Abstract
The free-floating American duckweed, Lemna minuta, is an invasive species now widespread in Europe. Yet, its impact on freshwater ecosystems has been poorly investigated. In this study, the effects of the presence of this invasive duckweed on water quality, and aquatic plant and invertebrate communities were evaluated in sites in Central Italy. Water chemical and physical factors and community descriptors were analyzed to identify these effects. Surveys were carried out across 17 paired aquatic sites. Site pairs were similar in microclimate, hydrogeology and water quality, but differed in relation to the presence/absence of L. minuta floating mats. In sites with mats, light and dissolved oxygen in water were negatively correlated with increasing mat coverage and thickness. The limited light and hypoxic conditions under mats inhibited plant growth and had a selective impact on the invertebrate community. Sites with L. minuta had aquatic communities with a lower plant taxa richness and a contrasting composition, compared with those in sites without. At sites with mats some plants were unaffected, but the majority of plant taxa documented at sites without Lemna were no present at sites with Lemna or were very rare (macroalgae, submerged rhizophytes). As for invertebrates, hypoxic-tolerant taxa dominated under mats (Ostracoda, Copepoda, Isopoda), whilst those more sensitive to oxygen depletion, or obligate herbivores, or those with a winged stage or swimming on water surface, were rare or absent (Ephemeroptera, Amphipoda, Chironomus, Notonecta). Lemna minuta mats presence was associated with alterations in the underlying aquatic ecosystem, severely threatening the conservation of these habitats. Active management strategies, including spread-prevention techniques, or mechanical removal combined with biological control, are required to conserve these habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Globally, freshwaters habitats have become highly threatened due to multiple anthropogenic pressures, such as hydrological and hydro-morphological alterations, water pollution, water over-exploitation, and introduction of alien species (Ormerod et al. 2010; Carpenter et al. 2011). Therefore, the need for management protocols to monitor and preserve these habitats have been identified in several international agreements and Directives (e.g. Ramsar Convention on wetlands protection 1971; European Habitat Directive 92/43/CEE; Water Frame Directive 2000/60/CEE).
A variety of invasive alien plant species continue to spread and colonise aquatic habitats and some have been shown to severely alter ecological functioning and biodiversity of the invaded habitats (Howard and Harley 1998; Sala et al. 2000; Dudgeon et al. 2006; Stiers and Triest 2017). Free-floating aquatic alien plants can have a severe impact on aquatic habitats, especially when they form extensive, dense and multilayer mats that can cover the entire surface of a waterbody. In these cases, they physically prevent most of water–air interactions causing adverse chemical and physical modifications of the underlying water (Pokorny and Rejmánková 1983; Abdel-Tawwab 2006; Sengupta et al. 2010). In particular, these mats can sometimes cause severe reductions in light and dissolved oxygen concentrations (Morris and Barker 1977; Janes et al. 1996), which can especially cause deleterious effects on submerged plant and animal communities (Cronk and Fennessy 2001; Meerhoff et al. 2003; Ricciardi and MacIsaac 2011).
The effects on aquatic plants and animals by invasive free-floating plants, such as Eichhornia crassipes (C. Martius) Solms-Laubach (Brendonck et al. 2003; Midgley et al. 2006), Salvinia molesta D.S. Mitchell (Giardini 2004), Azolla filiculoides Lam (Gratwicke and Marshall 2001) and A. pinnata R.Br. (Abdel-Tawwab 2006) have been described. Comparatively, the impact of the American duckweed Lemna minuta Kunth on aquatic ecosystems has been poorly studied, despite its known invasiveness and continued spread across Europe (DAISIE 2009; Ceschin et al. 2018a). Lemna minuta is the smallest of the species of the genus, with fronds 1–2 mm wide that float on the surface, and one rhizoid that extends into the water (Landolt 1986). This duckweed grows in slow-moving and stagnant freshwater habitats that range from meso- to eutrophic waters (Iamonico et al. 2010; Iberite et al. 2011). Due to its high competitiveness and rapid growth (Ziegler et al. 2015), the accumulated biomass of L. minuta can be extensive, fully cover some waterbodies, and competitively exclude other Lemna spp. (Ceschin et al. 2016a). The dispersal mechanisms of L. minuta, that involve transport by birds and also by water currents, as described by some authors (DAISIE 2008; Hussner 2012; Coughlan et al. 2015), can drastically reduce the effectiveness of any control management practice of this species and allow to it to widely spread. Indeed, at several sites in Central Italy, the congeneric common native L. minor, very similar morphologically to L. minuta (Ceschin et al. 2016c), has been partially or totally replaced by this alien duckweed within only a few years from its initial arrival (Ceschin et al. 2016a). This phenomenon could signify that it has a wider ecological range than the native L. minor that allows it to colonize a wider variety of sites (e.g. very shady ponds, waters more neutro-alkaline and more nutrient rich) (Ceschin et al. 2018b). In addition, it could also reflect the higher growth rate of L. minuta than the native duckweed (Njambuya et al. 2011; Ceschin et al. 2016b) that leads it to the faster formation of larger and thicker mats (Dussart et al. 1993; Leng et al. 2004; Ceschin et al. 2016b). The production of thick mats by L. minuta is evident as its growth does not seem to be limited by contact inhibition, as in the case of L. minor (Driever et al. 2005). This seemingly uninhibited growth leads to a bidimensional mat development that can result in a myriad of water quality problems (Ceschin et al. 2019b).
Janes et al. (1996), and more recently Ceschin et al. (2019a), have investigated the adverse effects of L. minuta on submerged macrophytes and different animal groups, respectively. However, the evidence from these studies is derived from laboratory experiments and so suffer from some limitations, such as short time frames, small volumes, and less complex biotic and abiotic interactions than those occurring in (semi-)natural conditions (Carpenter 1996; Schindler 1998). In this respect, it is essential to develop the information gathered from these laboratory experiments to design field investigations to have a better insight on the effect of L. minuta invasions on aquatic ecosystems. With this in mind, the primary aim of this study was to evaluate the effects of L. minuta mats on invaded aquatic habitats. To do so, we comparatively assessed the diagnostics of water chemical and physical factors, and aquatic plant and invertebrate communities, in paired sites within the same hydrographic setting, and of similar chemical and physical characteristics but different in the fact that one is with and one is without a L. minuta mat.
Materials and methods
Sampling sites
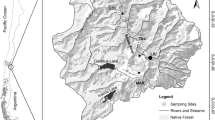
Surveys were carried out in 17 paired aquatic sites located in Central Italy (Fig. 1). For geographic coordinates of the sampling sites see Table S1. The sites ranged from ponds to small lakes of 20 to 100 cm depth. The sites in each pair were spatially close (less than 2 km apart), belonging to the same hydrographic network, and were similar in terms of microclimate, hydrogeology and water quality, as documented by previous studies (Mazzini et al. 2014; Ceschin et al. 2016a, 2018b). However, sites differed in relation to the presence (> 80% coverage) and absence (no coverage) of thick (> 10 mm) free-floating L. minuta mats. The occurrence of L. minuta mats was therefore the main discriminating variable between the paired sites, and each site without Lemna was considered as an un-impacted reference site, with the differences between the paired sites indicating the L. minuta effects on the aquatic ecosystem. Samplings of water chemical and physical factors, and aquatic communities, were carried out once at each of the paired sites in either 2017 or 2018, from April to October, coinciding with the main growing season of L. minuta.
Location of sampling sites. Each acronym is inclusive of a site pair with and without L. minuta for a total of 17 paired sites (i.e. 34 sampling sites). Alv, Alviano WWF Oasis (TR); Aum, Nursury Aumenta (LT); Bot, Botanical garden of Rome (RM); Bor, Borghese property (RM); Caf, Caffarella Valley (RM); Fog, Foglino wood-Nettuno (RM); Paq, Aqueduct park (RM); Tma, Tor Marancia Park (RM)
Water chemical and physical analysis
At each sampling site, air and water temperature (°C), conductivity (µS/cm), pH, and dissolved oxygen (mg/l) were measured in the field using a multi-parameter probe (Hach-Lange HQ40d). Transmitted light (%) was calculated considering the difference between the irradiance levels (μmol photon m2/s) measured above and below the L. minuta mat by a light meter (LI-250A light meter and LI-193SA quantum sensor, LI-COR GmbH). Measurements were made by having the probe just lightly in contact with the upper and lower surface of the Lemna mat.
Aquatic plants and invertebrates analysis
The coverage (%) of aquatic plants, including macroalgae and vascular plants, were estimated visually within a standard area of 10 m2 in each sampling site. As it was impossible to observe the submerged plants under the dense L. minuta mat, five sections of it were removed (ca. 30 × 30 cm) using a wide wooden spatula at intervals of approximately 1 m across a representative transect of the waterbody. Plant samples that were not immediately identified in field were taken to the laboratory for identification using a light microscope (Zeiss AxioScope) or stereoscope (Olympus SZX2-ILLT) and following the dichotomous keys of John et al. (2002) for algae and Pignatti (1982) for vascular plants. Total taxa richness and coverage of the plant community (excluding L. minuta) were calculated for each site. Regarding L. minuta, the average mat thickness (mm) was determined by measuring the thickness of three mat samples taken randomly from each site, using a precision digital calliper.
Aquatic benthic invertebrates were sampled with a standard net (25 × 25 cm frame, 500 μm mesh) in the same area where plants were collected. The net was placed above the substrate and then it kicked the substrate in front of the net to catch the individuals present in the first centimeters of the substrate. Three kicks along each transect were carried out. For sampling planktonic invertebrates, the net was made to pass through the water column along the same transect. The identification of individuals was performed directly in field, otherwise uncertain samples were fixed in 95% ethanol and transported to laboratory for identification using a stereoscope (Olympus SZX2-ILLT) and following the taxonomic guides of Campaioli et al. (1999) and Tachet et al. (2000). For each taxon, the number of individuals (abundance) was reported per site. Total taxa richness and total abundance of invertebrate community were calculated for each site.
Statistical analysis
Principal Component Analysis (PCA) was used to identify the water chemical and physical factors influenced by the free-floating L. minuta mats. Analyses were run on datasets of abiotic and Lemna mat parameters (coverage, thickness). Spearman correlations between L. minuta mat and abiotic parameters, aquatic plant and invertebrate community descriptors (taxa richness, coverage, abundance), were carried out to evaluate the general influence of L. minuta mats on these parameters. Subsequently, comparisons on abiotic and community data sets were made between the paired sites with and without L. minuta, using the Mantel Test with Bray–Curtis similarity measures. The Mantel Test was used for verifying the degree of similarity between two data sets. Two data sets are significantly similar for p < 0.05, otherwise dissimilar. SIMPER analyses of aquatic plant and invertebrate datasets were specifically used to determine which taxa contributed mostly to the difference between the paired sites. Exclusive taxa of one of the two subsets of sites, but found only once, were not considered in the analysis. The overall significance of the difference was assessed by ANOSIM. All statistical analyses were conducted with Past software version 3.07.
Results
Effect of L. minuta mats on water chemical and physical factors
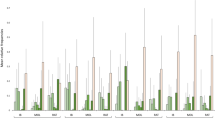
PCA analysis shows a clear distinction between sites with and without L. minuta mats in relation to water quality, with the first axis explaining around 70% of the total variance (Fig. 2). A summary of these differences in relation to the water chemical and physical factors is shown in Fig. 3. Light and dissolved oxygen were the factors that most differentiated sites and were both negatively correlated with coverage (p < 0.05, r = − 0.70 and − 0.69, respectively) and thickness of the Lemna mats (p < 0.05, r = − 0.82 and − 0.71) (Table 1). In the sites with Lemna, dissolved oxygen was significantly lower (over 60%) than in those without (p < 0.05). Light was blocked almost entirely by the Lemna mat with a mean of only 1.8% of the incident light (i.e. amount of light that arrives at surface) passing through the mat, compared to a mean about 46% at the sites without Lemna (p < 0.05) (Fig. 4a).
The water temperatures in sites with Lemna were generally lower than those measured in the sites without (mean 1.5 °C ± SD 0.26), but this difference was not significantly correlated with Lemna mat coverage and thickness (Table 1). Water temperatures in sites with Lemna were lower than those of the air (mean 2 °C ± SD 0.26), whilst in sites without Lemna the difference was less pronounced (mean 0.7 °C ± SD 0.31; Fig. 4b). Differences in pH and conductivity of the sites with and without Lemna were relatively small (mean 0.3 pH units ± SD 0.15 and 20 µS/cm ± 9.28, respectively).
Effect of L. minuta mats on aquatic plant community
The coverage of all plant taxa collected at sites with and without L. minuta mats are shown in Table S1. In sites with L. minuta, the plant communities generally have lower taxa richness and total coverage than the sites without (Table 2). Lemna minuta mat thickness was significantly and negatively correlated with the number of plant taxa (p < 0.05, r = − 0.57), and the mat coverage with plant total coverage (p < 0.05, r = − 0.46).
The Mantel Tests performed on the plant dataset showed that 15 out of 17 site pairs with and without Lemna were dissimilar (p > 0.05). In detail, SIMPER tests showed that differences in composition of the plant community between sites with and without Lemna was mainly due to some taxa (Table 3). All algal taxa recorded were exclusive to sites without Lemna (e.g. Phormidium sp., Spirogyra sp., Oedogonium sp., Chara spp., Draparnaldia sp., Bacillariophyta) and contributed about 25% to the observed differences. Cladophora glomerata (L.) Kützing was the only alga present in some sites with Lemna, but always with low frequency respect to these sites (around 12%) and very low coverage. Other plant species that commonly occurred in sites without Lemna, such as Veronica anagallis-aquatica L., Apium nodiflorum (L.) Lag., Nelumbo nucifera Gaertn and Equisetum telmateja Ehrh, contributed around 25% to the total difference between waterbodies with and without Lemna. Also other species were exclusive to the sites lacking of L. minuta, even if present with less frequency (e.g. Vallisneria spiralis L., Alisma plantago-aquatica L.). Conversely, Typha latifolia L. and native duckweeds, such as L. minor and secondarily L. gibba L., were more frequently found in sites with L. minuta mats than in those without, accounting for 24% of the total difference. However, these other Lemna species were present at sites with L. minuta with low coverage (mean coverage < 12%). Juncus bufonius L. and Zannichellia palustris L. were exclusively found in presence of L. minuta, but at very low frequency and coverage.
Effect of L. minuta mats on aquatic invertebrate community
The abundance of all aquatic invertebrates recorded at sampling sites with and without L. minuta are listed in Table S1. Invertebrate community ranged from 2 to 7 taxa in the sites with Lemna (mean 5.2 taxa ± SD 0.53) and from 3 to 13 taxa in sites where L. minuta was absent (mean 7.2 taxa ± SD 0.62). In the sites without Lemna, there was on average a greater taxa richness and a higher abundance of individuals (Table 2). There was about a 40% decrease in taxa richness in the sites with Lemna compared to those without. The number of taxa, and not the number of individuals per taxa, was significantly and negatively correlated with Lemna mat coverage (p < 0.05, r = − 0.32) and thickness (p < 0.05, r = − 0.33).
The Mantel tests showed a high significant dissimilarity (12 out 17 pairs) between sites with and without L. minuta mats based on invertebrate community descriptors. SIMPER tests showed that some invertebrate taxa contribute mostly than others to differentiate these two site groups (Table 3). In particular, Diptera with Chironomus sp. and Culex sp., Ephemeroptera with Baetis rhodani Pictet and Cloeon sp., Amphipoda with Gammarus sp., Anellida with Naididae (formerly known as Tubificidae), ciliates with Amoeba sp., Coleoptera with Dytiscidae, and Hemiptera with Notonecta sp., make up 20% of this difference, occurring preferentially or exclusively in waterbodies without Lemna. Conversely, Ostracoda mainly with Cypria ophtalmica (Jurine) Brady & Norman, Isopoda with Asellus aquaticus L., Cladocera with Daphnia sp., and Copepoda, that contribute over 60%, were more frequent and in greater abundance in sites with Lemna.
Discussions
Here we show that the occurrence of thick and extended floating mats of L. minuta was associated with alterations of the underlying aquatic habitat. In particular, there was a direct negative association of this alien duckweed with water quality (mainly in relation to light and dissolved oxygen). Lemna minuta presence was also associated with lower richness and coverage/abundance of aquatic plants and invertebrates, as well as diverse community compositions.
As much of the information on the effects of Lemna mats on water quality and community was based on low-complex in vitro (and ex situ) experiments (Janes et al. 1996; Ceschin et al. 2019a), the data of this in situ study can be important to aid further understanding the ecological impacts of this species in natural complex environments. Here, the chemical and physical data aided the elaboration of the biological data, however it would be interesting to expand upon this by including detailed analyses on nutrients in water to give a more comprehensive view of the driving processes involved in Lemna population dynamics and environmental impacts.
Influence of L. minuta mats on water abiotic factors
High reductions of irradiance to near-darkness and oxygen to hypoxic levels were found in the water of sites with Lemna. The severity of these alterations increases with mat thickness and higher proportional surface coverage, since dense L. minuta mats would tend to form a barrier between air and water, confirming also previous observations of indoor experiments (Janes et al. 1996; Ceschin et al. 2019a). The overall impacts on the aquatic ecosystem by Lemna mats would seem to be a combination of direct effects (blocking light and oxygen exchange at the air–water interface) that likely are further intensified indirectly by the synchronous elimination of photosynthetic oxygen production and increase in heterotrophic respiration (Hamilton et al. 1995; Takamura et al. 2003).
The barrier effect of L. minuta mats seemed to be also thermal as the differences between ambient and aquatic temperatures were significantly different with lower temperatures found in the sites with the mats compared to those measured in the sites without. It is already known that temperature can vary widely between air and water in the presence of floating mats (Dale and Gillespie, 1976) and differences of more than 2 °C have often been found highlighting the insulating capacity of the Lemna mats (Ceschin et al. 2019b).
The other environmental parameters (pH, conductivity) were not significantly affected by the presence of duckweed mats. There was, however, a general decrease in pH with increasing mat coverage and thickness, a relationship that has been shown to be significant in previous indoor experiments (Ceschin et al. 2019a), as a result of increasing dissolved carbon dioxide concentrations, due to the prevalence of respiratory over photosynthetic activity in the water column (Morris and Barker 1977; Janes et al. 1996).
Influence of L. minuta mat on aquatic plant communities
The changes in water quality conditions likely caused by the presence of a L. minuta mat exerts a strong control on the growth of underlying aquatic plants, resulting in assemblages with low coverage, low taxa richness and altered taxonomic composition. Lemna mat coverage, over prolonged periods, would seem to lead to an absence of other aquatic plants, with only the most plastic and eurytopic species surviving. Some species, such as the fast-growing, phenotypically plastic Elodea sp., can respond by elongation and colonising the immediate subsurface layer where there is a greater potential to obtain light (Janes et al. 1996), whilst some algae and plants can produce spores, resting stages or propagules to survive through these stressful conditions (Maberley 1993; McMinn and Martin 2013).
Lemna minuta mat cover would seem severely impact algal communities since they were completely absent or, at most, sporadic. As L. minuta mats blocked nearly all light penetration into the water column, the total inhibition of algal growth would be expected. This phenomenon has also been noted to occur under other free-floating plants (De Tezanos Pinto et al. 2007; O’Farrell et al. 2009). When Lemna coverage is less 30%, algae are still able to compete for resources (space and nutrients) (Szabó et al. 1999; Roijackers et al. 2004), a situation, in fact, that in this study has sometimes occurred in the sites without Lemna or with more restricted L. minuta coverage where there was a greater algal taxa richness and abundance (see Table S1).
On the whole, also submerged rhizophytes, present in sites lacking L. minuta, were not found in those with, where the occurrence of floating L. minuta mats on the water surface likely limits the growth of these plants that would not receive enough light. The limiting effect of L. minuta mats on a submerged rhizophyte, such as Potamogeton crispus L., had already been previously observed within laboratory experiments (Janes et al. 1996).
Emergent rhizophytes would likely have problems overwintering if mats of L. minuta are established, this is because the regular germination of their submerged seeds, winter buds or turions is likely to be impeded by the lack of light, oxygen and lower temperatures present under a persistent mat. Indeed, in this study, emergent rooted species, such as V. anagallis-aquatica, A. nodiflorum and E. telmateja were found only very sporadically in the sites with L. minuta mats (see Table S1). Other rhizophyte species, such as T. latifolia, can overcome the deleterious effects of the Lemna mats as it has floral and perennial vegetative structures that are emergent and a well-developed aerenchyma which allows it to tolerate also very low dissolved oxygen concentrations (Brix 1993).
At sites with L. minuta, other duckweed species, such as the native L. minor and L. gibba, were co-occurring, even if only with small populations (see Table S1). Indeed, here it seems the presence of L. minuta limits the growth of these other duckweeds and actually there is empirical evidence showing that L. minuta does have higher growth rates than L. minor (Ziegler et al. 2015; Ceschin et al. 2016b), especially under medium–high nutrient concentrations (Njambuya et al. 2011; Paolacci et al. 2016; Ceschin et al. 2018b) and medium–high light intensities (Paolacci et al. 2018a). However, it should be noted that some recent transplant experiments have demonstrated that the two Lemna species are able to grow in ponds with various physical and chemical characteristics during the summer period (Paolacci et al. 2018b).
Influence of L. minuta mat on invertebrate community
It was shown that the water column beneath L. minuta mats is a sub-optimal environment for aquatic invertebrates, and resulted in a decreased taxa richness and an altered taxa composition. Similar consequences have been shown for other invasive free-floating plants, such as S. molesta (Giardini 2004), A. filiculoides (Gratwicke and Marshall 2001) and A. pinnata (Abdel-Tawwab 2006). The reduction of dissolved oxygen levels under L. minuta mats caused a reduction of taxa sensitive to a depletion in oxygen, such as Ephemeroptera, namely Baetis rhodani and Cloeon sp., and Amphipoda as Gammarus sp.. The phenomenon of selective pressure by hypoxia on zooplankton communities (Dejen et al. 2004; Fontanarrosa et al. 2010) has also been shown under the free-floating mats of E. crassipes (Midgley et al. 2006) that have similar impacts to the Lemna mats. A significant reduction of herbivorous taxa (e.g. Ephemeroptera as Baetis rhodani and Cloeon sp.) was most likely due to food scarcity (Bramm et al. 2009). Also surface-swimming taxa, such as Notonecta sp. (Hemiptera), were absent at sites with the Lemna mats, likely because their typical movement on the surface is physically impeded by the mat presence. Similarly, it was observed a small number of taxa with a life cycle including an aquatic larval and a winged adult stage (e.g. Diptera as Chironomus sp. and Culex sp., and Ephemeroptera as Baetis rhodani and Cloeon sp.), likely because L. minuta mats can act as physical barriers preventing the completion of the life cycle of these taxa (Furlow and Hays 1972).
There are, however, other taxa that tolerate the aquatic conditions created under L. minuta mats. Among these taxa, Isopoda, Cladocera, Ostracoda and Copepoda even proliferate and dominate the invertebrate communities found in sites with Lemna, most probably because of the reduction of interspecific competition. High abundances of Ostracoda have also been previously observed in field under dense mats of duckweed communities (Mazzini et al. 2014) and the tolerance of Asellus aquaticus (Isopoda) to the hostile environmental conditions under L. minuta mats has also been noted in laboratory (Ceschin et al. 2019a). Although, how much the success of these species is due to reduced competition or physiological processes has not yet been illucidated.
Management strategies for L. minuta population control and spread-prevention
The high dispersal capacity of L. minuta combined with its capacity to alter the chemical and physical characteristics and ecological function of waterbodies highlights the need to find active management strategies to conserve these valuable habitats. There are limited methods available to control the spread of this invasive duckweed, among which, mechanical removal of the mats using nets with fine-mesh that could rapidly reverse any impacts. But, full removal is difficult, and considerable biomass can be reproduced in a very short time (Landolt 1986; Ceschin et al. 2016b). Even if full removal of Lemna is achieved, the likelihood of re-invasion from nearby populations in surrounding waterbodies could frustrate these management efforts. However, the spread of L. minuta can be monitored using remote sensing techniques (e.g. Villa et al. 2015), and mat formation could be prevented by timely removal of pioneering fronds. For greater success in controlling the spread of L. minuta populations, a combination of physical removal and biological control using natural local competitors or grazers could be suggested. At present, there is no field-evidence on a biological control of the species, but some preliminary laboratory observations would suggest that some native herbivorous insects might be suitable in controlling this aquatic alien plant (Mariani et al. 2020). More recently, a new biosecurity practice of steam treatment has been developed for the control of invasive macrophytes (Crane et al. 2019), and this could potentially be useful for spread-prevention of Lemna, especially as the possibility of successful in situ treatment may be likely linked to the floating lifestyle of this invasive duckweed.
References
Abdel-Tawwab M (2006) Effect of free floating macrophyte, Azolla pinnata R. Brown on water physico-chemistry, primary productivity, and the production of Nile Tilapia, Oreochromis niloticus (L.), and common carp, Cyprinus carpio L., in fertilized earthen ponds. J Appl Aquac 18:21–41. https://doi.org/10.1300/J028v18n01_02
Bramm ME, Lassen MK, Liborussen L et al (2009) The role of light for fish-zooplankton–phytoplankton interactions during winter in shallow lakes—a climate change perspective. Freshw Biol 54:1093–1109. https://doi.org/10.1111/j.1365-2427.2008.02156.x
Brendonck L, Maes J, Rommens W et al (2003) The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). II. Species diversity. Fundam Appl Limnol 158:389–405. https://doi.org/10.1127/0003-9136/2003/0158-0373
Brix H (1993) Macrophyte-mediated oxygen transfer in wetlands: transport mechanisms and rates. In: Moshiri GA (ed) Constructed wetlands for water quality improvement. Lewis Publishers, London, pp 391–398
Campaioli S, Ghetti PF, Minelli A, Ruffo S (1999) Manuale per il riconoscimento dei macroinvertebrati delle acque dolci Italiane. Vol. II. Provincia Autonoma di Trento, Museo di Storia Naturale di Trento, p 484
Carpenter SR (1996) Microcosm experimentations have limited relevance for community and ecosystem ecology. Ecology 77:677–680. https://doi.org/10.2307/2265490
Carpenter SR, Stanley EH, Zanden MJV (2011) State of the world’s freshwater ecosystems: physical, chemical and biological changes. Annu Rev Environ Resour 36:75–99. https://doi.org/10.1146/annurev-environ-021810-094524
Ceschin S, Abati S, Leacche I, Iamonico D, Iberite M, Zuccarello V (2016a) Does the alien Lemna minuta show an invasive behaviour outside its original range? Evidence of antagonism with the native L. minor L. in Central Italy. Int Rev Hydrobiol 101(5-6):173–181. https://doi.org/10.1002/iroh.201601841
Ceschin S, Della Bella V, Piccari F, Abati S (2016b) Colonization dynamics of the alien macrophyte Lemna minuta Kunth: a case study from a semi-natural pond in Appia Antica Regional Park (Rome, Italy). Fundam Appl Limnol 188(2):93–101. https://doi.org/10.1127/fal/2016/0870
Ceschin S, Leacche I, Pascucci S, Abati S (2016c) Morphological study of Lemna minuta Kunth, an alien species often mistaken for the native L. minor L. (Araceae). Aquat Bot 131:51–56. https://doi.org/10.1016/j.aquabot.2016.01.005
Ceschin S, Abati S, Ellwood NTW, Zuccarello V (2018a) Riding invasion waves: spatial and temporal patterns of the invasive Lemna minuta from its arrival to its spread across Europe. Aquat Bot 150:1–8. https://doi.org/10.1016/j.aquabot.2018.06.002
Ceschin S, Abati S, Leacche I, Zuccarello V (2018b) Ecological comparison between duckweeds in Central Italy: the invasive Lemna minuta vs. the native L. minor. Plant Biosyst 152(4):674–683. https://doi.org/10.1080/11263504.2017.1317671
Ceschin S, Abati S, Traversetti L, Spani F, Del Grosso F, Scalici M (2019a) Effects of the invasive duckweed Lemna minuta on aquatic animals: evidence from an indoor experiment. Plant Biosyst. https://doi.org/10.1080/11263504.2018.1549605
Ceschin S, Sgambato V, Ellwood NTW, Zuccarello V (2019b) Phytoremediation performance of Lemna communities in a constructed wetland system for wastewater treatment. Environ Exp Bot 162:67–71. https://doi.org/10.1016/j.envexpbot.2019.02.007
Coughlan NE, Kelly TC, Jansen MA (2015) Mallard duck (Anas platyrhynchos)-mediated dispersal of Lemnaceae: a contributing factor in the spread of invasive Lemna minuta? Plant Biol 17(1):108–114. https://doi.org/10.1111/plb.12182
Crane K, Cuthbert RN, Dick JTA, Kregting L, MacIsaac HJ, Coughlan NE (2019) Full steam ahead: direct steam exposure to inhibit spread of invasive aquatic macrophytes. Biol Invasions 21:1311–1321. https://doi.org/10.1007/s10530-018-1901-2
Cronk JK, Fennessy MS (2001) Wetland plants: biology and ecology. CRC Press, Boca Raton
DAISIE (2009) Handbook of alien species in Europe. Springer, Berlin
Dale HM, Gillespie T (1976) Influence of floating vascular plants on diurnal fluctuations of temperature near water surface in early spring. Hydrobiologia 49:245–256. https://doi.org/10.1007/BF00014518
De Tezanos Pinto P, Luz A, O’Farrell I (2007) Influence of free-floating plants on the structure of a natural phytoplankton assemblage: an experimental approach. J Plankton Res 29(1):47–56. https://doi.org/10.1093/plankt/fbl056
Dejen E, Vijverberg J, Nagelkerke LAJ, Sibbing FA (2004) Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (Lake Tana, Ethiopia). Hydrobiologia 513:39–49. https://doi.org/10.1023/B:hydr.0000018163.60503.b8
Driever SM, Nes EH, Roijackers RMM (2005) Growth limitation of L. minor due to high plant density. Aquat Bot 81:245–251. https://doi.org/10.1016/j.aquabot.2004.12.002
Dudgeon D, Arthington AH, Gessner MO et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182. https://doi.org/10.1017/S1464793105006950
Dussart G, Robertson J, Bramley J (1993) Death of a lake. Biol Sci Rev 5(5):8–10
Fontanarrosa MS, Chaparro G, de Tezanos Pinto P, Rodriguez P, O’Farrell I (2010) Zooplankton response to shading effects of free-floating plants in shallow warm temperate lakes: a field mesocosm experiment. Hydrobiologia 646:231–242. https://doi.org/10.1007/s10750-010-0183-1
Furlow BM, Hays KL (1972) Some influences of aquatic vegetation on the species and number of Culicidae (Diptera) in small pools of water. Mosq News 32(4):595–599
Giardini M (2004) Salvinia molesta DS Mitchell (Salviniaceae): seconda segnalazione per l’Italia (Lazio) e considerazioni sul controllo di questa specie infestante. Webbia 59:456–467. https://doi.org/10.1080/00837792.2004.10670778
Gratwicke B, Marshall BE (2001) The impact of Azolla filiculoides Lam. on animal biodiversity in streams in Zimbabwe. Afr J Ecol 38:1–4. https://doi.org/10.1046/j.0141-6707.2000.00284.x
Hamilton SK, Sippel SJ, Melack JM (1995) Oxygen depletion and carbon dioxide and methane production in waters of the Pantanal wetland of Brazil. Biogeochemistry 30:115–141. https://doi.org/10.1007/BF00002727
Howard GW, Harley KLS (1998) How do floating aquatic weeds affect wetland conservation and development? How can these effects be minimized? Wetl Ecol Manag 5:215–225. https://doi.org/10.1023/a:1008209207736
Hussner A (2012) Alien aquatic plant species in European countries. Weed Res 52:297–306. https://doi.org/10.1111/j.1365-3180.2012.00926.x
Iamonico D, Abati S, Iberite M (2010) Lemna minuta Kunth (Araceae) nel Lazio (Italia centrale): note morfologiche e osservazioni sui caratteri d’invasività. In: Proceedings of the 18th meeting forum natura mediterraneo on “Le specie aliene nel Mediterraneo”, 2010 March 20–21; Paliano, Italy 2010. https://www.naturamediterraneo.com/primoconvegnoNM/Iamonico_Iberite.pdf
Iberite M, Iamonico D, Abati S, Abbate G (2011) Lemna valdiviana Phil. (Araceae) as a potential invasive species in Italy and Europe: taxonomic study and first observations on its ecology and distribution. Plant Biosyst 145:751–757. https://doi.org/10.1080/11263504.2011.633112
Janes AR, Eaton WJ, Hardwick K (1996) The effects of floating mats of Azolla filiculoides Lam and Lemna minuta Kunth on the growth of submerged macrophytes. Hydrobiologia 340:23–26. https://doi.org/10.1007/BF00012729
John DM, Whitton BA, Brook AJ (2002) The freshwater algal flora of the British Isles: an identification guide to freshwater and terrestrial algae. Cambridge University Press, Cambridge
Landolt E (1986) The family of Lemnaceae—a monographic study, vol 1. Veröff. Geobot. Inst. ETH. Stift. Rübel, Zurich
Leng RA, Preston TR, Rodriguez L (2004) The duckweed invasion of Lake Maracaibo: an evaluation of the causes and proposals for future action. The University of Tropical Agriculture Foundation: UTA, Bogotá
Maberley SC (1993) Morphological and photosynthetic characteristics of Potamogeton obtusifolius from different depths. J Aquat Plant Manag 31:34–39
Mariani F, Di Giulio A, Fattorini S, Ceschin S (2020) Experimental evidence of the consumption of the invasive alien duckweed Lemna minuta by herbivorous larvae of the moth Cataclysta lemnata in Italy. Aquat Bot. 161. https://doi.org/10.1016/j.aquabot.2019.103172
Mazzini I, Ceschin S, Abati S, Gliozzi E, Piccari F, Rossi A (2014) Ostracod communities associated to aquatic macrophytes in an urban park: the example of the Caffarella Valley (Park of the Appia Antica, Rome, Italy. Intl Rev Hydrobiol 99:425–434. https://doi.org/10.1002/iroh.201301728
McMinn A, Martin A (2013) Dark survival in a warming world. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2012.2909
Meerhoff M, Mazzeo N, Moss B, Rodriguez Gallego L (2003) The structuring role of free floating versus submerged plants in a subtropical shallow lake. Aquat Ecol 37:377–391. https://doi.org/10.1023/b:aeco.0000007041.57843.0b
Midgley JM, Hill MP, Villet MH (2006) The effect of water hyacinth, Eichhornia crassipes (Martius) Solms Laubach (Pontederiaceae), on benthic biodiversity in two impoundments on the New Year’s River, South Africa. Afr J Aquat Sci 31(1):25–30. https://doi.org/10.2989/16085910609503868
Morris PF, Barker WG (1977) Oxygen transport rates through mats of Lemna minor and Wolffia sp. and oxygen tension within and below the mat. Can J Bot 55(1):1927–1932. https://doi.org/10.1139/b77-220
Njambuya J, Stiers I, Triest L (2011) Competition between Lemna minuta and Lemna minor at different nutrient concentrations. Aquat Bot 94:158–164. https://doi.org/10.1016/j.aquabot.2011.02.001
O’Farrell I, de Tezanos Pinto P, Rodrìguez PL, Chaparro G, Pizarro HN (2009) Experimental evidence of the dynamic effect of free-floating plants on phytoplankton ecology. Freshw Biol 54:363–375. https://doi.org/10.1111/j.1365-2427.2008.02117.x
Ormerod SJ, Dobson M, Hildrew AG, Townsend CR (2010) Multiple stressors in freshwater ecosystems. Freshw Biol 55:1–4. https://doi.org/10.1111/j.1365-2427.2009.02395.x
Paolacci S, Harrison S, Jansen MAK (2016) A comparative study of the nutrient responses of the invasive duckweed Lemna minuta, and the native co-generic species Lemna minor. Aquat Bot 134:47–53. https://doi.org/10.1016/j.aquabot.2016.07.004
Paolacci S, Harrison S, Jansen MAK (2018a) The invasive duckweed Lemna minuta Kunth displays a different light utilisation strategy than native Lemna minor Linnaeus. Aquat Bot 146:8–14. https://doi.org/10.1016/j.aquabot.2018.01.002
Paolacci S, Jansen MAK, Harrison S (2018b) Competition between Lemna minuta, Lemna minor, and Azolla filiculoides. Growing fast or being steadfast? Front Chem 6:207. https://doi.org/10.3389/fchem.2018.00207
Pignatti S (1982) Flora d’Italia. Edagricole, Bologna
Pokorny J, Rejmánková E (1983) Oxygen regime in a fishpond with duckweeds (Lemnaceae) and Ceratophyllum. Aquat Bot 17:125–137. https://doi.org/10.1016/0304-3770(83)90109-2
Ricciardi A, MacIsaac HJ (2011) Impacts of biological invasions on freshwater ecosystems. In: Richardson DM (ed) Fifty years of invasion ecology: the legacy of Charles Elton. Wiley, Hoboken, pp 398–432
Roijackers R, Szabó S, Scheffer M (2004) Experimental analysis of the competition between algae and duckweed. Arch Hydrobiol 160:401–412. https://doi.org/10.1127/0003-9136/2004/0160-0401
Sala OE, Chapin FS, Armesto JJ et al (2000) Global Biodiversity scenarios for the year 2100. Science 287:1770–1774. https://doi.org/10.1126/science.287.5459.1770
Schindler DW (1998) Replication versus realism: the need for ecosystem-scale experimentations. Ecosystems 1:323–334. https://doi.org/10.1007/s100219900026
Sengupta S, Medda C, Dewanji A (2010) The impact of duckweed growth on water quality in sub-tropical ponds. Environmentalist 30:353–360. https://doi.org/10.1007/s10669-010-9293-6
Stiers I, Triest L (2017) Impact of non-native invasive plant species cover on phytoplankton and zooplankton communities in temperate ponds. Aquat Inv 12(3):385–395. https://doi.org/10.3391/ai.2017.12.3.11
Szabó S, Braun M, Borics G (1999) Elemental flux between algae and duckweeds (Lemna gibba) during competition. Archiv Hydrobiol 146:355–367. https://doi.org/10.1127/archiv-hydrobiol/146/1999/355
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2000) Invertébrés d’eau douce. CNRS, Paris, p 587
Takamura N, Kadono Y, Fukushima M, Nakagawa M, Kim BHO (2003) Effects of aquatic macrophytes on water quality and phytoplankton communities in shallow lakes. Ecol Res 18:381–395. https://doi.org/10.1046/j.1440-1703.2003.00563.x
Villa P, Bresciani M, Bolpagni R, Pinardi M, Giardino C (2015) A rule-based approach for mapping macrophyte communities using multi-temporal aquatic vegetation indices. Remote Sens Environ 171:218–233. https://doi.org/10.1016/j.rse.2015.10.020
Ziegler P, Adelmann K, Zimmer S, Schmidt C, Appenroth KJ (2015) Relative in vitro growth rates of duckweeds (Lemnaceae)—the most rapidly growing higher plants. Plant Biol 17(1):33–41. https://doi.org/10.1111/plb.12184
Acknowledgements
The Authors are grateful to Staff of the Regional Park of Appia Antica (Rome), and to Dr. Silverio Abati and Dr. Amii Bellini, for their support during fieldwork and data collection. They also thank Prof. Vincenzo Zuccarello for his support in statistical analyses of data. The Grant of Excellence Departments, MIUR (ARTICOLO 1, COMMI 314-337 LEGGE 232/2016), is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ceschin, S., Ferrante, G., Mariani, F. et al. Habitat change and alteration of plant and invertebrate communities in waterbodies dominated by the invasive alien macrophyte Lemna minuta Kunth. Biol Invasions 22, 1325–1337 (2020). https://doi.org/10.1007/s10530-019-02185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-019-02185-5