica.htm. Acessed 03 Oct 2012
Köeppen WP (1948) Climatología. Fondo de Cultura Econômica, México, p 478
Kremen C, Colwell RK, Erwin TL, Murphy DD, Noss RF, Sanjayan MA (1993) Terrestrial arthropod assemblages: their use in conservation planning. Conserv Biol 7:796–808
Leandro MJF, D′Almeida JM (2005) Levantamento de Calliphoridae, Fanniidae, Muscidae e Sarcophagidae em um fragmento de mata na Ilha do Governador, Rio de Janeiro, Brasil. Iheringia Ser Zool 95:377–381
Linhares AX (1981) Synanthropy of Calliphoridae and Sarcophagidae (Diptera) in the city of Campinas, São Paulo, Brazil. Rev Bras Entomol 25:189–215
Linhares AX, Thyssen PJ (2007) Miíases de Importância Médica—Moscas e Entomologia Forense. In: De Carli GA (ed) Parasitologia Clínica—Seleção de Métodos e Técnicas de Laboratório para o Diagnóstico das Parasitoses Humanas, 2nd edn. Ed. Atheneu, São Paulo, p 906
Magurran AE (1988) Ecological diversity and Its measurement. Princeton University, Princeton, p 192
Margalef R (1951) Diversidad de especies en las comunidades naturales. Publ Inst Biol Apl Barcelona 9:5–27
Mariluis JC, Schnack JA, Muzón J, Spinelli GR (1990) Flies of the families Calliphoridae and Mesembrinellidae from Puerto Iguazú. Species composition and ecology (Insecta, Diptera). Graellsia 46:7–18
Mata RA, McGeoch M, Tidon R (2008) Drosophilid assemblages as a bioindicator system of human disturbance in the Brazilian Savanna. Biodivers Conserv 17:2899–2916
Mata RA, McGeoch MA, Tidon R (2010) Drosophilids (Insecta, Diptera) as tools for conservation biology. Nat Cons 8:60–65
McGeoch MA (1998) The selection, testing and application of terrestrial insects as bioindicators. Biol Rev Camb Philos Soc 73:181–201
Mello RP (2003) Chave para identificação das formas adultas das espécies da família Calliphoridae (Diptera, Brachycera, Cyclorrhapha) encontradas no Brasil. Entomol Vect 10:255–268
Moreno CE (2001) Métodos para medir la biodiversidad. vol.1. M&T–Manuales y Tesis SEA, Zaragoza, p 84
Nave AG (2005) Banco de sementes autóctone e alóctone, resgate de plantas e plantio de vegetação nativa na Fazenda Intermontes, município de Ribeirão Grande, SP. PhD. Thesis, Universidade de São Paulo
Offerman HL, Dale VH, Pearson SM, Bierregaard-Jr RO, O’Neill RV (1995) Effects of forest fragmentation on neotropical fauna: current research and data availability. Environ Rev 3:191–211
Rodrigues WC (2007) DivEs - Diversidade de Espécies - Guia do Usuário. Seropédica: Entomologistas do Brasil. http://www.ebras.bio.br/dives/. Accessed 15 Aug 2012
Shuey JA (1997) An optimizing portable bait trap for quantitative sampling of butterflies. Trop Lep 8:1–4
Sousa JRP, Esposito MC, Carvalho Filho FS (2011a) Composition, abundance and richness of Sarcophagidae (Diptera: Oestroidea) in forests and forest gaps with different vegetation cover. Neotrop entomol 40:20–27
Sousa JRP, Esposito MC, Carvalho Filho FS (2011b) Diversity of Calliphoridae and Sarcophagidae (Diptera, Oestroidea) in continuous forest and gaps at different stages of regeneration in the Urucu oilfield in western Brazilian Amazonia. Rev Bras Entomol 55:578–582
Summerville KS, Boulware MJ, Veech JA, Crist TO (2003) Spatial variation in species diversity and composition of forest Lepidoptera in eastern deciduous forests of North America. Conserv Biol 17:1045–1057
Acknowledgments
The Department of Environment Company “Cimento Ribeirão Grande” by financial support and permission to access the collection area. To Dr. Paulo R. S. Bunde (UFPel) for critical reading and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabrini, I., Grella, M.D., Andrade, C.F.S. et al. Richness and composition of Calliphoridae in an Atlantic Forest fragment: implication for the use of dipteran species as bioindicators. Biodivers Conserv 22, 2635–2643 (2013). https://doi.org/10.1007/s10531-013-0545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-013-0545-x