Abstract
Measures of geodiversity may provide a potentially useful surrogate for biodiversity patterns in insufficiently surveyed areas. However, their reliability in modelling the spatial variation in species richness is inadequately understood. We investigated whether the explanatory and predictive power of species richness models can be improved by considering explicit measures of geodiversity (variability of earth surface materials, forms and processes) in addition to climate and topography variables. Vascular plant species richness was modelled in two study areas in Northern Europe, Finland at the resolution of 500 or 1000 m, and as a function of three geodiversity (geological, geomorphological and hydrological diversity) variables, and six climate and topography variables. Variation partitioning was used to identify the independent and shared contributions of the geodiversity, climate and topography variable groups in explaining the spatial patterns of species richness. Generalized additive models were used to explore the ability of the different explanatory variables in predicting plant species richness within and between the study areas. In both the study areas, the inclusion of measures of geodiversity improved the explanatory power, predictive ability and robustness of the plant species richness models. In conclusion, the explicit measures of geodiversity appear to be promising surrogates of biodiversity, which reflect important abiotic resource factors, and may thus provide an equally, or even more reliable basis for transferring biodiversity models to new areas than models based on climate and topography variables.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The preservation of biodiversity is of paramount importance under current global change (Sala et al. 2000; Brooks et al. 2006; Barnosky et al. 2011). However, effective conservation planning is often difficult because spatial data on biodiversity patterns are sparse in many areas and expensive to acquire. Thus, a long-term challenge in conserving biologically valuable areas is the development of an efficient, but yet at the same time comprehensive approach for identification of biologically diverse landscapes, and thereby establishing robust methods for targeting of conservation actions (Whittaker et al. 2005). The most straightforward approach to assessing conservation priorities would be the direct mapping of all biological features of the target area. As this is rarely feasible, numerous indirect surrogates of biodiversity, especially for species richness, have been developed (e.g. Ferrier 2002; Sarkar et al. 2006; Grantham et al. 2010). Desirable attributes for robust and cost-effective surrogates include rapidity and ease of data gathering, sufficient data coverage in the study area, and ecologically plausible links between the species richness patterns and the given surrogate(s).
Biological diversity is determined by internal biotic factors, or extrinsic abiotic factors, or both (Huston 1994). In recent years, the concept of geodiversity has been put forward as a novel alternative and potentially useful means to assess and model spatial biodiversity patterns (see Parks and Mulligan 2010, and the references therein). According to the classical definition, geodiversity refers to the variability of geological, geomorphological and soil features (Gray 2004). In contrast, in the broadest sense geodiversity includes geology, geomorphology, topography, hydrology and climate (Benito-Calvo et al. 2009; Parks and Mulligan 2010). These five components are intimately linked with key abiotic drivers of biodiversity, such as energy, water and nutrients (Richerson and Lum 1980; Kerr and Packer 1997). In other words, geodiversity captures features from the geosphere (geology, geomorphology and topography), hydrosphere (e.g. streams and springs) and atmosphere (e.g. temperature) and their spatial variation, and by these means displays the heterogeneity of abiotic features of Earth surface. Thus, in cases in which biodiversity patterns are mainly governed by abiotic factors, geodiversity may provide a useful surrogate for various aspects of biodiversity (Whittaker et al. 2001; Willis and Whittaker 2002; Hjort and Luoto 2010; Parks and Mulligan 2010).
A better understanding of the relationship between geodiversity (in a broad sense) and biodiversity has been one of the main aims of biogeographical research for many decades (e.g. Harner and Harper 1976; Swanson et al. 1988; Nichols et al. 1998), but the topic is still insufficiently understood. Firstly, this is because most of the previous studies have been conducted on broad scales and covered only one or some aspects of geodiversity. The focal point in such modelling studies has predominantly been on the impacts of factors such as climate and topography (e.g. Richerson and Lum 1980; Owen 1989; Kerr and Packer 1997; Kocher and Williams 2000; Ferrer-Castán and Vetaas 2005; Davidar et al. 2007). Thus, the classical geodiversity attributes have largely been ignored and studies focussing on aspects of hydrology or soils, for example, have clearly been in the minority (e.g. Burnett et al. 1998; Lobo et al. 2001; Pausas et al. 2003; Titeux et al. 2009; Illán et al. 2010). Secondly, most of the previous studies have used oversimplified (e.g. digital elevation model, DEM) measures of geodiversity (Nichols et al. 1998; Mutke and Barthlott 2005; Barthlott et al. 2007; Randin et al. 2009). Thirdly, the relationship between species diversity and measures of geodiversity has not been unequivocally described or explicitly tested (Parks and Mulligan 2010). As a result of these shortcomings, there is a lack of studies in which the potential of geodiversity as a surrogate of biodiversity has systematically been assessed using comprehensive species richness data and unambiguous measures of geodiversity.
In this study, we specifically aimed at comparing the performance of explicitly measured variables of geodiversity (i.e. measures of geological, geomorphological and hydrological variability) and commonly used abiotic surrogates of biodiversity in modelling plant species richness. This was done by developing species richness models for two study areas in the boreal vegetation zone in Finland, Northern Europe, with comprehensive plant species distribution and environmental data. We investigated whether (i) the explanatory power and (ii) predictive ability of the vascular plant species richness models was improved by including measures of geodiversity as an explanatory variable to complement the set of variables describing climatic and topographical conditions. The predictive ability of the models was assessed with respect to within area (within-area prediction, alias model interpolation) and between areas (between-area prediction, alias model extrapolation) model performance.
Study area
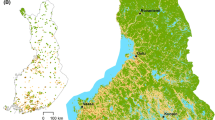
The study areas are located in Finland, Northern Europe (Fig. 1). The Kevo study area (69°39′N, 26°46′E; 362 km2) lies north of the northern limit of continuous pine forests in the transition zone between the northern boreal and hemiarctic zones (Ahti et al. 1968; Oksanen and Virtanen 1995). The study area is within the Kevo Strict Nature Reserve. The area is characterized mostly by open uplands with forests of subalpine mountain birch Betula pubescens ssp. czerepanovii, shallow peats supporting mires, and gently sloping hills and treeless fells (Heikkinen et al. 1998, Hjort and Luoto 2009). The Kevo area includes most of the catchment area of the Kevojoki River which runs in a steep-sided valley, the walls of which can be over 150 m. The climate is subarctic with a mean annual air temperature (MAAT) of −1.7 °C (−14.8 °C in January, 13.0 °C in July) and mean annual precipitation (MAP) of 414 mm (1971–2000; Drebs et al. 2002). The growing season (>5 °C) in the area is 110–120 days.
Locations of the study areas in Finland, Northern Europe: a Kevo (362 km2) and b Oulanka (~ 110 km2) (HB hemiboreal, SB southern boreal, MB middle boreal, NB northern boreal). The terrain views were produced using the shaded relief surface model derived from digital elevation models (sun angle = 45°, azimuth = 315°). The elevations ranged from 95 to 550 m above sea level (a.s.l.) in Kevo and from 140 to 385 m a.s.l. in Oulanka
The Oulanka study area (66°22′N, 29°19′E; ~110 km2), part of Oulanka National Park, is located in the northern boreal zone (Ahti et al. 1968; Fig. 1). The area is characterized by forested hills, and a mosaic of river valleys, water bodies and open wetlands is typical (Parviainen et al. 2009a). Despite the rather harsh climatic conditions, the vegetation is relatively rich with Arctic, eastern, Siberian and southern species (Vasari et al. 1996), and with Norway spruce (Picea abies), Scots pine (Pinus sylvestris) and birches (Betula spp.) as the dominant tree species. The climate of the region is rather continental with a MAAT of −0.7 °C (−15.0 °C in January, 14.7 °C in July) and MAP of 568 mm (1971–2000; Drebs et al. 2002). The growing season in the area is ca. 120–130 days.
Materials and methods
Species richness data
A grid system at a mesoscale resolution was utilized as a spatial study design (Fig. 2). The grid cell size was determined by the resolution of the plant species data. A grid cell size of 1,000 × 1,000 m was used in Kevo (n = 362). In Oulanka (n = 440) most of the grid cells were 500 × 500 m, but the sizes of the marginal grid cells were often less than 25 hectares. The species data were obtained from Söyrinki and Saari (1980) and Heikkinen and Kalliola (1990) for Oulanka and Kevo, respectively. Both of the two areas have been intensively surveyed, as they have been subject to a number of academic theses conducted by the Turku and Oulu university research stations, several hundred days of floristic mapping activities, as well as vegetation mapping of the whole area. Moreover, a number of successful earlier studies have been based on these two plant species data sets, including Heikkinen and Neuvonen (1997), Heikkinen et al. (1998), and Parviainen et al. (2009b). Due to such a high intensity of the surveys, we consider that our two study areas represent some of the floristically best-known areas in their size category in the entire circumboreal vegetation zone. A total of 313 vascular plant species were recorded in the Kevo area, whereas 476 were recorded in Oulanka, respectively. In the subsequent analysis, the total number of vascular plant species recorded in each grid cell was used as a measure of biodiversity (Araújo et al. 2004; Fig. 2).
Vascular plant species richness and measures of geodiversity in Kevo (a–d) and Oulanka (e–h). Spatial patterns of species richness are shown in (a) and (e), geological diversity in (b) and (f), geomorphological diversity in (c) and (g) and hydrological diversity in (d) and (h). The grid cell size is 1,000 × 1,000 m in Kevo and 500 × 500 m in Oulanka
Explanatory variables
In this study, we compiled a total of 17 climate and topography-based variables to cover the most commonly used abiotic resource factors affecting the vascular plant species richness at the mesoscale resolution (Table 1; e.g. Whittaker et al. 2001; Willis and Whittaker 2002; Field et al. 2009). In addition, we compiled three explicit (‘direct’) measures of geodiversity (i.e. diversity of geology, geomorphology and hydrology) to be used in the comparative modelling setting. In this study, we specifically focused (based on the stricter definition of geodiversity) on directly measured geodiversity variables which are hereafter referred to as measures of geodiversity, and the explanatory power of climate and topography variables were assessed and treated separately. A starting hypothesis for our study was that the geology, geomorphology and hydrology variables have the potential to reflect the variability of abiotic conditions more intimately than climate and topography variables. Especially, plant species richness was expected to have rather weak relationship with climate variables in our mesoscale analysis.
We compiled measures of geodiversity following Hjort and Luoto (2010) by simply summing the total number of different geological, geomorphological and hydrological features in the study grid cells (Table 1; Fig. 2). For example, the geomorphological diversity is the sum of different geomorphological feature types regardless of the number and cover of the specific features in the study grid cells. We mapped the features of the geology, geomorphology and hydrology groups using a comprehensive survey of orthorectified aerial photographs (taken 2003–2009; ground resolution ~30 cm) and topographical and geological maps (1:20,000–1:400,000). The group of geological features included rock types and surficial ground materials. We classified the features of geomorphology according to the national system of geomorphological mapping (Fogelberg and Seppälä 1986), which is based on international recommendations (Demek 1972). Geomorphological features included process units and landforms from different geomorphological process groups (aeolian, biogenic, cryogenic, fluvial, glacigenic, glaciofluvial, littoral and marine, polygenetic bedrock, slope and mass-wasting and weathering). The group of hydrological features included springs, streams, rivers, ponds and lakes. The sizes of the smallest mapped features varied from 1 to 2 m for point features (e.g. tors and erratic blocks) to ca. 5 m for areal features (e.g. sand bars and wind deflation sites) (Supplementary material 1).
We estimated that the costs of compiling geodiversity data were higher (ca. 50–100 %) when compared to the costs of the other explanatory variables (assuming free access to the source data sets). However, the data costs are highly dependent on the study area (e.g. availability of aerial photographs, geological maps, topographical and climate data). Thus, the costs for topography and climate data may also be several times higher than geodiversity data. However, the costs to compile geodiversity data were considered to be several tens of times less expensive than direct mapping of plant species distributions of the target study areas.
We derived the climate variables from data provided by the Finnish Meteorological Institute (1961–1990) (Venäläinen and Heikinheimo 2002) and topographical data (DEM, grid cell size = 25 × 25 m) from the National Land Survey of Finland (NLS 2009). We used a trend surface based on multivariate linear regressions to relate climatic variables to the latitude, longitude and elevation of each study grid cell, downscaling climate data from 10 × 10 km resolution to a 1 and 0.25 km2 resolution (for details on the method, see Vajda and Venäläinen 2003). The trend surface consisted of the nine terms of cubic trend surface analysis [centred geographical coordinates (x, y) and the higher and cross-product terms (x2, xy, y2, x3, x2y, xy2 and y3)] and the mean elevation of a grid cell (m above sea level, a.s.l.) calculated for each study square. Besides linear terms, adding the quadratic and cubic terms of the coordinates and their interactions into the model permits the detection of more complex spatial features, e.g. spatial clumping or clustering. The elevation variable enabled us to take into account the effect of local relief on the climatic variables. Vajda and Venäläinen (2003) have shown in a study based on Finnish conditions that latitude (y) and elevation (m a.s.l.) have the most pronounced influence on annual temperature (correlation coefficients = −0.86 and −0.56), whereas the influence of longitude (x) was minor (correlation coefficient = −0.07).
The compiled temperature and moisture-related climate variables were MAAT, mean temperature of the coldest month (MTCM), MAP, potential evapotranspiration (PET) and water balance (WAB) (Skov and Svenning 2004). Water balance was calculated as the monthly difference between precipitation and potential evapotranspiration, and then summing the separate monthly differences. The potential evapotranspiration was calculated following Skov and Svenning (2004):
WAB is a simple but widely used index that requires only monthly average values of temperature and precipitation [Skov and Svenning (2004) and the references therein]. Despite its simplicity, this measure of WAB has been found to provide a valuable variable for the distribution modelling of different taxa [see Skov and Svenning (2004), Luoto and Heikkinen (2008) and Heikkinen et al. (2010) for successful applications]. Moreover, we computed mean, standard deviation and range of theoretical solar radiation (SRAD) in the study grid cells using a computer model of clear sky insolation and exposure of different slopes (McCune and Keon 2002). The DEM-based topographical variables were mean, standard deviation and range of elevation, slope angle and topographical wetness index (TWI). The TWI was calculated using pit-filled DEMs (i.e. sinks were removed to ensure a continuous drainage network) and the formula:
where ln denotes the natural logarithm, As represents the upslope-contributing area and α represents the slope angle (Beven and Kirkby 1979).
We acknowledge here one basic difference between the geodiversity and climate and topography variables. Namely, the geodiversity variables were measures of diversity (i.e. they described how many different elements were present in each grid cell). In contrast, the climate and topography based explanatory variables were not diversity measures (e.g. how many topographically different conditions were present in a given grid cell) but descriptions of which particular elements were present in each grid cell. Nevertheless, the performances of these variable groups were compared here with each other in the modelling, because we deliberately selected the climate and topography variables to represent traditionally employed explanatory variables in species richness analyses and models, whereas the geodiversity variables represented a new, alternative approach to compile explanatory data (Field et al. 2009; Parks and Mulligan 2010).
Statistical analysis
The relationship between species richness and explanatory variables was analysed using two different approaches, partial generalized linear modelling (GLM; McCullagh and Nelder 1989) analyses (i.e. variation partitioning, VP) and generalized additive modelling (GAM). More precisely, VP was used to identify the independent and shared contributions of the geodiversity (i.e. measures of geological, geomorphological and hydrological diversity), climate and topography variable groups in explaining the spatial patterns of species richness in the study areas (Borcard et al. 1992; Hawkins et al. 2003). GAMs were used to explore the ability of the different explanatory variable combinations in predicting the plant species richness patterns within and between the study areas.
In VP, we applied the approach of Hawkins et al. (2003) by which the coefficients of determination [here D2 = (null deviance − residual deviance)/null deviance] were used to determine the independent and shared contributions of the explanatory variable groups. Consequently, we calibrated seven GLMs and computed D2 using (i) climate, (ii) topography, (iii) geodiversity, (iv) climate and topography, (v) climate and geodiversity, (vi) topography and geodiversity variables and (vii) variables from all three groups. Based on D2 values extracted from these seven separate GLM runs, we calculated the independent and shared fractions for the three explanatory variable groups [for more details on the calculation process, see Hawkins et al. (2003) and Heikkinen et al. (2004)]. GLMs were calibrated using the statistical package R version 2.11.1 with standard glm functions (R Development Core Team 2011), a Poisson distribution with a log link function in the model fitting, and quadratic terms in addition to the linear terms of the explanatory variables to capture potential curvilinear responses. The selection of the explanatory variables for the analyses was made so that correlations (Spearman’s rank order correlation, Rs) within the variable groups were <0.7 in order to minimize collinearity problems (e.g. Zimmermann et al. 2007). If Rs between two explanatory variables was >0.7, the one correlated higher with the species richness variable was selected. In cases where the correlation analysis showed rather equal performance of two explanatory variables, an ecologically more relevant variable was selected. The variables for the final GLMs were selected using a step-wise approach and Akaike’s Information Criterion (AIC) (Akaike 1974).
GAMs are semi-parametric extensions of GLMs in which the linear explanatory variable is substituted with a smoothing function that can take various forms (Hastie and Tibshirani 1990). The high performance of GAMs in predictive modelling has been evident in biogeography and ecology studies (e.g. Guisan et al. 2007). GAMs are parameterized just like GLMs, except that some explanatory variables can be modelled non-parametrically in addition to linear and polynomial terms for other variables. GAMs relate the expected value (μ) to the explanatory variables (x j ) by
where g is a link function, α is the constant (i.e. intercept) and f j are unspecified smooth functions. [In practice, the f j are estimated from the data by using techniques developed for smoothing scatterplots (Hastie and Tibshirani 1990).]
In GAMs, Rs between all explanatory variables was determined to be <0.7. The selected variables were fitted to the species richness using a smoothing spline with the degrees of freedom (d.f.) permitted to vary between one (i.e. straight-line relationship) and three (i.e. nonlinear relationship). Similarly to GLMs, GAMs were calibrated using a step-wise AIC approach, with standard gam function (R Development Core Team 2011). The spatial autocorrelation (SAC) in the residuals of GLMs and GAMs were explored using Moran’s I (e.g. Dormann et al. 2007; Bini et al. 2009).
GAMs were calibrated and evaluated using interpolation (i.e. within-area) and extrapolation (i.e. between-area) assessment of model performance. First, a split-sample approach was used to split the study areas into semi-independent calibration and evaluation data sets. The study areas were split into four parts (geographical split of the study areas into south, west, north and east parts; each area = 25 % of the samples). Thus, the calibrated models were evaluated with three data sets (within-area or interpolative evaluation). Second, the calibrated models were evaluated with data from the other study area (between-area or extrapolative evaluation). We utilized the original data sets in the assessment of between-area predictive ability of the GAMs to keep the extrapolation setting straightforward. Differences in the predictive performances of the GAMs with different sets of explanatory variables were tested using Student’s paired t test (Sokal and Rohlf 1995).
Results
A total of nine explanatory variables were selected for the VP and six for the GAM analyses (Tables 1, 2; Fig. 3). The set of variables for VP included all three measures of geodiversity and six variables describing the climatic and topographical conditions of the modelling grid cells. The climate variables MAAT, MTCM, PET and WAB correlated equally well with species richness, but were highly intercorrelated (Rs commonly >0.9). Due to the importance of temperature conditions at high latitudes, the MAAT variable was selected for further analyses. In the GAM model building, all these nine explanatory variables were considered simultaneously and again here, the highly intercorrelated variables were identified and excluded from the actual modelling. Consequently, mean elevation, standard deviation of the slope angle and range of SRAD variables were excluded from the following GAM analysis because they correlated highly with the MAAT and geomorphological diversity variables.
Univariate generalized additive modelling-based response curves for vascular plant species richness and explanatory variables used in variation partitioning analysis in Kevo (a) and Oulanka (b). The explanatory variables were fitted to species richness using a smoothing spline with the degrees of freedom permitted to vary between one (i.e., straight-line relationship) and three (i.e. nonlinear relationship) (y axis = fitted function with estimated degrees of freedom). Explained variation (%) also appears in this figure. GeolDiv geological diversity, GeomDiv geomorphological diversity, HydroDiv hydrological diversity, MAAT mean annual air temperature, MAP mean annual precipitation, SradRange range of theoretical solar radiation, ElevMean mean of elevation, SlopeStd standard deviation of slope angle, TwiRange range of topographical wetness index
Overall, the general form of the relationship between measures of geodiversity and plant species richness was rather constant, i.e. the shape of the response functions showed a positive trend in both areas indicating increasing species richness with increasing geodiversity (Fig. 3). However, in some of the cases an asymptotic curvilinear relationship was observed in the model outputs. According to the residual plots of the final GLMs and GAMs, the assumption of Poisson errors was appropriate. Moreover, SAC in the residuals of the final models was generally rather low (Supplementary material 2). In Kevo, the Moran’s I values in the first distance class ranged from −0.01 to 0.14 and from −0.19 to 0.22 for GLMs and GAMs, respectively. In Oulanka, the comparable values were 0.18–0.33 for GLMs and −0.10–0.35 for GAMs.
The results of VP were surprisingly similar in the two study areas (Fig. 4). The GLMs with explanatory variables from all the three groups explained 51.7 % (Kevo) and 53.4 % (Oulanka) of the variation in the plant species richness data. The shared contribution of all the three groups of variables was notably high, explaining 23.6 % (Oulanka) and 25.6 % (Kevo) of the variation. Considering pure fractions, the group of geodiversity measures showed the highest pure explanatory power for the species richness patterns (9.1 and 11.2 %), while the climate variables showed the lowest (Fig. 4).
Results of the variation partitioning (VP) analyses in the study areas, based on a series of partial regression analyses with generalized linear models. The figures describe the amount of explained variation (%) in the species richness data as independent and shared contributions (U undetermined variation). Note that the shared variation may be negative due to suppressor variables (i.e., a explanatory variable having low correlation with the response variable and a correlation with another explanatory variable, which in turn is correlated with the response variables) or due to two strongly correlated predictors with strong effects on response of opposite signs (one positive and the other negative) (e.g. Legendre and Legendre 1998). The climate group included the following variables: mean annual air temperature, mean annual precipitation and range of theoretical solar radiation. Topographical variables were mean elevation, standard deviation of slope angle and range of topographical wetness index. The geodiversity group included the variables geological diversity, geomorphological diversity and hydrological diversity
GAMs calibrated with measures of geodiversity were on average more robust than models with only climate and topography variables, especially GAMs that included only measures of geodiversity (Table 3). In both evaluation settings, models with measures of geodiversity were better (statistically, from nearly significant to significant differences) in predicting the plant species richness than models with climate and topography variables (‘GD-models’ vs. ‘CLIMTOPO-models’) (Tables 3, 4). The inclusion of geodiversity variables into the set of explanatory variables improved the predictive abilities of the GAMs significantly in the within-area but not significantly in the between-area evaluation (CLIMTOPO vs. ALL). Interestingly, models including only measures of geodiversity showed a better predictive performance than models including variables from all the groups (GD vs. ALL) (Table 4). Moreover, the models with measures of geodiversity seemed to be more accurate in intra-area prediction of the plant species richness when assessed based on the optimal fit line between predicted and observed values (i.e. slopes were closer to 1 and intercepts to 0) (Fig. 5).
Scatter plots between predicted and observed vascular plant species richness in the intra-area evaluation data. The predictions were derived using generalized additive modelling (GAM) (see the text for further details). The example plots were selected to describe good (a–c, Oulanka) and moderate (d–f, Kevo) predictive success. Plots (a) and (d) were derived using GAMs based on geodiversity variables (i.e. measures of geodiversity); plots (b) and (e) were derived using GAMs based on the climate and topography variables; and plots (c) and (f) were derived using GAMs based on the geodiversity, climate and topography variables
Discussion
We have shown here that consideration of explicit measures of geodiversity—not just DEM-based topographical or “geomorphological” variables—can improve species richness models in three fundamental ways at the mesoscale resolution. Firstly, the inclusion of variables describing geological, geomorphological and hydrological variability increased the explanatory power of our species richness models. Secondly, the predictive ability and accuracy of the species richness models were higher when measures of geodiversity were included in the set of explanatory variables in addition to the commonly used variables describing abiotic resources. In fact, our results suggested that models with only measures of geodiversity showed a better predictive performance than models with explanatory variables from all three groups. Thirdly, the inclusion of measures of geodiversity improved the robustness of the models.
The two key rationales for using features of geodiversity to model and predict the spatial variation in species richness patterns are the following: (i) the potential that long-term targets in biodiversity conservation may be better achieved by focusing conservation actions on preserving heterogenic abiotic units rather than on biological communities, which are more subject to changes under changing environmental conditions, and (ii) lower survey costs in mapping the geodiversity features of the areas compared to running comprehensive biological surveys. In other words, when geodiversity is the focal site-selection criterion and when sites with high geodiversity are protected, the aim is not to preserve certain types of static biotic communities. Rather, the focus is on protecting areas with a high probability of harbouring high biodiversity, even if the species composition of these areas would change over time (e.g. Hunter et al. 1988). Consequently, geodiversity conservation has the potential to provide a useful approach for long-term preservation of biodiversity that may be applied across a range of spatial scales (Anderson and Ferree 2010; Beier and Brost 2010; Parks and Mulligan 2010).
The measures of geodiversity used here, i.e. diversity of geology, hydrology and geomorphology, were rather clearly linked to plant species richness, probably because of their contribution to one or more important resource factors (cf. Vanreusel et al. 2007). Geology (e.g. rock type) and geological processes (e.g. weathering) contribute to the availability of important resources for plants by providing them with a substrate and nutrients (e.g. Birks 1996; Heikkinen et al. 1998; Moser et al. 2005). Moreover, different rock types and surficial deposits increase the environmental heterogeneity and number of different habitats in an area. The positive relationship between plant species richness and hydrological diversity supports earlier findings that moisture availability is one of the critical factors for plant species distribution and richness (Gosselink and Turner 1978). However, the linkage between species richness and hydrological diversity goes beyond this general maxim and supports the observations of Nichols et al. (1998) that the within-area variation of water availability also significantly contributes to the number of species that a given landscape can support (see also Lavers and Field 2006). For example, streams and rivers were considered as different features in hydrological diversity because their characteristics can differ significantly: drastic floods in river environments and ephemerality of small streams give rise to different growing conditions for plants.
In the context of space and spatial heterogeneity, geomorphological diversity is linked to plant species richness because it partly controls the variability of habitats. Moreover, geomorphological diversity contains features of various sizes, from small-sized fluvial features to extensive fracture valleys, which provide habitats with different sizes and ecological characteristics. A more diverse landscape consisting of various different abiotic habitats and structural organisms provides a setting for a wider number of niches available for species to occupy (Menge and Sutherland 1976; Tilman 1994; Moser et al. 2005).
Physical disturbance is regarded as an important driver of biodiversity (e.g. Tilman 1982; Huston 1994) which may have a particularly notable significance in high-latitude landscapes (Birks 1996; Virtanen et al. 2010). Physical disturbance associated with soil instability and other geomorphological processes causes a decrease in biomass (Grime 2001), and changes in resource availability (Tilman 1982) and species interactions (Crawley 1986). Disturbances often counteract competitive exclusion and enhance the spatial heterogeneity of vegetation, and both of these factors may have a positive influence on species richness (Huston 1994). In our study, geomorphological diversity included several process types that actively modify the landscape under current conditions (Hjort and Luoto 2009, 2010). Physical processes, especially those related to running water and slopes, generate fine-scale dynamical disturbances and patches free of competition in the vegetation cover. These processes often results in overall enhanced biodiversity on a given site. In an extreme case, geomorphological processes may create novel ecospaces that can be colonized by species new to the area (e.g. Benton 2009).
The measures of geodiversity used in this study are simple and easy and rapid to employ, but yet rather explicit. Moreover, they seem to convey information that is not covered by the commonly utilized abiotic variables (Whittaker et al. 2001; Willis and Whittaker 2002; Field et al. 2009). However, our measures of geodiversity did not include direct estimates of climate or other energy-related components (e.g. Serrano and Ruiz-Flaño 2007; Benito-Calvo et al. 2009). Earlier studies have shown that the energy-related factors (e.g. evapotranspiration, temperature and productivity) may explain up to 90 % of the variation in biodiversity patterns (Mittelbach et al. 2001), and the importance of energy-related factors is especially pronounced in broad-scale studies (Currie and Paquin 1987; Wright et al. 1993; Kerr and Packer 1997; Parks and Mulligan 2010). However, our analyses were performed at the landscape scale at which the abiotic heterogeneity (e.g. geomorphology and hydrology), in addition to properties of vegetation, controls microclimatological variability (Geiger 1965; Hayden 1998). Thus, the energy component was indirectly included in our measures of geodiversity. In addition, it should be noted that other factors often begin to show relatively more importance for species richness than energy-related factors when switching the resolution from broad biogeographical scales to a landscape or local scale (Wright et al. 1993; Whittaker et al. 2001; Willis and Whittaker 2002; Luoto et al. 2007). Nevertheless, we acknowledge that when the exploration of process-based links between geodiversity and biodiversity (i.e. mechanistic analysis) or prediction of biodiversity at national or continental scales is the focus of the study, the incorporation of energy-related variables in geodiversity will no doubt have a higher relevance (Parks and Mulligan 2010).
Our analyses indicate that measures of geodiversity were the best single variable group for explaining plant species richness at the landscape scale. However, a well-known limitation is that species richness is a measure that conveys no information on the actual species composition of a community or on the habitat composition of a landscape (Luoto et al. 2004). Correspondingly, geodiversity is a landscape summary measure that does not take into account the uniqueness or potential ecological importance of different habitats. This may limit its usefulness under certain circumstances. Further, there are situations in which increasing geodiversity may contradict management objectives for threatened species that require large and homogeneous habitat patches of a specific habitat type, since higher habitat diversity may actually be a reflection of undesirable fragmentation of critical habitats (McGarigal and Marks 1994). These challenging features of the geodiversity measures may give rise to biased predictions in the models from the conservation and management point of view, especially for species that require large areas of suitable habitat (see Luoto et al. 2004; Hanski 2005).
There are also some other potential data- and method-related limitations to be acknowledged. First, our study included plant species data from only two study areas with particular environmental conditions. Thus, without testing, our findings cannot be applied to other types of environments, landscapes and biomes. For example, vegetation in more topographically uniform boreal landscapes can be rather homogenous when compared with mountainous regions. Second, the utilized air temperature and precipitation variables were downscaled from a coarser resolution, which probably underestimated the importance of the climate variables and may have influenced the results. On the other hand, it should be noted that the solar radiation and topographical variables employed in our study very likely cover part of the microclimatological variability at the modelling resolution (Geiger 1965; Hayden 1998).
A general assumption in biogeographical species richness modelling studies has been that climate plays a less significant role than other abiotic conditions at the landscape scale (Pearson and Dawson 2003). Thus, the concept used in this study should be developed further and tested on a broader sampling scale, particularly on a wider sampling extent. Also the approach to measure geodiversity could be developed further. For example, more ecologically relevant measures of geodiversity might be outlined by considering the habitat requirements of the plants in the target area. This target could be approached by weighting different features of geodiversity according to their importance for plants (e.g. higher weights for limestone and gully than granite and erratic block, respectively) (cf. Ruban 2010). Overall, the development of ecologically relevant measures of geodiversity can be arguably included among the key tasks in geodiversity research in future (Gordon et al. 2012).
Methodologically, the relatively small data sets employed here hampered the fully independent evaluation of the models. Given this, our statistical test results may be over-optimistic. Moreover, the presence of SAC in model residuals may increase type I error rates (falsely rejecting the null hypothesis of no effect). However, SAC in the residuals of GLMs and GAMs developed in this study were rather low (Dormann et al. 2007; Bini et al. 2009). Thus, we consider that the potential influence of residual SAC for the interpretation of our results would be low. Another point which should be acknowledged is that the relationships between species richness and measures of geodiversity were in all cases positive, but they were not always linear throughout (cf. Nichols et al. 1998). If the threshold is not known, the asymptotic levelling off effect may complicate the accurate prediction of high species diversity sites.
Despite these limitations, our results provide a clear signal that the inclusion of explicit and directly measured geodiversity variables have the potential to improve species richness models and provide novel insights into biodiversity surveys (Swanson et al. 1988; Nichols et al. 1998; Lobo et al. 2001; Pausas et al. 2003; Illán et al. 2010). When the interpretation of the variability of geological, geomorphological and hydrological features is done systematically and with caution, they may provide a more reliable basis for transferring biodiversity models to new areas than topographical or climatic variables especially, at scales, where the effect of climate is expected to be rather weak.
Conclusions
A long-term challenge in biodiversity research has been the development of robust methods for cost-effective targeting of conservation actions. Here we compared the explanatory and predictive performance of directly measured variables of geodiversity and commonly used abiotic surrogates of biodiversity in modelling and have shown that the inclusion of explicit measures of geodiversity can improve the explanatory power, predictive ability and robustness of the mesoscale plant species richness models in the boreal zone. Thus, the measures of geodiversity employed, namely geological, geomorphological and hydrological diversity, appear to be promising surrogates of biodiversity which both directly and indirectly reflect important abiotic resource factors. In areas with insufficient climate and topography data, simple measures of geodiversity may provide the best surrogates for biodiversity patterns.
References
Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in northwestern Europe. Ann Bot Fennici 5:169–211
Akaike H (1974) A new look at the statistical identification model. IEEE Trans Autom Contr 19:716–723
Anderson MG, Ferree CE (2010) Conserving the stage: climate change and the geophysical underpinnings of species diversity. PLoS ONE 5:e11554
Araújo MB, Densham PJ, Williams PH (2004) Representing species in reserves from patterns of assemblage diversity. J Biogeogr 31:1037–1050
Barnosky AD, Matzke N, Tomiya S, Wogan GOU, Swartz B, Quental TB, Marshall C, McGuire JL, Lindsey EL, Maguire KC, Mersey B, Ferrer EA (2011) Has the Earth’s sixth mass extinction already arrived? Nature 471:51–57
Barthlott W, Hostert A, Kier G, Koper W, Kreft H, Mutke J, Rafiqpoor MD, Sommer JH (2007) Geographic patterns of vascular plant diversity at continental to global scales. Erdkunde 61:305–315
Beier P, Brost B (2010) Use of land facets to plan for climate change: conserving the arenas, not the actors. Conserv Biol 24:701–710
Benito-Calvo A, Pérez-González A, Magri O, Meza P (2009) Assessing regional geodiversity: the Iberian Peninsula. Earth Surf Proc Land 34:1433–1445
Benton MJ (2009) The Red Queen and the Court Jester: species diversity and the role of biotic and abiotic factors through time. Science 323:728–732
Beven KJ, Kirkby MJ (1979) A physically based, variable contributing area model of basin hydrology. Hydrolog Sci Bull 24:43–69
Bini LM, Diniz-Filho JAF, Rangel TFLV, Akre TSB, Albaladejo RG, Albuquerque FS, Aparicio A, Araújo MB, Baselga A, Beck J, Bellocq MI, Böhning-Gaese K, Borges PAV, Castro-Parga I, Chey VK, Chown SL, De Marco P, Dobkin DS, Ferrer-Castán D, Field R, Filloy J, Fleishman E, Gómez JF, Hortal J, Iverson JB, Kerr JT, Kissling WD, Kitching IJ, León-Cortés JL, Lobo JM, Montoya D, Morales-Castilla I, Moreno JC, Oberdorff T, Olalla-Tárraga MÁ, Pausas JG, Qian H, Rahbek C, Rodríguez MÁ, Rueda M, Ruggiero A, Sackmann P, Sanders NJ, Terribile LC, Vetaas OR, Hawkins BA (2009) Coefficient shifts in geographical ecology: an empirical evaluation of spatial and non-spatial regression. Ecography 32:193–204
Birks HJB (1996) Statistical approaches to interpreting diversity patterns in the Norwegian mountain flora. Ecography 19:332–340
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Brooks TM, Mittermeier RA, da Fonseca GAB, Gerlach J, Hoffmann M, Lamoreux JF, Mittermeier CG, Pilgrim JD, Rodrigues ASL (2006) Global biodiversity conservation priorities. Science 313:58–61
Burnett M, August P, Brown J, Killingbeck K (1998) The influence of geomorphological heterogeneity on biodiversity. I. A patch-scale perspective. Conserv Biol 12:363–370
Crawley MJ (1986) The population biology of invaders. Phil Trans R Soc B 314:711–731
Currie DJ, Paquin V (1987) Large-scale biogeographical patterns of species richness of trees. Nature 329:326–327
Davidar P, Rajagopal B, Mohandass D, Puravaud J, Ccondit R, Wright SJ, Leigh EG Jr (2007) The effect of climatic gradients, topographic variation and species traits on the beta diversity of rain forest trees. Global Ecol Biogeogr 16:510–518
Demek J (1972) ed) Manual of detailed geomorphological mapping. Academia, Praque
Dormann CF, McPherson JM, Araújo MB, Bivand R, Bolliger J, Carl G, Davies RG, Hirzel AH, Jetz W, Kissling WD, Kühn I, Ohlemüller R, Peres-Neto PR, Reineking B, Schröder B, Schurr F, Wilson R (2007) Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609–628
Drebs A, Nordlund A, Karlsson P, Helminen J, Rissanen P (2002) Climatological statistics of Finland 1971–2000. Clim Stat Finland 2002 1
Ferrer-Castán D, Vetaas OR (2005) Pteridophyte richness, climate and topography in the Iberian Peninsula: comparing spatial and nonspatial models of richness patterns. Global Ecol Biogeogr 14:155–165
Ferrier S (2002) Mapping spatial pattern in biodiversity for regional conservation planning: where to from here? System Biol 51:331–363
Field R, Hawkins BA, Cornell HV, Currie DJ, Diniz-Filho JAF, Guégan JF, Kaufman DM, Kerr JT, Mittelbach GG, Oberdorff T, O’Brien EM, Turner JRG (2009) Spatial species-richness gradients across scales: a meta-analysis. J Biogeogr 36:132–147
Fogelberg P, Seppälä M (1986) Geomophology. In: Alalammi P (ed) Geomophology, Atlas of Finland, Folio 122. National Board of Survey & Geographical Society of Finland, Helsinki, pp 1–19
Geiger R (1965) The Climate Near the Ground. Harvard University Press, Cambridge
Gordon JE, Barron HF, Hansom JD, Thomas MF (2012) Engaging with geodiversity – why it matters. Proc Geol Assoc 123:1–6
Gosselink JG, Turner RE (1978) The role of hydrology in freshwater wetland ecosystems. In: Good RE, Whigham DF, Simpson RL (eds) Freshwater wetlands: ecological processes and management potential. Academic, New York, pp 63–78
Grantham HS, Pressey RL, Wells JA, Beattie AJ (2010) Effectiveness of biodiversity surrogates for conservation planning: different measures of effectiveness generate a kaleidoscope of variation. PLoS ONE 5:e11430
Gray M (2004) Geodiversity valuing and conserving abiotic nature. Wiley, Chichester
Grime JP (2001) Plant strategies, vegetation processes, and ecosystem properties, 2nd edn. Wiley, Chichester
Guisan A, Graham CH, Elith J, Huettmann F, the NCEAS Species Distribution Modelling Group (2007) Sensitivity of predictive species distribution models to change in grain size. Divers Distrib 13:332–340
Hanski I (2005) The shrinking world: ecological consequences of habitat loss. International Ecology Institute, Oldendorf
Harner RF, Harper KT (1976) The role of area, heterogeneity, and favorability in plant species diversity of pinyon-juniper ecosystems. Ecology 57:1254–1263
Hastie TJ, Tibshirani RJ (1990) Generalized additive models. Chapman and Hall, London
Hawkins BA, Porter EE, Diniz-Filho JAF (2003) Productivity and history as predictors of the latitudinal diversity gradient for terrestrial birds. Ecology 84:1608–1623
Hayden BP (1998) Ecosystem feedbacks on climate at the landscape scale. Phil Trans R Soc B 353:5–18
Heikkinen RK, Kalliola RJ (1990) The vascular plants of the Kevo nature reserve (Finland); an ecological-environmental analysis. Kevo Notes 9:1–56
Heikkinen RK, Neuvonen S (1997) Species richness of vascular plants in the subarctic landscape of northern Finland: modelling relationships to the environment. Biodivers Conserv 6:1181–1201
Heikkinen RK, Birks HJB, Kalliola RJ (1998) A numerical analysis of the mesoscale distribution patterns of vascular plants in the subarctic Kevo nature reserve, northern Finland. J Biogeogr 25:123–146
Heikkinen RK, Luoto M, Virkkala R, Rainio K (2004) Effects of habitat cover, landscape structure and spatial variables on the abundance of birds in an agricultural-forest mosaic. J Appl Ecol 41:824–835
Heikkinen RK, Luoto M, Leikola N, Pöyry J, Settele J, Kudrna O, Marmion M, Fronzek S, Thuiller W (2010) Assessing the vulnerability of European butterflies to climate change using multiple criteria. Biodivers Conserv 19:695–723
Hjort J, Luoto M (2009) Interaction of geomorphic and ecologic features across altitudinal zones in a subarctic landscape. Geomorphology 112:324–333
Hjort J, Luoto M (2010) Geodiversity of high-latitude landscapes in northern Finland. Geomorphology 115:109–116
Hunter ML Jr, Jacobson GL Jr, Webb T III (1988) Paleoecology and the coarse-filter approach to maintaining biological diversity. Conserv Biol 4:375–385
Huston MA (1994) Biological diversity: the coexistence of species on changing landscapes. Cambridge University Press, Cambridge
Illán JG, Gutiérrez D, Wilson RJ (2010) Fine-scale determinants of butterfly species richness and composition in a mountain region. J Biogeogr 37:1706–1720
Kerr JT, Packer L (1997) Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature 385:252–254
Kocher SD, Williams ED (2000) The diversity and abundance of North American butterflies vary with habitat disturbance and geography. J Biogeogr 27:785–794
Lavers CP, Field R (2006) A resource-based conceptual model of plant diversity that reassesses causality in the productivity–diversity relationship. Global Ecol Biogeogr 15:213–224
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
Lobo JM, Castro I, Moreno JC (2001) Spatial and environmental determinants of vascular plant species richness distribution in the Iberian Peninsula and Balearic Islands. Biol J Linn Soc 73:233–253
Luoto M, Heikkinen RK (2008) Disregarding topographic heterogeneity biases species turnover assessments based on bioclimatic models. Glob Change Biol 14:483–494
Luoto M, Virkkala R, Heikkinen RK, Rainio K (2004) Predicting bird species richness using remote sensing in boreal agricultural–forest mosaics. Ecol Appl 14:1946–1962
Luoto M, Virkkala R, Heikkinen RK (2007) The role of land cover in bioclimatic models depends on spatial resolution. Global Ecol Biogeogr 16:34–42
McCullagh P, Nelder JA (1989) Generalized linear models, 2nd edn. Chapman and Hall, London
McCune B, Keon D (2002) Equations for potential annual direct incident radiation and heat load. J Veg Sci 13:603–606
McGarigal K, Marks BJ (1994) FRAGSTATS: spatial pattern analysis program for quantifying landscape structure. Version 2.0. Forest Science Department, Oregon State University, Corvallis
Menge BA, Sutherland JP (1976) Species diversity gradients: synthesis of the roles of predation, competition and temporal heterogeneity. Am Nat 101:351–369
Mittelbach GG, Steiner CF, Scheiner SM, Gross KL, Reynolds HL, Waide RB, Willig MR, Dodson SI, Gough L (2001) What is the observed relationship between species richness and productivity? Ecology 82:2381–2396
Moser D, Dullinger S, Englisch T, Niklfeld H, Plutzar C, Sauberer N, Zechmeister HG, Grabherr G (2005) Environmental determinants of vascular plant species richness in the Austrian Alps. J Biogeogr 32:1117–1127
Mutke J, Barthlott W (2005) Patterns of vascular plant diversity at continental to global scales. Biol Skrift 55:521–537
Nichols WF, Killingbeck KT, August PV (1998) The influence of geomorphological heterogeneity on biodiversity II. A landscape perspective. Conserv Biol 12:371–379
NLS (2009) Digital elevation model. National land survey of Finland, Helsinki
Oksanen L, Virtanen R (1995) Topographic, altitudinal and regional patterns in continental and suboceanic heath vegetation in northern Fennoscandia. Acta Bot Fennica 153:1–80
Owen JG (1989) Patterns of herpetofaunal species richness: relation to temperature, precipitation and variance in elevation. J Biogeogr 16:141–150
Parks KE, Mulligan M (2010) On the relationship between a resource based measure of geodiversity and broad scale biodiversity patterns. Biodivers Conserv 19:2751–2766
Parviainen M, Luoto M, Heikkinen RK (2009a) The role of local and landscape level productivity in modelling of boreal plant species richness. Ecol Model 220:2690–2701
Parviainen M, Marmion M, Luoto M, Thuiller W, Heikkinen RK (2009b) Using summed individual species models and state-of-the-art modelling techniques to identify threatened plant species hotspots. Biol Conserv 142:2501–2509
Pausas JG, Carreras J, Ferre A, Font X (2003) Coarse-scale plant species richness in relation to environmental heterogeneity. J Veg Sci 14:661–668
Pearson RG, Dawson TP (2003) Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecol Biogeogr 12:361–371
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Randin CF, Vuissoz G, Liston GE, Vittoz P, Guisan A (2009) Introduction of snow and geomorphic disturbance variables into predictive models of alpine plant distribution in the Western Swiss Alps. Arctic Antarctic Alpine Res 41:347–361
Richerson PJ, Lum K (1980) Patterns of plant species and diversity in California: relation to weather and topography. Am Nat 116:504–536
Ruban DA (2010) Quantification of geodiversity and its loss. Proc Geol Assoc 121:326–333
Sala OE, Chapin SF III, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, Poff NL, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sarkar S, Pressey RL, Faith DP, Margules CR, Fuller T, Stoms DM, Moffett A, Wilson KA, Williams KJ, Williams PH, Andelman S (2006) Biodiversity conservation planning tools: present status and challenges for the future. Ann Rev Env Res 31:123–159
Serrano E, Ruiz-Flaño P (2007) Geodiversity: a theoretical and applied concept. Geogr Helv 62:140–147
Skov F, Svenning J-C (2004) Potential impact of climatic change on the distribution of forest herbs in Europe. Ecography 27:366–380
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biology research, 3rd edn. WH Freeman, New York
Söyrinki N, Saari V (1980) Die Flora im Nationalpark Oulanka, Nord-Finnland. Acta Florestica Fennica 114:1–150
Swanson FJ, Kratz TK, Caine N, Woodmansee RG (1988) Landform effects on ecosystems pattern and processes. Bioscience 38:92–98
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Titeux N, Maes D, Marmion M, Luoto M, Heikkinen RK (2009) Inclusion of soil data improves the performance of bioclimatic envelope models for insects species distributions in temperate Europe. J Biogeogr 36:1459–1473
Vajda A, Venäläinen A (2003) The influence of natural conditions on the spatial variation of climate in Lapland, Northern Finland. Int J Clim 23:1011–1022
Vanreusel W, Maes D, Van Dyck H (2007) Transferability of species distribution models: a functional habitat approach for two regionally threatened butterflies. Conserv Biol 21:201–212
Vasari Y, Tonkov S, Vasari A, Nikolova A (1996) The late-quaternary history of the vegetation and flora in northeastern Finland in the light of a re-investigation of Aapalampi in Salla. Aquilo Ser Bot 36:27–41
Venäläinen A, Heikinheimo M (2002) Meteorological data for agricultural applications. Phys Chem Earth 27:1045–1050
Virtanen R, Luoto M, Rämä T, Mikkola K, Hjort J, Grytnes JA, Birks HJB (2010) Recent vegetation changes in the high-latitude tree-line ecotone are controlled by geomorphological disturbance, productivity and diversity. Global Ecol Biogeogr 19:810–821
Whittaker RJ, Willis KJ, Field R (2001) Scale and species richness: towards a general, hierarchical theory of species diversity. J Biogeogr 28:453–470
Whittaker RJ, Araújo MB, Paul J, Ladle RJ, Watson JEM, Willis KJ (2005) Conservation biogeography: assessment and prospect. Divers Distrib 11:3–23
Willis KJ, Whittaker RJ (2002) Species diversity: scale matters. Science 295:1245–1248
Wright DH, Currie DJ, Maurer BA (1993) Energy supply and patterns of species richness on local and regional scales. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities: historical and geographical perspectives. University of Chicago Press, Chicago, pp 66–74
Zimmermann NE, Edwards TC, Moisen GG, Frescino TS, Blackard JA (2007) Remote sensing-based predictors improve distribution models of rare, early successional and broadleaf tree species in Utah. J Appl Ecol 44:1057–1067
Acknowledgments
We would like to express our gratitude to Paul Beier and two anonymous reviewers for their helpful and constructive comments. Moreover, we would like to acknowledge Lauren Martin for her help in checking the English of the revised manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hjort, J., Heikkinen, R.K. & Luoto, M. Inclusion of explicit measures of geodiversity improve biodiversity models in a boreal landscape. Biodivers Conserv 21, 3487–3506 (2012). https://doi.org/10.1007/s10531-012-0376-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0376-1