Abstract
Human-modified habitats often harbor non-native populations and may facilitate the transport and establishment of invasive species. Disturbed areas are typically warmer than adjacent natural habitats (e.g. urban heat island effect), and thus, ectotherms may benefit from landscape modifications. Although much research has considered how urban areas facilitate invasion, greenhouses may enhance successful invasion to rural areas via transport of exotics through plant shipments and increased temperatures. However, for non-native species to establish and become invasive, populations must adapt to the local climate outside the protection of greenhouses. We studied a population of non-native lizards (Anolis sagrei) that have been naturalized for at least 10 years in a greenhouse in Opelika, Alabama, USA, which is far above the northernmost point of the species’ continuous invasive range. The greenhouse was warmer than adjacent outdoor habitat at all hours of the day and often reached temperatures above the critical thermal maximum of lizards. Our measurements of behavior and thermal physiology suggest that lizards have not physiologically adapted to these novel conditions, but rather, behavioral modifications likely allow the population to persist. Moreover, we found no evidence that lizards are dispersing from the greenhouse, and we conclude that this population is dependent on this structure for survival. While human structures and human-modified habitats may facilitate the transport of non-natives, these environments potentially shield populations from selective pressures necessary for species to spread throughout non-native areas. This may contribute to a long lag phase which characterizes many invasions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urbanization creates novel environmental conditions to which many native species are unable to adapt or acclimate, resulting in extirpation. Although some native species can persist or even thrive in cities (Gahbauer et al. 2015), urban biodiversity is often composed of non-native species for several reasons (McKinney 2006). First, epicenters of human activity are replete with shipping connections with distant locations; therefore, the accidental transport of non-native species is high in urban areas (Davis 1974; Latella et al. 2011). Second, structural modifications in cities alter abiotic conditions (e.g. temperature; Tiatragul et al. 2019), which often favor the establishment of some species over others (McKinney 2006). One such phenomenon is the “urban heat island effect”, which is the tendency for urban areas to have warmer temperatures than surrounding natural habitats (Arnfield 2003). These increased temperatures can be more favorable for the establishment of non-native species, especially ectotherms (Battles and Kolbe 2019).
Because ectotherms rely on environmental heat to regulate their body temperature, the thermal environment greatly influences their growth, reproduction, and survival (Angilletta 2009). Therefore, non-native ectotherms are likely to establish and spread in areas with climates like their native range (Bomford et al. 2009; van Wilgen and Richardson 2012). Because the establishment and spread of non-native ectotherms is often limited by cold temperatures (Kolbe et al. 2010; Suzuki-Ohno et al. 2017), the urban heat island effect may enhance the probability of successful establishment (Leniaud et al. 2009; Suzuki-Ohno et al. 2017; Wessels et al. 2018). Moreover, urban thermal environments can lower the cost of thermoregulation (Battles and Kolbe 2019), which frees up energy for growth and reproduction, potentially enhancing invasion success. For some species, however, urban areas may be too hot during certain hours of day or seasons of the year, which can restrict the spread of ectotherms throughout the urban landscape (Kolbe et al. 2016; Hall and Warner 2018). Populations may mitigate the effects of these extreme temperatures in several ways. First, they can behaviorally thermoregulate by using microhabitats that limit their exposure to suboptimal temperatures (Vickers et al. 2011; Battles and Kolbe 2019). Alternatively, physiological adaptation or acclimation can enable ectotherms to tolerate extreme temperatures. Indeed, physiological responses to novel thermal environments is well documented in ectotherms, particularly arthropods (Angilletta et al. 2007; Leal and Gunderson 2012; Logan et al. 2014; Martin et al. 2019); however, the extent to which urban environments facilitate invasion success is less studied (Hufbauer et al. 2012).
Like cities, greenhouses are human-made structures that produce unnatural biotic and abiotic conditions, but on a smaller scale. First, greenhouses are designed to be warmer than surrounding areas to provide optimal conditions for year-round plant growth. Second, greenhouses receive a large quantity of non-native plants, which can harbor non-native animal species. For example, the greenhouse frog (Eleutherodactylus planirostris) is named for its widespread occurrence in greenhouses across the United States (Somma 2019). Thus, like cities, greenhouses may serve as springboards for biological invasion; however, this possibility has received little attention (Wang et al. 2015). Although greenhouses can serve as reservoirs for non-native populations (Wang et al. 2015), adaptation to the local climate outside the protection of greenhouses is required for populations to become invasive.
The invasion biology of the brown anole (Anolis sagrei) has received considerable attention. This small, tropical lizard is native to the Bahamas and Cuba but has spread to various parts of the world (Tan and Lim 2012; Norval et al. 2016; Powell et al. 2016). Most notably, A. sagrei has colonized the southeastern United States where it was first observed in the Florida Keys in the 1880s. By the 1940s, A. sagrei was well established in south Florida, and its present non-native range is continuous from south Florida to the upper portion of the Florida peninsula, but many isolated populations occur outside of this continuous range (e.g. North and South Carolina, Georgia, Alabama, Mississippi, Texas, California; Powell et al. 2016). Brown anoles occur in both natural and urban areas in their non-native range but are most associated with urban structures (Battles and Kolbe 2019). Moreover, abiotic factors associated with urbanization enhance their growth and reproduction (Thawley and Kolbe 2020). Shipments of plants have likely been a major contributor to their current distribution (Steffen and Birkhead 2007; Perry et al. 2008). Indeed, observations on iNaturalist.com report individuals in plant nurseries and greenhouses well outside their continuous range, occasionally at high latitudes where this species is unlikely to persist due to cold winter temperatures.
An established population of non-native A. sagrei was discovered at a plant nursery in Opelika, Alabama, USA in 2006 (Steffen and Birkhead 2007). The brown anoles likely arrived via transport of commercial plants from Florida, and the population has mainly resided in and around the greenhouse. This is one of the most northern occurrences of an established A. sagrei population, even though winter temperatures in Opelika, AL occasionally fall below freezing and frequently fall below the critical thermal minimum (i.e. CTmin) of this species (6–12 °C; Kolbe et al. 2013). Additionally, the greenhouse has not been in operation, and consequently not ventilated since spring of 2016, resulting in extremely warm indoor temperatures during the summer (e.g. > 50 °C) which exceed the critical thermal maximum (i.e. CTmax) for A. sagrei (Kolbe et al. 2013). Although the greenhouse is not in an urban center, the building represents a model urban structure surrounded by semi-natural and natural habitat that experience normal climatic temperatures. Intriguingly, this population potentially experiences temperatures that exceed the CTmax in summer, as well as the CTmin during winter. Thus, this established population provides an important and unique opportunity to examine how behavior and thermal adaptation might facilitate population persistence of a non-native species in a novel, anthropogenic habitat.
We quantified spatiotemporal variation in thermal characteristics of the greenhouse and adjacent outdoor habitat during the summer and examined associations between thermal variation and lizard habitat use. We predicted that individuals retreat to microhabitats with suitable thermal conditions to avoid extreme ambient temperatures. We also examined the potential for local thermal adaptation (CTmax and CTmin) of lizards by comparing their thermal physiology to that of brown anole populations in the southeast United States along a latitudinal gradient. We considered that CTmax of the greenhouse population may be high compared to other populations due to unusually warm summer temperatures. However, because CTmax and CTmin likely covary (Angilletta 2009), positive shifts in CTmax may be constrained by selection favoring lower CTmin. Indeed, the northern latitude of this population may place significant directional selection on cold tolerance due to relatively low winter temperatures. Thus, although we do not explicitly examine winter temperatures in this study, we also quantify whether CTmin shifts across latitude. This work is a novel assessment of how a non-native species responds to unusual thermal characteristics of anthropogenic structures that have facilitated their naturalization. Additionally, this study provides insight on how ectotherms may respond to increased temperatures in urban centers and due to climate change.
Materials and methods
Study species
Anolis sagrei is an extremely successful invader and has dispersed both naturally and via human transport to many parts of the Caribbean, the south-eastern United States, and many other places that are well outside this range (e.g. California, Hawaii, Singapore, Taiwan; Goldberg and Bursey 2000; Norval and Chen 2012; Mahrdt et al. 2014). Females frequently lay eggs across a broad reproductive season, offspring mature within 3–6 months, and the lifespan is generally less than 2 years (Mitchell et al. 2018). High population densities (1.2 individuals/m2; Campbell and Echternacht 2003), high fecundity, short time to maturity, and tolerance of a diversity of microhabitats (especially disturbed habitats) by both adults and eggs are characteristics that likely contribute to its success as an invader (Losos et al. 2003; Tiatragul et al. 2017; Hall and Warner 2019; Battles and Kolbe 2019). Anolis sagrei is a diurnal, generalist predator that consumes a wide variety of invertebrate prey. This species is considered a “trunk-ground” ecomorph (Losos 2011); thus, individuals typically perch 1–2 m above the ground on a vertical surface (e.g. tree trunk) with their head down, surveying the ground and lower branches for food. They often move to the ground to forage, and commonly utilize cover objects (e.g. rocks, logs) and subterranean retreats to sleep at night or escape predation (Delaney et al. 2014). Across the non-native range, this species has potential to cause ecological harm via competition with native lizards and depredation of native arthropods (Huang et al. 2008; Stuart et al. 2014; Stroud et al. 2017).
Site description
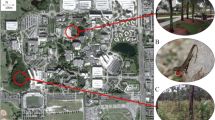
Our study population resides in a greenhouse at a plant nursery in Opelika, Alabama (Fig. 1a). The surrounding area is mostly rural with a mix of forest, creeks, farmland, and residential properties. The greenhouse has not been well-maintained for several years prior to the start of our study (e.g., tears in greenhouse walls and roof). The immediate vicinity outside the greenhouse is a combination of ornamental shrubs and trees, concrete cinderblocks, grassy areas, and a few storage sheds. The first record of A. sagrei at this site reports a minimum of 50 adults of both sexes observed over just 1 h of searching (Steffen and Birkhead 2007), which suggests that this population was large and was present for several generations prior to its discovery in 2006.
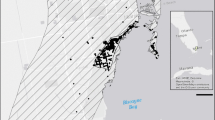
a Satellite view via Google Earth of the greenhouse and surrounding area. Outside surveys were conducted between the red and blue lines, while inside surveys were within the blue lines. Note that the greenhouse consisted of seven inter-connected parallel sections. b Male A. sagrei clinging to a wood table. c Inside view of the greenhouse with wood tables, metal pipes, and bare ground. d Map of anole populations sampled for thermal tolerance trials
The greenhouse contained seven interconnected sections that comprised an area of 3300 m2 (Fig. 1a). Airflow was minimal within the greenhouse because ventilation fans were not functional during our study. The greenhouse frames were mostly metal, while the roof and walls were polycarbonate plastic, which further increased indoor temperature and humidity. The plastic walls had many slits that enabled lizards and other animals to move in and out of the greenhouse. Center aisles within each greenhouse structure consisted of tables made from a combination of wood, metal wire, and/or cinderblocks (Fig. 1b, c). Plastic pots and flattened cardboard boxes were scattered on the tables and the ground, which were often used as retreat sites for lizards. Two concrete walkways extended along the length of each section; thus, the substrate was a mix of concrete, exposed soil, and weed mat overtop soil. Many hollow steel pipes, that provide structural support for tables, served as retreat sites that enabled lizards to get beneath the concrete or travel below ground. Low vegetation was abundant in areas that received water from leaks in the roof. Invertebrate prey (e.g. ants, grasshoppers, moths) were highly abundant.
Temperatures
We placed temperature loggers (Thermochron iButtons; Embedded Data Systems) inside (n = 13) and outside (n = 6) the greenhouse from 7 June to 31 August, 2016. Each iButton was wrapped in Parafilm for protection from water damage and solar radiation and programmed to record hourly. In natural populations, lizards move among a variety of microhabitats to thermoregulate (e.g. shade cover, subterranean retreats, rocks warmed by the sun). Thus, temperature loggers inside the greenhouse were placed in four microhabitat types that were commonly used by lizards based on our preliminary observations: (1) under cover (n = 2), (2) on wood (n = 3), (3) inside metal pipes (n = 4), or (4) subterranean (n = 4). Loggers for undercover microhabitats were placed beneath various objects, such as plastic pots and tarps that were above ground. Loggers on wood microhabitats were placed on wooden tables and stands. Loggers for metal microhabitats were placed within pipes that provided structural support for tables, benches, and the greenhouse. Thus, loggers in the wood microhabitats were exposed to sun, whereas those in the pipes tended to be shaded by the wooden tables, benches, and pipes that enclosed the loggers. Loggers for subterranean microhabitats were placed beneath concrete slabs or in holes in the ground that lizards were commonly observed using as refugia. Temperature loggers placed outside in the visual survey area (details below) were either under cover (e.g. beneath logs) or exposed. Our goal in deploying iButtons was to quantify the thermal environment commonly used by lizards (based on our observations) and not to quantify the general thermal conditions of the greenhouse. For example, when we placed iButtons under cover objects or inside metal pipes, we did not randomly select locations, but chose locations that we had observed lizards utilize. Additionally, we recorded air temperatures with a thermocouple (Omega model HH801A) at chest level outside and inside the greenhouse before each visual survey (details below).
To quantify spatiotemporal variation in temperature, we averaged the mean, minimum, maximum, and range of temperatures for each day that each logger was deployed. We used general linear mixed effects models with these temperature metrics as the response variables and location (inside vs. outside) as the independent variable. IButton ID was the random effect. To compare temperature variables among microhabitats inside the greenhouse, we performed similar models, but used microhabitat as the independent variable. Because visual encounter surveys spanned different locations (inside vs. outside) and times (7:00–18:59), we used a two-way ANOVA to analyze variation in survey temperature due to both these factors and their interaction. Time was considered a factor with three groups: morning (7:00–10:59; n = 7), mid-day (11:00–14:59; n = 8), and evening (15:00–18:59; n = 6). We used Levene’s test and visual inspection of the model residuals to assess the assumptions of ANOVA. We log-transformed survey temperatures to reduce heteroskedasticity and used Tukey Post Hoc tests for pairwise comparisons.
To assess the suitability of thermal conditions for lizards, for each iButton, we calculated the percentage of typical active hours (i.e., 6:00–20:00) that temperatures were within the range of preferred body temperatures (30–36 °C; Battles and Kolbe 2019). Henceforth, we refer to this as the PTtime. For A. sagrei, preferred body temperature does not differ between populations exposed to different ambient temperatures (e.g. urban vs. forest; Battles and Kolbe 2019; Thawley et al. 2019). Moreover, across the native and non-native range, body temperatures tend to fall within the aforementioned temperature range (Lister 1976, Losos et al. 1993). Our use of PTtime makes two notable assumptions. First, we assume that the iButton temperatures reflect operative body temperatures of lizards. Second, we assume that all potential microhabitats are equally available for use by lizards. Although these assumptions are tenuous, our primary goal was to quantify temperatures of microhabitats most commonly used by lizards (based on our observations) and not to quantify the general thermal conditions of the greenhouse. Thus, although there are many microhabitats that are not represented in our temperature data (because we did not randomly distribute iButtons), these microhabitats were also not commonly used by lizards. To validate these assumptions, we would need to deploy copper lizard models (rather than iButtons) and randomly deploy temperature loggers rather than place them strategically. Therefore, PTtime should only be interpreted as the percentage of active hours that commonly used microhabitats are within the preferred body temperature and not as a proxy of the opportunity for lizards to thermoregulate. To compare PTtime between inside and outside the greenhouse, we performed a general linear mixed effects model with PTtime as the response variable and location (inside vs. outside) as the independent variable. iButton ID was the random effect. To compare PTtime among microhabitats inside the greenhouse, we performed a similar model, but using microhabitat as the independent variable.
Visual surveys
We conducted 21 visual surveys from 28 May to 8 August, 2016 (Supplemental Table S1). Surveys were conducted at various times during the day (i.e. morning, afternoon, evening) so we could quantify daily variation in lizard behavior. Before each survey, we recorded the air temperature inside and outside of the greenhouse. To conduct surveys, a single individual slowly walked through each section of the greenhouse and the surrounding outside area while carefully searching for lizards. Each survey took approximately 30 min. The inside and outside areas that were surveyed were 3300 m2 and 2600 m2, respectively (Fig. 1a). Since there were seven interconnected sections of the greenhouse, we randomized the order of the sections we walked through during each survey. Upon observing a lizard, we recorded its location (e.g. inside vs. outside the greenhouse) and the substrate it was on. We walked through each compartment once during each survey to minimize the chance of double counting lizards.
To quantify the effects of location (inside vs. outside), time (morning, mid-day, evening) and their interaction on lizard activity (indexed by counts during surveys), we initially used a generalized linear model with a Poisson distribution. Due to mild overdispersion (dispersion parameter = 1.84), we fit our final model with a quasi-Poisson distribution. To determine if extreme temperatures inside the greenhouse reduced lizard activity, we tested for a relationship between temperature and the total number of lizards sighted during each survey using two separate generalized linear models: one for surveys inside the greenhouse and one for outside surveys. These also assumed a quasi-Poisson distribution due to mild overdispersion (dispersion parameters = 1.37, 2.42).
To assess how microhabitat use was influenced by temperature inside the greenhouse, we divided the substrates on which lizards were seen into three microhabitat categories: ground, wood, or metal. Ground included lizards standing on the ground or on horizontal substrates resting on the ground (e.g. bricks, weed mat, grass, concrete walkways). Wood and metal are the structures from which tables and benches were constructed, so these categories included lizards perched above ground on tables. Two lizards were perched on small shrubs growing in the greenhouse. These were included in the wood category. We performed a multinomial logistic regression with perch microhabitat as the response variable and air temperature inside the greenhouse as the independent variable to quantify how air temperature influenced microhabitat use.
Thermal tolerances
To quantify potential thermal adaptation, we measured the CTmax and CTmin of A. sagrei from the greenhouse and from three other non-native populations collected across a broad latitude within the invasive range in Florida and Georgia (Fig. 1d). CTmax and CTmin are the upper and lower body temperatures at which a lizard loses its righting response (i.e. cannot right themselves after being placed on their back; Lutterschmidt and Hutchison 1997).
We collected adult male (2.8–8.1 g) A. sagrei from the Opelika, AL greenhouse on 9 April 2016 (n = 14), Miami, FL on 9 April 2016 (n = 16), Palm Coast, FL on 1 April 2016 (n = 18), and Tifton, GA on 9 May 2016 (n = 17). Like the focal study population, the A. sagrei population in Tifton, GA is disjunct from the continuous invasive range in Florida, and Tifton and Opelika experience similar temperatures (Table 1). Additionally, lizards from Tifton, GA were captured on the outside of hotel buildings and are likely reliant on these structures for winter survival at this latitude (AH, JMH personal observation). Miami, FL is near the first point of colonization of A. sagrei into Florida and experiences the warmest temperatures among our study populations (Table 1). Latitudinally, Palm Coast, FL is approximately mid-way between Miami, FL and Opelika, AL and experiences intermediate temperatures (Table 1).
Lizards were transported to Auburn University and kept in ReptiBreeze screen cages (46 × 46 × 91 cm) at a room temperature of 25 °C. Each cage consisted of multiple bamboo perches, fresh cut branches from landscaping bushes, and plastic vines. Five or six lizards were housed in each cage. We provided each cage with three crickets per lizard (dusted with vitamins and calcium) twice weekly, and cages were misted with water daily. Lights (Reptisun 5.0 UVB and Tropic Sun 5500 K daylight bulbs, Zoo Med Inc.) were kept on a 12-h photoperiod. To reduce field effects on thermal tolerances, we acclimated lizards for 2 weeks before conducting trials (Leal and Gunderson 2012; Kolbe et al. 2013). We fasted lizards for 48 h before each trial to reduce effects of food availability on our measures of CTmin and CTmax, although fasting does not influence thermal tolerance in another species of lizard (Gilbert and Miles 2016).
After 2 weeks of acclimation, individuals were randomly assigned by coinflip to be tested for CTmax or CTmin on the first day, and the other measurement on the next day. Before measuring CTmax, most lizards were kept inside a VWR INCU-line incubator for ~ 60 min at 37 °C, which is 6 °C lower than the mean CTmax of brown anoles (Kolbe et al. 2013). However, for logistical reasons many of the Palm Coast lizards were held in the incubator for a variety of durations (60–120 min). Lizards were placed in small, plastic containers (GladWare Designer Series Rectangular Containers with Lids, UPC:0001258778514) while inside the incubator. We drilled a small hole in a waterproof case (Pelican 1040 Micro Case Series, model number: 1040-025-100) and threaded a thermocouple wire inside the case. Each lizard was removed from the incubator and transferred from the plastic container to the open case. To measure lizard body temperature, we inserted the thermocouple probe 1 cm into each lizard’s cloaca and held the probe in place with surgical tape (Leal and Gunderson 2012; Campbell-Staton et al. 2016). We raised body temperatures by approximately 1 °C per minute by holding the case containing the lizard above a 60-W heat lamp (Kolbe et al. 2013). To later calculate warming rates, we recorded cloacal temperatures every 15 s. Each time the lizard temperature increased by 1 °C, the lizard was flipped on its back by turning the container upside down. CTmax was determined as the temperature that the lizard could not right itself within 30 s (Kolbe et al. 2013; Campbell-Staton et al. 2016). If the lizard righted itself before 30 s, we continued to increase its body temperature (Kolbe et al. 2013; Campbell-Staton et al. 2016).
Before testing for CTmin, lizards were placed inside a watertight container and kept inside a VWR circulating water bath for ~ 60 min at 16 °C, which is 3 to 6 °C warmer than the mean CTmin of brown anoles (Kolbe et al. 2013). However, for logistical reasons, many of the Palm Coast lizards were held in the water bath for a variety of durations (60–120 min). We determined CTmin in the same manner as CTmax, except we attempted to decrease lizard body temperatures by approximately 1 °C per minute by placing the case containing the lizard in a cooler filled with ice. To later calculate cooling rates, we recorded cloacal temperatures every 15 s.
One lizard from Palm Coast and one from Opelika died during or shortly after trials, and these were excluded from analyses. For each anole, we calculated the temperature difference for every 15 s interval, which gave us a set of warming or cooling rates for each lizard with Δ°C/15 s as units, which we then averaged and converted to Δ °C/min. We performed two Analyses of Covariance (ANCOVA): one to determine effects of locality on CTmax and one for CTmin. Body mass (g), warming rate (Δ °C/min), and pre-trial duration were covariates. Initially, we also included all possible interactions between locality and covariates; however, in a backwards stepwise fashion, we dropped interaction terms from the final model if they were not statistically significant (alpha = 0.05).
Results
Temperatures
The average daily minimum, mean, maximum, and range of temperatures were 1.9, 3.6, 7.57, and 5.67 °C greater inside the greenhouse than outside, respectively (Fig. 2a); however, only the effect on mean temperature was statistically clear (Table 2). Given these large effects, the lack of statistical significance is likely due to low sample sizes. Moreover, temperatures varied considerably among microhabitats within the greenhouse; however, these differences were primarily between the subterranean microhabitat and the other locations. Subterranean sites had cooler mean and maximum temperatures and a smaller temperature range; however, the minimum temperature of subterranean sites was warmer than all other microhabitats (Table 3). The effect of microhabitat on mean temperature was not statistically clear (Table 3).
Summer temperatures throughout the day at the greenhouse. Vertical dotted lines denote the boundary between time groups (morning, mid-day, evening) from visual surveys. Shaded areas represent the preferred body temperatures of A. sagrei (30–36 °C; Battles and Kolbe 2019). a Inside and outside temperatures at the greenhouse. The lines represent mean temperatures from hourly iButton logger recordings from June 7 to August 31, 2016. The points represent air temperatures taken before visual surveys. b Mean hourly substrate temperatures inside the greenhouse from iButton logger recordings from June 7 to August 31, 2016
During visual surveys, temperatures were warmer inside the greenhouse than outside (F1,32 = 11.39, p = 0.017) (Fig. 2a). Mean (± S.E.) temperatures for inside and outside the greenhouse were 37.76 (± 1.77) °C and 29.14 (± 0.82) °C, respectively. Survey temperatures differed among time groups (F1,32 = 6.33, p < 0.001), where mid-day (11:00–14:59) and evening (15:00–18:59) temperatures were warmer than morning temperatures (7:00–10:59) (p < 0.001, p = 0.007, respectively) (Fig. 2a). The mean (± S.E.) survey temperatures during morning, mid-day, and evening were 28.74 (± 1.55) °C, 37.41 (± 2.01) °C, 34.76 (± 2.08) °C, respectively. The interaction between time group and location did not influence survey temperatures (F2,32 = 2.34, p = 0.113).
The PTtime outside the greenhouse was 33.0% (± 3.8 S.E.) which was 6.7% (± 6.8 S.E.) greater than inside, but this difference was not statistically significant (t17 = 1.0; p = 0.33) (Fig. 2a). We suspected this lack of effect might be due to subterranean temperatures inside the greenhouse being much cooler than the other microhabitats (see Fig. 2b), so we repeated the analysis without the subterranean temperatures. PTtime was then 14.3% (± 5.3 S.E.) greater outside versus inside (t13 = 2.7; p = 0.02). The PTtime inside the greenhouse differed among substrates (F3,9 = 20.11, p = 0.0003). Post hoc analysis revealed that metal, under cover, and wood did not differ from one another in PTtime; however, all three had a lower PTtime than subterranean (all p < 0.002). PTtime (± S.E.) was 19.5 (± 2.6), 18.4 (± 3.7), 17.9 (± 3.0), and 43.2 (± 2.6) % for metal, under cover, wood, and subterranean, respectively (Fig. 2b).
Visual surveys
The number of anoles observed per survey was 2.99 (1.11–8.08; 95% C.L.) times greater inside (n = 67) the greenhouse than outside (n = 27) (p = 0.033; Fig. 3). Inside the greenhouse, we observed fewer anoles during mid-day than in the morning or evening; however, this was not statistically significant (χ2 = 2.38; df = 2; p = 0.30; Fig. 3). Additionally, the interaction between location and time did not influence the number of anoles observed per survey (χ2 = 3.19; df = 2; p = 0.20).
Counts of Anolis sagrei inside (grey bars) and outside (white bars) the greenhouse from surveys conducted during morning (7:00–10:59), mid-day (11:00–14:59), and evening (15:00–18:59) hours. Box plots show the median value (line within box), middle 50% of data (box), and values within 1.5 times the interquartile range (whiskers), with individual points as outlying data
We observed a negative correlation between survey temperature and number of lizards observed inside the greenhouse: for each 1 °C decrease in survey temperature, we observed 1.06 (1.02–1.10, 95% CL; p = 0.005) times as many lizards (Fig. 4a). There was, however, no relationship between temperature and the number of lizards observed outside the greenhouse (χ2 = 0.11; df = 1; p = 0.75). Air temperature influenced microhabitat use inside the greenhouse: at warmer temperatures, lizards were more likely found on the ground than on wood or metal (Fig. 4b). For every 1 °C increase in air temperature, lizards were 1.63 (± 1.22 SE) and 1.12 (± 1.04 SE) times as likely to perch on the ground than metal (z = − 2.41; p = 0.02) or wood (z = − 2.73; p = 0.01), respectively. For each 1 °C increase in temperature, lizards were 1.45 (± 1.22 SE) times as likely to perch on wood than metal, but this was not significant (z = − 1.87; p = 0.06).
Relationships between air temperature and a anoles sighted during surveys and b anole microhabitat usage. a Number of anoles seen inside (closed circles) and outside (open circles) the greenhouse versus survey temperature. The solid line shows the model estimates for lizard count inside the greenhouse versus temperature. Dotted lines show 95% CI, and circles show the raw data. The relationship between lizard count and temperature was not significant outside the greenhouse (see results). b The probability of a lizard being sited on a microhabitat against air temperature. Symbols show raw data for ground (closed circles), wood (open circles), and metal (triangles). Each symbol represents a single lizard sited at a particular temperature; thus, y values have no meaning for the raw data points. Data are jittered to reduce over plotting
Thermal tolerances
Heat tolerance did not vary among localities (Table 4). The mean (± S.E.) CTmax measurements for the Opelika greenhouse, Tifton, Palm Coast, and Miami anoles were 43.16 (± 0.31) °C, 42.48 (± 0.27) °C, 43.18 (± 0.20), and 43.52 (± 0.12) °C, respectively (Fig. 5a). Neither warming rate, incubator duration, nor body mass influenced CTmax (Table 4).
Cold tolerance did not differ among localities (Table 4). After adjusting for cooling rate, the mean (± S.E.) CTmin measurements for the Opelika greenhouse, Tifton, Palm Coast and Miami lizards were 9.74 (± 0.50) °C, 8.95 (± 0.48) °C, 8.76 (± 0.47) °C, and 8.74 (± 0.48) °C, respectively (Fig. 5b). Cooling rate influenced CTmin (Table 4). For every 0.2 °C/min decrease in cooling rate, we observed a 1.17 (± 0.39; ± S.E.) °C increase in CTmin (Supplemental Fig. S1). Neither body mass nor water bath duration influenced CTmin (Table 4).
Discussion
The establishment and spread of non-native species can be facilitated by human structures due to the creation of novel thermal environments and high rates of shipping (Locey and Stone 2006; McKinney 2006). Although large urban areas, such as cities, are often the focus of invasion studies, greenhouses also serve as a reservoir of non-native species due, in part, to their warm temperatures (Wang et al. 2015). We quantified spatiotemporal variation in thermal characteristics of a greenhouse inhabited by a non-native lizard. We found that summer temperatures inside the greenhouse were much warmer than outside temperatures and often exceeded the CTmax of brown anoles. Moreover, inside the greenhouse, temperatures of commonly used microhabitats were outside the range of preferred body temperatures for brown anoles throughout much of the day, except in subterranean retreats. Regardless, A. sagrei were observed more frequently inside the greenhouse than outside, even at times when temperatures were extremely hot. The thermal physiology of our study population did not differ from those of other populations, suggesting that brown anoles might mitigate the adverse effects of extremely warm temperatures via behavioral, rather than physiological means.
Despite the extremely warm and suboptimal temperatures inside the greenhouse, we observed more anoles inside than outside. The extreme thermal environment inside the greenhouse must be costly to these lizards. Not only was the greenhouse warmer than the surrounding habitat which should reduce the time available for foraging, air temperatures much more closely matched surface temperatures inside the greenhouse compared to outside (Fig. 2a). Excepting the subterranean habitat, commonly used microhabitats in the greenhouse were somewhat thermally homogenous, which could make thermoregulation more costly compared to a heterogenous environment (Huey and Slatkin 1976, Basson et al. 2017). Moreover, the only microhabitat inside the greenhouse with a high percentage of PTtime were the subterranean spaces. Thus, for much of the day, lizards probably shelter underground or shuttle between shelter and open spaces to maintain preferred body temperatures. These activities would come at costs due to reduced foraging time and/or increased energy expenditure on thermoregulation (Sinervo et al. 2010; Basson et al. 2017). Because lizards were concentrated inside the greenhouse, these potential costs are likely matched by significant benefits. The greenhouse likely reduces predation pressure by excluding many potential predators (e.g. birds; though some predators were observed—i.e. snakes), and food appeared to be highly abundant inside the greenhouse. Lizards may also benefit from reduced competition with native wildlife. Although we regularly encountered a diversity of native competitors in the greenhouse (Supplemental Table S2), brown anoles were by far the most abundant species. Brown anoles appear much better able to exploit this disturbed habitat than native lizards (e.g. green anoles, Anolis carolinensis). The ability of this population to grow so prolifically in this environment is a testament to their hardiness in disturbed areas, which certainly contributes to their success as invaders.
We saw no evidence of brown anoles shuttling outside the greenhouse to avoid extreme temperatures, but we observed an inverse relationship between anole sightings and temperature inside the greenhouse (Fig. 4). Moreover, lizards were much more likely to be seen on or near the ground when temperatures were warm. Given the proximity of ground microhabitat to subterranean retreats, they might move to these cooler microhabitats to avoid the hottest hours of the day. Additionally, anoles may be transferring body heat to the cooler ground via conduction. Such behavioral flexibility can allow populations to adjust to novel conditions and may be a common trait among invasive species (Wright et al. 2010; Stroud et al. 2019). Moreover, changes in behavior are often a first step in the invasion process and may precede adaptive responses in physiology (Webb et al. 2014). Importantly, however, while temperatures measured with iButtons provide information about the thermal conditions of each microhabitat, we do not know the operative temperatures of the lizards, which may have been lower than what was measured in each microhabitat. Indeed, on two occasions, we opportunistically measured lizard body temperatures and found that body temperatures were substantially lower than the air temperature inside the greenhouse; however, body temperatures were greater inside than outside the greenhouse (Supplemental Table S3). Overall, our observations of behavior may explain, in part, why we found no evidence for differences in thermal physiology (which would be suggestive of thermal adaptation) in our study population compared to other populations.
Neither CTmax nor CTmin differed between the greenhouse population and other invasive populations. Although thermal physiology of A. sagrei can rapidly adapt to novel conditions (Logan et al. 2014), thermal physiology and behavior exhibit low additive genetic variation (Logan et al. 2018), which can constrain the pace of adaptation (but see Martin et al. 2019; Logan et al. 2019). The lack of physiological adaptation in CTmax provides additional evidence that lizards utilize behavioral adjustments to deal with extremely hot temperatures. Likely, A. sagrei are seeking cooler microhabitats, such as subterranean crevices, inside the greenhouse for refuges, which shields them from selection on thermal physiology (Huey et al. 2003). Indeed, our anecdotal observations of lizards emerging from inside pipes and beneath concrete sidewalks supports this speculation. Upper thermal tolerance is thought to be relatively conserved, so strong selection would be required for the adaptation of this trait (Hoffmann et al. 2013). We also found no evidence of adaptation to cold temperatures, which would be necessary for our population to spread to the rural area outside the greenhouse. Opelika commonly experiences winter temperatures that fall below the lower thermal limits of A. sagrei. Although we did not quantify winter temperatures in the greenhouse, air temperatures inside the greenhouse likely stay above the lizard’s CTmin, since the greenhouse traps heat from solar radiation in the winter (Wang et al. 2015). Additionally, thermally stable greenhouse microhabitats, such as subterranean crevices, likely serve as ideal refuges for A. sagrei during the winter, even if temperatures drop close to the lizards’ lower thermal tolerances. One caveat of our study is that we only measured CTmax and CTmin, while other aspects of ectotherm physiology, such as optimal performance temperatures, preferred body temperature, field body temperatures, thermal tolerance breadth or the thermal sensitivity of metabolism might have been responsive to selection.
Since minimum temperatures are major limits on the spread and establishment of non-native ectotherms (Kolbe et al. 2010; Suzuki-Ohno et al. 2017), the thermal buffering of the greenhouse is likely responsible for the establishment of this northern A. sagrei population. Virtually nothing is known about how brown anoles utilize human structures to survive winter temperatures; however, the Mediterranean gecko (Hemidactylus turcicus) is a small, nocturnal lizard that is naturalized on a global scale. These lizards depend heavily on human structures to survive winter temperatures; consequently, their diffusion dispersal is slow, and they almost never invade natural habitats in their non-native range (Locey and Stone 2006). We expect that brown anoles follow a similar invasion process whereby jump dispersal to northern latitudes is facilitated by humans, but once established, populations remain dependent on human structures for survival. The Tifton, GA population provides additional support for this hypothesis. ACH and JMH collected these specimens from hotel buildings. Lizards utilized crevices in the buildings as retreats. Despite thoroughly searching the area, we found no lizards away from buildings. This population has been established since at least the early 1990’s (Kolbe et al. 2013), yet the lizards have not spread into the surrounding landscape. Like the greenhouse population, the lizards in Tifton are likely dependent on human structures for winter survival. Had these introductions occurred in more urbanized areas, the spread of these lizards would probably have been more extensive.
Much research in urban ecology and invasion biology assumes that human-altered landscapes facilitate the invasion of non-native species (e.g. Hufbauer et al. 2012; Battles and Kolbe 2019). Our research, however, implies that these novel habitats might simply shield non-native species from the very selective pressures that are required for them to adapt to the local climate. Often, urban populations are characterized by smaller home ranges and increased population density, and many reasons have been given for these phenomena (e.g. increased density of food) (Fernández-Juricic 2001; Chamberlain et al. 2009; Lowry et al. 2013). In our study system, we expect there is strong selection against dispersal of lizards far from the greenhouse since local thermal conditions (i.e. winter temperatures) are unfavorable for long-term survival. Indeed, soon after our study, a severe storm removed the roof from the greenhouse, and the population appears to have gone extinct over the subsequent winter (Warner et al. 2019). This evidence further illustrates the important role the greenhouse played in facilitating persistence of this invasive population. In addition to the surveys reported here, we have on numerous occasions searched for brown anoles far from the greenhouse (i.e. up to 30 m) and almost never find brown anoles at this distance, despite observing native lizards. Selection against dispersal may result in reduced home range size. It is, thus, conceivable that greenhouses do not facilitate biological invasion per se, but rather provide a habitat for naturalization. Through random mutation, alleles that increase cold-tolerance may appear in the population; however, if the greenhouse buffers individuals against the cold, there will be little selective pressure to propagate those novel genes in future generations unless cold-adapted genotypes are able to disperse to novel areas that might increase fitness. In addition, it is unlikely that standing genetic variation in thermal physiology traits is great enough for selection to effectively operate given that winter temperatures drop far below what A. sagrei can tolerate. Moreover, many generations of strong selection against dispersal would reduce the likelihood that potentially cold-hardy individuals would travel far from the greenhouse. If our greenhouse, which could be considered a microcosm for human-altered habitat at a grander scale, does facilitate biological invasion, then the pace at which it does so may be rather slow for these reasons.
North of their continuous range, brown anoles likely depend on human structures for survival, and their use of these structures may limit physiological adaptation to local conditions. Our focal population clearly inhabits a novel thermal landscape that experiences temperatures well above and below their thermal limits. As such, behavioral flexibility and use of human structures may be necessary to avoid and mitigate the costs associated with such thermal extremes. We conclude that human altered habitats may enhance the probability of naturalization of non-native species, but adaptation to surrounding natural areas may be slow if these habitats shield populations from selection on thermal traits. This could potentially increase the lag time that precedes invasion.
References
Angilletta MJ (2009) Thermal adaptation: a theoretical and emprical synthesis. Oxford University Press, Oxford
Angilletta MJ, Wilson RS, Niehaus AC, Sears MW, Navas CA, Ribeiro PL (2007) Urban physiology: city ants possess high heat tolerance. PLoS ONE 2(2):e58
Arnfield AJ (2003) Two decades of urban climate research: a review of turbulence, exchanges of energy and water, and the urban heat island. Int J Climatol 23:1–26
Basson CH, Levy O, Angilletta MJ, Clusella-Trullas S (2017) Lizards paid a greater opportunity cost to thermoregulate in a less heterogeneous environment. Funct Ecol 31(4):856–865
Battles AC, Kolbe JJ (2019) Miami heat: urban heat islands influence the thermal suitability of habitats for ectotherms. Glob Change Biol 25:562–576
Bomford M, Kraus F, Barry SC, Lawrence E (2009) Predicting establishment success for alien reptiles and amphibians: a role for climate matching. Biol Invasions 11:713–724
Campbell TS, Echternacht AC (2003) Introduced species as moving targets: changes in body sizes of introduced lizards following experimental introductions and historical invasions. Biol Invasions 5:193–212
Campbell-Staton SC, Edwards SV, Losos JB (2016) Climate-mediated adaptation after mainland colonization of an ancestrally subtropical island lizard, Anolis carolinensis. J Evol Biol 29(11):2168–2180
Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, Gaston KJ (2009) Avian productivity in urban landscapes: a review and meta-analysis. Ibis 151:1–18
Davis WK (1974) The Mediterranean gecko, Hemidactylus turcicus in Texas. J Herpetol 8(1):77–80
Delaney DM, Cates CD, Warner DA (2014) Anolis sagrei (Brown Anole). Gopherus polyphemus burrow commensalism. Herpetol Rev 45(4):694
Fernández-Juricic E (2001) Avian spatial segregation at edges and interiors of urban parks in Madrid, Spain. Biodivers Conserv 10:1303–1316
Gahbauer MA, Bird DM, Clark KE, French T, Brauning DW, McMorris FA (2015) Productivity, mortality, and management of urban peregrine falcons in northeastern North America. J Wildl Manag 79(1):10–19
Gilbert AL, Miles DB (2016) Food, temperature and endurance: effects of food deprivation on the thermal sensitivity of physiological performance. Funct Ecol 30:1790–1799
Goldberg SR, Bursey CR (2000) Transport of helminths to Hawaii via the brown anole, Anolis sagrei (Polychrotidae). J Parasitol 86(4):750–755
Hall JM, Warner DA (2018) Thermal spikes from the urban heat island increase mortality and alter physiology of lizard embryos. J Exp Biol 221:jeb181552
Hall JM, Warner DA (2019) Thermal tolerance in the urban heat island: thermal sensitivity varies ontogenetically and differs between embryos of two sympatric ectotherms. J Exp Biol 222:jeb210708
Hoffmann AA, Chown SL, Clusella-Trullas S (2013) Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct Ecol 27:934–949
Huang S-C, Norval G, Wei C-S, Tso I-M (2008) Effects of the brown anole invasion and betelnut palm planting on arthropod diversity in southern Taiwan. Zool Sci 25:1121–1129
Huey RB, Slatkin M (1976) Coast and benefits of lizard thermoregulation. Q Rev Biol 51:363–384
Huey RB, Hertz PE, Sinervo B (2003) Behavioral drive versus behavioral inertia in evolution: a null model approach. Am Nat 161:357–366
Hufbauer RA, Facon B, Ravigné V, Turgeon J, Foucaud J, Lee CE, Rey O, Estoup A (2012) Anthropogenically induced adaptation to invade (AIAI): contemporary adaptation to human-altered habitats within the native range can promote invasions. Evol Appl 5:89–101
Kolbe JJ, Kearney MR, Shine R (2010) Modeling the consequences of thermal trait variation for the cane toad invasion of Australia. Ecol Appl 20(8):2273–2285
Kolbe JJ, Ehrenberger JC, Moniz HA, Angilletta MJ (2013) Physiological variation among invasive populations of the brown anole (Anolis sagrei). Physiol Biochem Zool 87(1):92–104
Kolbe JJ, VanMiddlesworth P, Battles AC, Stroud JT, Buffum B, Forman RTT, Losos JB (2016) Determinants of spread in an urban landscape by an introduced lizard. Landsc Ecol 31:1795–1813
Latella IM, Poe S, Giermakowski JT (2011) Traits associated with naturalization in Anolis lizards: comparison of morphological, distributional, anthropogenic, and phylogenetic models. Biol Invasions 13:845–856
Leal M, Gunderson AR (2012) Rapid change in the thermal tolerance of a tropical lizard. Am Nat 180(6):815–822
Leniaud L, Pichon A, Uva P, Bagnres AG (2009) Unicoloniality in Reticulitermes urbis: a novel feature in a potentially invasive termite species. Bull Entomol Res 99:1–10
Lister BC (1976) The nature of niche expansion in West Indian Anolis lizards I: ecological consequences of reduced competition. Evolution 30:659–676
Locey KJ, Stone PA (2006) Factors affecting range expansion in the introduced Mediterranean gecko, Hemidactylus turcicus. J Herpetol 40(4):526–530
Logan ML, Cox RM, Calsbeek R (2014) Natural selection on thermal performance in a novel thermal environment. Proc Natl Acad Sci 111(39):14165–14169
Logan ML, Curlis JD, Gilbert AL, Miles DB, Chung AK, McGlothlin JW, Cox RM (2018) Thermal physiology and thermoregulatory behaviour exhibit low heritability despite genetic divergence between lizard populations. Proc R Soc B 285:20180697
Logan ML, Minnaar IA, Keegan KM, Clusella-Trullas S (2019) The evolutionary potential of an insect invader under climate change. Evolution. https://doi.org/10.1111/evo.13862
Losos JB (2011) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. University of California Press, Berkeley
Losos JB, Marks JC, Schoener TW (1993) Habitat use and ecological interactions of an introduced and a native species of Anolis lizard on Grand Cayman, with a review of the outcomes of anole introductions. Oecologia 95:525–532
Losos JB, Schoener TW, Spiller DA (2003) Effect of immersion in seawater on egg survival in the lizard Anolis sagrei. Oecologia 137:360–362
Lowry H, Lill A, Wong BBM (2013) Behavioural responses of wildlife to urban environments. Biol Rev 88:537–549
Lutterschmidt WI, Hutchison VH (1997) The critical thermal maximum: history and critique. Can J Zool 75:1561–1574
Mahrdt CR, Ervin EL, Nafis G (2014) Geographic distribtution: Anolis sagrei. Herpetol Rev 45(4):658–659
Martin RA, Chick LD, Yilmaz AR, Diamond SE (2019) Evolution, not transgenerational plasticity, explains the divergence of acorn ant thermal tolerance across an urban-rural temperature cline. Evol Appl 12:1678–1687
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127:247–260
Mitchell TS, Hall JM, Warner DA (2018) Female investment in offspring size and number shifts seasonally in a lizard with single-egg clutches. Evol Ecol 32:231–245
Norval G, Chen CK (2012) The diet of an introduced lizard species, the brown anole (Anolis sagrei), in Chiayi County, Taiwan. Russ J Herpetol 17(2):131–138
Norval G, Mao J-J, Slater K, Brown LR (2016) A morphological comparison of Anolis sagrei Duméril et Bibron 1837, from two localities in Taiwan. Russ J Herpetol 23(3):195–204
Perry G, Powell R, Watson H (2008) Keeping invasive species off Guana Island, British Virgin Islands. Iguana 13(4):273–277
Powell R, Conant R, Collins JT (2016) Peterson field guide to reptiles and amphibians of eastern and central North America. Houghton Mifflin Harcourt Publishing Company, New York
Sinervo B, Méndez-de-la-Cruz F, Miles DB, Heulin B, Bastiaans E, Villagrán-Santa Cruz M, Lara-Resendiz R, Martínez-Méndez N, Calderón-Espinosa ML, Meza-Lázaro RN, Gadsden H et al (2010) Erosion of lizard diversity by climate change and altered thermal niches. Science 328(5980):894–899
Somma LA (2019) Eleutherodactylus planirostris (Cope, 1862). US Geological Survey Nonindigenous Aquatic Species Database. https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=61. Accessed 25 June 2019
Steffen J, Birkhead R (2007) Geographic distribution: Norops sagrei. Herpetol Rev 38(3):353
Stroud JT, Giery ST, Outerbridge ME (2017) Establishment of Anolis sagrei on Bermuda represents a novel ecological threat to Critically Endangered Bermuda skinks (Plestiodon longirostris). Biol Invasions 19:17231731
Stroud JT, Colom M, Ferrer P, Palermo N, Vargas V, Cavallini M, Lopez J, Jones I (2019) Behavioral shifts with urbanization may facilitate biological invasion of a widespread lizard. Urban Ecosyst 22(3):425–434
Stuart YE, Campbell TS, Hohenlohe PA, Reynolds RG, Revell LJ, Losos JB (2014) Rapid evolution of a native species following invasion by a congener. Science 346(6208):463–466
Suzuki-Ohno Y, Morita K, Nagata N, Mori H, Abe S, Makino T, Kawata M (2017) Factors restricting the range expansion of the invasive green anole Anolis carolinensis on Okinawa Island, Japan. Ecol Evol 7:4357–4366
Tan HH, Lim KKP (2012) Recent introduction of the brown anole Norops sagrei (Reptilia: Squamata: Dactyloidae) to Singapore. Nat Singap 5:359–362
Thawley CJ, Kolbe JJ (2020) Artificial light at night increases growth and reproductive output in Anolis lizards. Proc R Soc B 287(1919):20191682
Thawley CJ, Moniz HA, Merritt AJ, Battles AC, Michaelides SN, Kolbe JJ (2019) Urbanization affects body size and parasitism but not thermal preferences in Anolis lizards. J Urban Ecol 5(1):juy031
Tiatragul S, Kurniawan A, Kolbe JJ, Warner DA (2017) Embryos of non-native anoles are robust to urban thermal environments. J Therm Biol 65:119–124
Tiatragul S, Hall JM, Pavlik NG, Warner DA (2019) Lizard nest environments differ between suburban and forest habitats. Biol J Linn Soc 126:392–403
van Wilgen NJ, Richardson DM (2012) The roles of climate, phylogenetic relatedness, introduction effort, and reproductive traits in the establishment of non-native reptiles and amphibians. Conserv Biol 26:267–277
Vickers M, Manicom C, Schwarzkopf L (2011) Extending the cost-benefit model of thermoregulation: high-temperature environments. Am Nat 177:452–461
Wang C, Zhang X, Pan X, Li Z (2015) Greenhouses: hotspots in the invasive network for alien species. Biodivers Conserv 24:1825–1829
Warner DA, Hall JM, Hulbert A, Tiatragul S, Pruett J, Mitchell TS (2019) Recent extinction of a viable tropical lizard population from a temperate area. Integr Comp Biol 59:E431
Webb JK, Letnic M, Jessop TS, Dempster T (2014) Behavioural flexibility allows an invasive vertebrate to survive in a semi-arid environment. Biol Lett 10:7–10
Wessels JL, Carter ET, Hively CL, Hayter LE, Fitzpatrick BM (2018) Population viability of nonnative Mediterranean house geckos (Hemidactylus turcicus) at an urban site near the northern invasion front. J Herpetol 52(2):215–222
Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA (2010) Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404
Acknowledgements
We thank D. Douglas and C. Guiffre for assistance with animal care and visual surveys, J. Stroud for collection of lizards, S. Campbell-Staton for advice on methodology, B. Dozier for access to the greenhouse, and two anonymous reviewers that provided helpful feedback on the manuscript. All applicable institutional and/or national guidelines for the care and use of animals were followed. Research was approved by the Auburn University Institution Animal Care and Use Committee (2016-2905) and supported by a grant from the Department of Biological Sciences at Auburn University (to ACH) and the National Science Foundation (DEB-1564563 to DAW and DBI-1402202 to TSM). JMH acknowledges financial support from the Alabama Graduate Research Scholars Program (GRSP) funded through the Alabama Commission for Higher Education and administered by the Alabama EPSCoR. This is contribution no. 904 of the Auburn University Museum of Natural History. The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. S1.
Relationship between critical thermal minimum (CTmin) and cooling rate. Quicker reductions in temperatures resulted in higher CTmin measurements. (TIFF 79 kb)
Rights and permissions
About this article
Cite this article
Hulbert, A.C., Hall, J.M., Mitchell, T.S. et al. Use of human-made structures facilitates persistence of a non-native ectotherm. Biol Invasions 22, 2017–2031 (2020). https://doi.org/10.1007/s10530-020-02236-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-020-02236-2