Abstract
Wetlands around the world face unprecedented threats, including from invasive species. In North America, the invasive cattail hybrid Typha x glauca dominates wetlands around the Laurentian Great Lakes; more recently it was found in high abundance across the central and eastern Prairie Pothole Region, an area that includes one of the world’s largest wetland complexes. Surveys of the Typha hybrid zone have so far been largely conducted in areas where hybrids are well established, and it therefore remains unclear whether the range expansion of this invasive hybrid occurs after the establishment of its maternal species, T. angustifolia. We surveyed 50 wetlands in the western PPR and found that while 75% of plants were native T. latifolia, the second most common group was F1 hybrids, which had greater abundance and occupancy than T. angustifolia despite the fact that T. angustifolia produces relatively few hybrid seeds; our findings therefore highlight the importance of long-distance dispersal for this hybrid range expansion. The distribution of hybrids combined with the paucity of non-F1 hybrids suggest that the western PPR represents a leading edge of the range expansion by invasive T. × glauca. Our results show that T. × glauca has the capacity for continued range expansion that does not rely on the presence of T. angustifolia, and the impacts of this range expansion should be monitored because of its potential to impede ecosystem services and reduce local biodiversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Wetlands provide essential ecosystem services and accommodate high levels of biodiversity across an estimated 5–10% of the Earth’s surface; however, despite their importance, > 70% of natural wetlands have been lost globally since 1900 (Davidson 2014; Kingsford et al. 2016). Threats to wetlands include development, climate change, pollution, and invasive species (Zedler and Kercher 2004; Xu et al. 2019). In wetlands within the region surrounding the Laurentian Great Lakes and St. Lawrence Seaway in North America, one problematic invader is the cattail hybrid complex involving Typha × glauca, which is the interspecific F1 hybrid of native T. latifolia and naturalized T. angustifolia (Grace and Harrison 1986). Typha × glauca is fertile, and can interbreed to form advanced-generation hybrids, or backcross with either of its parent species to form backcrossed hybrids (Pieper 2017); because advanced-generation and backcrossed hybrids are difficult to differentiate, we refer to these collectively as non-F1 hybrids. In regions around the Great Lakes and St. Lawrence Seaway the hybrid displaces parental species and dominates wetlands (Travis et al. 2010; Freeland et al. 2013; Pieper et al. 2020), and is considered invasive because it reduces biodiversity and alters wetland functions (reviewed in Bansal et al. 2019).
The invasion of wetlands around the Laurentian Great Lakes has led to multiple management programs aimed at controlling or eliminating T. × glauca (e.g. Lawrence et al. 2016; Graham et al. 2022), and has also raised concerns about range expansion by this invasive hybrid, including into the Prairie Pothole Region (PPR) of North America. The PPR occurs within the Great Plains of central North America, and includes one of the world’s largest wetland complexes. Here there are an estimated 5–8 million wetlands commonly referred to as potholes, most of which are depressions < 1 ha in area, < 1 m in depth, and commonly within farmland (Doherty et al. 2016). Wetlands in the PPR provide essential ecosystem services, and harbour substantial levels of biodiversity, for example they enable millions of waterfowl to migrate through, and breed within, the PPR each year (Doherty et al. 2018; Pattison-Williams et al. 2018). Monitoring invasive wetland plants in the PPR is essential to our understanding of whether these wetlands are at risk from substantial modification following biological invasions. A recent study that surveyed 131 wetlands spread over approximately 350,000 km2 in the central and eastern PPR found hybrid cattails in over 80% of surveyed wetlands; in contrast, T. latifolia was found in 26% of wetlands, and T. angustifolia in 18% of wetlands (Tangen et al. 2022). Non-F1 hybrids comprised the most common taxonomic category in the central/eastern PPR (57% of genets compared to 30% that were F1 hybrids), which suggests that the hybrid zone in the central/eastern PPR is not very recent (Tangen et al. 2022).
While the Tangen et al. study (2022) provided important information about the abundance and occupancy of hybrid Typha throughout wetlands in the central and southeast PPR, some knowledge gaps remain. In this study we expanded the investigation of the Typha hybrid zone into previously unsampled regions of the west/northwest PPR, beyond the presumed range limit of T. angustifolia. F1 T. × glauca hybrids arise following asymmetric hybridization, in which T. angustifolia is normally the maternal parent and T. latifolia is the paternal parent (Pieper 2017). There are almost no records of T. angustifolia from the western PPR, which could preclude the local formation of hybrids, and we therefore addressed the following questions: (1) is the paucity of T. angustifolia records from the western PPR an accurate reflection of T. angustifolia occurrence in this region? (2) If T. angustifolia plants occur in the western PPR, are they producing seeds that are T. × glauca? (3) Does the range expansion of T. × glauca follow the range expansion of its maternal species, T. angustifolia?
Methods
Field work
Between August 19 and August 24, 2022, we sampled 246 Typha plants from 50 wetlands (34 wetlands in the province of Saskatchewan, 16 wetlands in the province of Alberta) in the western PPR (Fig. 1, Table S1). Sampled sites were mostly restricted to small wetlands accessible from roadsides because a substantial portion of the Prairie Pothole Region is privately owned agricultural land. This sampling strategy should not have influenced our findings because an earlier study found that Typha species and hybrids had comparable frequencies in wetlands versus ditches in eastern North America (Pieper et al. 2020). Typha latifolia is native to Saskatchewan and Alberta (Flora of North America 2008) and was thus expected to occur at the majority of sites; we therefore prioritized the sampling of T. angustifolia followed by T. × glauca. We did this by first visually inspecting the stands at each site and identifying plants that appeared to have the narrowest leaves and—for flowering plants—the largest gaps between the staminate and pistillate flowers: T. angustifolia has the narrowest leaves and largest flower gaps, T. latifolia has the widest leaves and typically no discernible flower gap, and F1 hybrids are intermediate in both traits (Smith 1967). Between two and eight plants with what appeared to be the narrowest leaves were sampled at each site (average number of plants per site = 4.9; Table S1); plants were separated by a minimum of 2 m to reduce the likelihood of sampling ramets from the same genet. From each sampled plant we collected two leaf fragments approximately 10 cm long. Leaf samples were placed into labelled coin envelopes, and the envelopes were placed in a plastic zippered bag containing Sor bead orange silica beads for desiccation. In addition, we collected mature fruits from nine plants growing at two sites (Craik and Regina; Table S1) that were provisionally identified as T. angustifolia, and stored these in paper bags that were enclosed within resealable plastic bags. Our sample size of T. angustifolia fruits was relatively small because it was the least common taxon, plus not all plants flower in any given year.
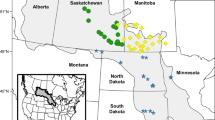
Distribution of Typha taxa across the 50 sampled wetlands. At each site a pie chart illustrates the percentage of each taxon. Black dots within the pie chart represent the site location, and leader lines were used to connect pie charts to site locations when nearby wetlands were also sampled. The shaded area in the inset represents the approximate geographic boundaries of the western prairie pothole region
Genotyping
Because the leaf widths and flower gaps of advanced-generation (AGH) and backcrossed hybrids (BCH) can overlap with parental taxa (Snow et al. 2010; Kirk et al. 2011; Geddes et al. 2021; Tangen et al. 2022), we used genetic information to identify the taxonomy of each sampled plant. In brief, we followed the methods described in Chambers et al. (2024) to extract DNA and genotype each plant at up to five loci with species-specific alleles (one microsatellite locus and four PCR–RFLP loci). Plants with exclusively Typha latifolia alleles (identified as T. latifolia), T. angustifolia alleles (identified as T. angustifolia), or heterozygous loci (one T. latifolia and one T. angustifolia allele at each locus; identified as F1 hybrids) were genotyped at all five loci. Plants that were heterozygous for Typha latifolia and Typha angustifolia alleles at some but not at all loci (genotyped at between two and five loci) were identified as advanced generation or backcrossed hybrids and pooled into a single category of non-F1 hybrids.
Seed germination and identification
The nine putative T. angustifolia plants from which we collected seeds were taxonomically confirmed from genetic data using the methods described above. To investigate whether these plants were generating exclusively F1 hybrids, we germinated their seeds and genotyped a subset of their offspring. The seeds were separated from the inflorescence stalk, and from each maternal plant approximately 0.5 g of fruit was prepared for germination following the protocol outlined in (Ahee et al. 2015). The seeds were left to germinate in petri dishes filled with DI water in a climate-controlled (15–20 °C) greenhouse at Trent University. After 6 days the seedlings were transferred into 10 cm pots filled with well-draining soil from Sunshine Professional growing mixture (germination grade; Sun Gro Horticulture, Brantford, Canada) and left in the greenhouse on plastic trays filled with tap water. Seedlings were fertilized after 45 days using 100 mL of 0.5% water-soluble 20:20:20 N:P: K general-purpose fertilizer (Plant-Prod, Leamington, Ontario). Ten seedlings from each maternal plant were harvested after approximately 80–90 days, by which time they had reached heights of approximately 8 cm. Harvested seedlings were put into coin envelopes in resealable plastic bags containing orange silica beads (Sor bead, USA) for desiccation. DNA extraction and genotyping followed the same methods described for the plant samples collected in the field.
Results
Distribution of taxa
Overall, the majority of the samples that we genotyped were T. latifolia, followed by F1 T. × glauca, T. angustifolia, and then non-F1 hybrids (Table 1). Hybrids were more abundant and identified at more sites than the maternal taxon T. angustifolia (Fig. 1) despite a sampling strategy that prioritized T. angustifolia.
T. angustifolia offspring
The 90 genotyped offspring of nine T. angustifolia plants comprised 78 (87%) T. angustifolia, 10 (11%) F1 hybrids, and 2 (2%) non-F1 hybrids (advanced-generation or backcrossed hybrids). Seed samples from five of the nine T. angustifolia plants included no hybrid offspring. The remaining four plants had 1/10, 1/10, 2/10, and 8/10 hybrids (either F1 or back-crossed hybrids) among their offspring.
Discussion
The data collected in this study allow us to address the three questions presented in our introduction. First, the paucity of T. angustifolia records from the western PPR does indeed seem to be a fairly accurate reflection of its occurrence in this region: despite a sampling strategy that prioritized T. angustifolia, we found this species at only four of the 50 sampled sites. At least one record suggests that T. angustifolia has been present in the western PPR since the late 1970s (Harms and Ledingham 1986). This record was not verified with genetic data but, if accurate, suggests that despite being in the western PPR for several decades T. angustifolia remains rare and thus may not be adapted to conditions in this region. Our second question asked whether T. angustifolia in the western PPR are producing hybrid seeds. Although our sample size was small (reflecting the small number of T. angustifolia in this region—and the even smaller number of flowering T. angustifolia), we found that T. angustifolia mostly produce T. angustifolia seedlings, with only 13% of our seedling sample comprising hybrids. This was an unexpected finding given the predominance of T. latifolia in the area (75% of plants despite a sampling strategy that favoured other taxa), which in turn should increase the likelihood of T. angustifolia plants being pollinated by heterospecifics and producing mostly hybrids. Previous studies have shown that seedling production by T. angustifolia is comparable when pollinated by T. angustifolia, T. latifolia, or T. × glauca (Pieper et al. 2017), and therefore the predominance of T. angustifolia seedlings may be at least partly explained by other forms of reproductive barriers such as different flowering times.
Although we did not find T. × glauca in any of the Alberta sites, it was found in 10/34 Saskatchewan wetlands, compared to T. angustifolia in only 3/34 Saskatchewan wetlands, suggesting a westwards expansion. Additionally, unlike in other regions (including the Great Lakes region and the central/eastern PPR), we found very few non-F1 hybrids. A paucity of non-F1 hybrids, combined with the fact that hybrids remain overall less common than T. latifolia (although dominate some of the more easterly sites that we sampled), suggests that hybrids have only recently colonized this area. The western PPR therefore appears to be the leading edge of the hybrid range expansion. Our third question asked whether the range expansion of T. × glauca into the western PPR is following that of its maternal species T. angustifolia, and our data suggest this is not the case: because the hybrid occurred at more sites, and across a larger area, than T. angustifolia, it does not appear reliant on its maternal species for successful establishment at new sites. Although we found that T. angustifolia produce only a small proportion of hybrid seeds, these have the potential for dispersal across short and long distances via wind, water, or animal vectors including waterfowl (Soons 2006; Soons et al. 2008), and thus can colonize sites from which the maternal species is absent. Additionally, backcrossed hybrids, advanced-generation hybrids, and vegetative propagation can perpetuate hybrid Typha in the absence of its maternal species. In eastern Canada and the central/eastern PPR T. × glauca has outcompeted both parental species (Freeland et al. 2013; Pieper et al. 2020; Tangen et al. 2022), and may be able to dominate wetlands even with relatively low propagule pressure. Our results collectively suggest that T. × glauca has the capacity for continued range expansion that is not reliant on the presence of T. angustifolia, and the impacts of this range expansion should be monitored because of the potential for this wetland plant to impede ecosystem services and reduce local biodiversity.
References
Ahee JE, Van Drunen WE, Dorken ME (2015) Analysis of pollination neighbourhood size using spatial analysis of pollen and seed production in broadleaf cattail (Typha latifolia ). Botany 93:91–100. https://doi.org/10.1139/cjb-2014-0169
Bansal S, Lishawa SC, Newman S et al (2019) Typha (Cattail) invasion in North American Wetlands: biology, regional problems, impacts, ecosystem services, and management. Wetlands 39:645–684
Chambers A, Chambers B, Bhargava D et al (2024) A simple method to genetically differentiate invasive F1 Typha hybrids (T. × glauca) and advanced-generation/backcrossed hybrids from parent species (T. Latifolia and T. angustifolia) in eastern Canada and northeastern USA. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2023.102257
Davidson NC (2014) How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar Freshw Res. https://doi.org/10.1071/MF14173
Doherty K, Howerter D, Devries J, Walker J (2016) Prairie Pothole Region of North America. In: Finlayson C, Milton G, Prentice R, Davidson N (eds) The Wetland Book. Springer, Cham
Doherty K, Howerter D, Devries J, Walker J (2018) Prairie pothole region of North America. In: Finlayson C, Milton R, Prentice R, Davidson N (eds) The Wetland Book II: Distribution, Description and Conservation. Springer, New York, pp 1–10
Flora of North America (2008) Typha latifolia. In: efloras. Missouri Botanical Garden, St Louis, MO & Harvard University Herbaria, Cambridge, MA
Freeland J, Ciotir C, Kirk H (2013) Regional differences in the abundance of native, introduced, and hybrid Typha spp. in northeastern North America influence wetland invasions. Biol Invasions 15:2651–2665. https://doi.org/10.1007/s10530-013-0481-4
Grace J, Harrison J (1986) The biology of Canadian weeds: 73. Typha latifolia L., Typha angustifolia L. and Typha x glauca Godr. Can J Plant Sci 66:361–379. https://doi.org/10.4141/cjps86-051
Geddes P, Murphy L, Astudillo-Scalia Y, et al (2021) Microsatellite markers reveal unprecedented high frequencies of hybridization among typha species in the midwestern US. Wetlands 41:24. https://doi.org/10.1007/s13157-021-01429-2
Graham A, Mudrzynski B, Polzer E, Wilcox DA (2022) Restoration of a Lake Ontario-connected fen through invasive Typha removal. Restor Ecol. https://doi.org/10.1111/rec.13562
Harms VL, Ledingham GF (1986) Narrow-leaved cattail, Typha angustifolia, and the hybrid cattail, Typha × glauca, newly reported form Saskatchewan. Can F Nat 100:107–110
Kingsford RT, Basset A, Jackson L (2016) Wetlands: conservation’s poor cousins. Aquat Conserv Mar Freshw Ecosyst 26:892–916. https://doi.org/10.1002/aqc.2709
Kirk H, Connolly C, Freeland JR (2011) Molecular genetic data reveal hybridization between Typha angustifolia and Typha latifolia across a broad spatial scale in eastern North America. Aquat Bot 95. https://doi.org/10.1016/j.aquabot.2011.05.007
Lawrence BA, Lishawa SC, Rodriguez Y, Tuchman NC (2016) Herbicide management of invasive cattail (Typha × glauca) increases porewater nutrient concentrations. Wetl Ecol Manag. https://doi.org/10.1007/s11273-015-9471-x
Pattison-Williams JK, Pomeroy JW, Badiou P, Gabor S (2018) Wetlands flood control and ecosystem services in the Smith Creek Drainage Basin: a case study in Saskatchewan Canada. Ecol Econ. https://doi.org/10.1016/j.ecolecon.2017.12.026
Pieper SJ, Nicholls AA, Freeland JR, Dorken ME (2017) Asymmetric hybridization in cattails (Typha spp.) and its implications for the evolutionary maintenance of native Typha latifolia. J Hered 108:479–487. https://doi.org/10.1093/jhered/esx036
Pieper S, Dorken M, Freeland J (2020) Genetic structure in hybrids and progenitors provides insight into processes underlying an invasive cattail (Typha × glauca) hybrid zone. Heredity (Edinb) 124:714–727. https://doi.org/10.1038/s41437-020-0307-y
Smith S (1967) Experimental and natural hybrids in North American Typha (Typhaceae). Am Midl Nat 78:257–287. https://doi.org/10.2307/2485231
Snow, A.A., Travis, S.E., Wildova, R., Fer, T., Sweeney, P.M., Marburger, J.E., Windels, S., Kubatova, B., Goldberg, D.E., Mutegi, E., 2010. Species-specific SSR alleles for studies of hybrid cattails (Typha latifolia x T. angustifolia; Typhaceae) in North America. Am J Bot 97: 2061–2067. https://doi.org/10.3732/ajb.1000187
Soons MB (2006) Wind dispersal in freshwater wetlands: knowledge for conservation and restoration. Appl Veg Sci. https://doi.org/10.1111/j.1654-109x.2006.tb00676.x
Soons MB, Van Der Vlugt C, Van Lith B et al (2008) Small seed size increases the potential for dispersal of wetland plants by ducks. J Ecol 96:619–627. https://doi.org/10.1111/j.1365-2745.2008.01372.x
Tangen BA, Bansal S, Freeland JR et al (2022) Distributions of native and invasive Typha (cattail) throughout the Prairie Pothole Region of North America. Wetl Ecol Manag. https://doi.org/10.1007/s11273-021-09823-7
Travis SE, Marburger JE, Windels S, Kubátová B (2010) Hybridization dynamics of invasive cattail (Typhaceae) stands in the Western Great Lakes Region of North America: a molecular analysis. J Ecol 98:7–16. https://doi.org/10.1111/j.1365-2745.2009.01596.x
Xu T, Weng B, Yan D et al (2019) Wetlands of international importance: Status, threats, and future protection. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16101818
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: Opportunities, opportunists, and outcomes. CRC Crit Rev Plant Sci 23:431–452. https://doi.org/10.1080/07352680490514673
Funding
Funding for this project was provided by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada awarded to J. Freeland (RGPIN-2017–04371) and M. Dorken (RGPIN-2018–04866).
Author information
Authors and Affiliations
Contributions
This research was part of Sanjuti Deb Joyee’s MSc thesis, co-supervised by Joanna Freeland and Marcel Dorken. Study conception and design by Joanna Freeland and Marcel Dorken. Field work (sample collection) was performed by Joanna Freeland, laboratory and greenhouse methods by Sanjuti Deb Joyee. The first draft of the manuscript was written by Joanna Freeland and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joyee, S.D., Dorken, M. & Freeland, J. Range expansion of the invasive hybrid cattail Typha × glauca exceeds that of its maternal plant T. angustifolia in the western Prairie Pothole Region of North America. Biol Invasions (2024). https://doi.org/10.1007/s10530-024-03439-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10530-024-03439-7