Abstract
There is increasing evidence that invasive species are threating montane ecosystems globally. However, trends in species distribution are difficult to observe directly due to a lack of data with suitable spatio-temporal resolution. Here, we aimed to detect spatio-temporal trends in exotic plants in a montane ecosystem, and to determine which drivers had a role affecting these trends. Each year, for a period of 7 years, we recorded the upper elevational range limit of exotic plant species in road verges along an elevational gradient of 1500–2874 m a.s.l. in southern Africa. We fitted repeated-measures ANOVA models to test if upper elevational range limits changed over time. Generalized least squares models showed that exotic richness of annuals increased by 3.9 species per year. Also, the upper elevational range limits of established exotics ascended by 24.5 m/year for annuals (N = 17 species), and by 9.7 m/year for perennials (N = 26). These upward trends were too rapid to be explained by slow-acting drivers such as climatic change or time since species introduction. The rates of increase indicate that many exotics were not yet in equilibrium with the environment and, therefore, had not been in the region long enough to have filled their potential niches. Exotic species could reach much higher elevations than expected, indicating that current prediction models are likely an underestimation of potential distributional ranges. The spatial clustering of upper elevational range limits around potential points of introduction indicates ongoing human-mediated propagule pressure as the major cause of rapid exotic range expansion, especially along roads and near dwellings. Montane road verges are regularly disturbed by erosion and maintenance, creating unoccupied habitats, while traffic in the form of vehicles and tourists facilitates the introduction of new species. This suggests that easily accessible montane ecosystems are much more susceptible to invasions than previously assumed, due to a combination of anthropogenic disturbance and ongoing propagule pressure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive alien species are a major global concern due to their widespread negative impacts on biodiversity and agriculture (Convention on Biological Diversity 2002; McGeoch et al. 2010). These impacts are likely to be exacerbated by anthropogenic drivers such as habitat conversion, propagule movements, and global climate change (Beaumont et al. 2011). Globally, scientists and conservation decision makers therefore aim to curb the spread and impact of invasive alien species (Convention on Biological Diversity 2002). For example, parties to the Convention on Biological Diversity are committed to take adequate measures to block pathways of introduction of new exotics, and to limit further spread of established invasive alien species (sensu Richardson et al. 2000). To assess if counter measures are successful, there is an ongoing need to cost-effectively detect trends in invasive species in both space and time (McGeoch et al. 2010). Recently, various datasets have been developed or collated in an attempt to provide baseline information on the current distributional range of invasive or exotic species at a regional or continental scale (Beck et al. 2013). An inevitable downside of such datasets is that their accuracy is biased towards areas and time periods with a high collection intensity (Kalwij et al. 2014a). As a result, these datasets do not have a sufficiently high spatio-temporal resolution to relate trends in species distribution to certain environmental drivers (Pyšek et al. 2011). This limitation in the data is particularly noticeable in our knowledge and understanding of invasive species in montane ecosystems (McDougall et al. 2011; Kueffer et al. 2013).
Montane ecosystems are important reservoirs for global taxonomic and genetic diversity (Lomolino 2001), evolutionary centers and refugia in times of rapid climate change (Stewart et al. 2010), and important sources of ecosystem services (Gret-Regamey et al. 2012). For example, tropical montane ecosystems are notably important areas of global biodiversity due to an elevation-related range of environmental conditions and relatively stable climates (Kier et al. 2009). Anthropogenic climate change, however, is predicted to be too fast for slow-adjusting species to adapt, or for slow-dispersing species to migrate to an available area with suitable environmental conditions (Thuiller et al. 2005; Beaumont et al. 2011). Climate change is therefore considered to be a leading threat to the intrinsic conservation value of mountain ecosystems globally.
Invasive species are often considered not to be a major threat to montane ecosystems due to the harsh environmental conditions at high elevations and a relatively low introduction rate of exotic propagules (Millennium Ecosystem Assessment 2003; Pauchard et al. 2009; Kueffer et al. 2013). For example, a global survey of 13 mountain areas across all continents (except Antarctica) revealed that 49 % of exotic species in lowlands were also found in the adjacent mountainous areas, even though this was likely an underestimation due to the lack of comprehensive local checklists of exotics (McDougall et al. 2011). A lower number of exotic species in a mountainous area, however, should not be misinterpreted as a high resistance to plant invasions (Pauchard et al. 2009). This is especially true for many exotics that have not yet occupied all suitable regions in their introduced range (Alexander and Edwards 2010). Whether an introduced species will successfully invade a montane ecosystem depends on a range of factors such as species plasticity, local disturbance regime, biotic and abiotic resistance, and propagule pressure (Alexander et al. 2011; McDougall et al. 2011; Molina-Montenegro et al. 2012; Seipel et al. 2012; Van Rensburg et al. 2013). The lack of adequate datasets on the distributional range of exotics, however, makes it difficult to disentangle the relative importance of each of these factors.
Elevational gradients are widely used as natural landscape experiments to provide insights into the role of climate and other factors in the upward spread of species (see e.g. Becker et al. 2005; le Roux and McGeoch 2008; Alexander et al. 2011). A change in the environmental conditions for a given species may be reflected in a change in the species distributional range. Climate change, for example, can result in indigenous species undergoing upward range expansion, upward range shift, or range contraction (Parmesan 2006; le Roux and McGeoch 2008; Lenoir et al. 2008; Walther et al. 2009). An underlying assumption of such studies is that species have already occupied their potential range, and that range expansion keeps pace with climate change. For exotic species, however, this assumption does not hold since many are still in the process of colonizing their potential niche (Wilson et al. 2007). In addition, the rate at which a potential niche becomes occupied depends on a range of indirectly measured or reconstructed variables, such as residence time and propagule pressure (Becker et al. 2005; Pyšek et al. 2010, 2011; le Roux et al. 2013). Species distribution data therefore need to be sufficiently detailed to detect spatio-temporal trends (Pauchard et al. 2009; Walther et al. 2009). However, data that are typically being used, such as atlas data or herbarium records lack the temporal precision and coverage to meet this need (Pyšek et al. 2011; Kalwij et al. 2014a), especially for inconspicuous exotics in montane ecosystems (Walther et al. 2009). Instead, repeated observations on exotics across an elevational gradient could be used to detect trends in distributional range and to gain better insights into the underlying mechanisms of range expansion.
In this study we document spatio-temporal trends in the richness and upper elevational limits of exotic plants in a montane ecosystem. We expect no change in upper elevational limits for exotic species that are in ecological equilibrium and that have already reached their potential distributional ranges. However, we expect upper elevational limits to ascend for exotic species that have not reached their full potential range. We also expect exotic species richness to increase if more species colonize the area than go extinct. Such patterns are expected to be more prominent for annual lifeforms than for perennials given the larger amount of propagules and quick generation turnover time of annuals. Since propagule pressure and disturbance are important drivers in the spread of exotic species, we expect upper elevational limits to be in close proximity to a potential point of introduction (Kalwij et al. 2008). We tested these expectations by monitoring the upper elevational range limits of all exotic plant species along an elevational gradient of 1500–2874 m a.s.l. over a period of 7 years, and by measuring the distance between upper elevational limits and potential points of introduction. This repeated monitoring enabled us to provide direct observations of annual rates of change in upper elevational limits and in the colonization success rate of exotic species in a new habitat. We then discuss the potential susceptibility of montane ecosystems to invasion and the role of environmental drivers, such as anthropogenic propagule pressure and disturbance.
Methods
Study area
Our study area was a 20-km road running through a largely pristine mountain valley located in the southern region of the Maloti-Drakensberg covering an elevational gradient of 1500–2874 m a.s.l. The natural vegetation mostly consisted of montane grasslands and was part of the uKhahlamba/Drakensberg Park; a UNESCO world heritage site characterized by, amongst others, pristine rolling grassland valleys harboring an exceptional high diversity of flora and fauna (UNESCO 2011). Average annual precipitation varied from 862 mm in the lowlands to 768 mm at the top, the majority falling during summer (October–March; Nel and Sumner 2008). During our sampling period, the monthly average soil temperature ranged from 6.8 to 14.8 °C at 3000 m a.s.l., and from 13.1 to 22.2 °C at 1500 m a.s.l. (Bishop et al. 2014).
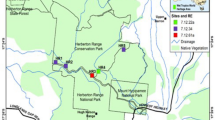
The 20-km road is the only road through the area, connecting the lowlands of South Africa with the highlands of eastern Lesotho. Starting from South Africa this road gradually climbs from 1500 m a.s.l. over a distance of 20 km, with sharply ascending hairpin bends as it reaches the international border at 2874 m a.s.l. (Fig. 1). Some 13 km into Lesotho the road reaches the Kotisephola Pass at 3240 m a.s.l. before continuing towards the town of Mokhotlong. The Sani Pass was originally opened as a bridal path in 1913, with only 4 × 4 vehicles using it since 1948. Currently almost 1000 4 × 4 vehicles drive up the pass monthly, and this number of vehicles did not vary significantly during the sampling period (988.3 vehicles per month ± 30.8 SE; Appendix A in Supplemental Material).
Overview of the study area in the uKhahlamba/Drakensberg Park. The study area was a cross-border arterial gravel road located in the uKhahlamba/Drakensberg Park at the border of South Africa and eastern Lesotho (inset). Four potential points of introduction are located along the annually monitored section of the altitudinal gradient, namely: (1) Lesotho border post at Sani Top village, (2) South African border post, (3) tourist cottages, and (4) a bus passengers transfer area. The border between Lesotho and South Africa is indicated with a thick dashed line. Projection: Albers equal-area conic
There are only four groups of buildings located along the 20-km road, namely a bus stop, a cluster of tourist cottages, a border check point, and a second border check at a rural village (Fig. 1). All visitors to the area, including travelers in transit, must stop and disembarked at both check points for immigration purposes. Previous surveys indicated that many exotics were spatially associated with such places of ongoing and strong presence of human activities (Kalwij et al. 2008, 2014b; Steyn 2014). Therefore, each of these groups of buildings was considered as a potential point from which exotic plants were introduced to the area.
Sampling of exotics
Data on the upper elevational range limit of exotic plants were collected from 2008 to 2014. Sampling aimed at annually detecting the upper elevational range limit of all exotic species in the area. An earlier study conducted in the same area indicated that such sampling was best done by monitoring the road verges of the 1500–2874 m gradient section on the South African side only (Kalwij et al. 2008). Therefore, annual monitoring involved a team of experienced observers walking down this entire 20-km stretch of road and surveying the verges of both sides of the road for the presence of exotics. Road verges consisted of a distinct zone varying between 0.5 and 4.0 m adjacent to the road and liable to be disturbed by road maintenance activities or road-related water run-off. For each exotic species in these verges the three highest occurrences were recorded using a handheld GPS (Garmin 60CSx). We recorded all exotic individuals as an occurrence, irrespective of whether they were likely to persist. To determine which species had their upper elevational range limit outside this annually-sampled gradient, we sampled the 2874–3200 m gradient in a similar way in 2012. Specimens were collected for future reference and, if deemed necessary, their identification confirmed at the herbarium of the South African National Biodiversity Institute (SANBI). For the nomenclature and life form categorization we followed Germishuizen and Meyer (2003).
Since the vertical accuracy of a GPS can vary considerably in mountainous areas we extracted elevation data from the Shuttle Radar Topography Mission (SRTM, version 4). This digital terrain model has a spatial resolution of 3 arc-seconds and a vertical accuracy of c. 9.8 m in Africa (Jarvis et al. 2008). A comparison of this digital terrain model with a calibrated barometric altimeter showed a vertical accuracy of 7.1 m for the steepest zone, which we considered as sufficiently accurate for the purpose of this study. Sampling between years was conducted consistently during the second half of January, i.e. mid-summer. This minimized the change of seasonal variation between sampling events, while maximizing our chances of recording plant specimens that could be identified to species level.
Sampling effort and observer experience are known factors affecting data quality, especially in the detection of rare, inconspicuous, or species with low-density populations (Fitzpatrick et al. 2009; Moore et al. 2011). To ensure optimal observer accuracy, field work was spread across three consecutive days to avoid tiredness. To keep sampling bias to a minimum, sampling was done by teams of a minimum of six people always including the first two authors of this study.
Data analysis
We used a paired t test to compare our observed upper elevational limits with the expected, known upper elevational limits for each species (sensu Kalwij et al. 2008). These expected elevational limits were based on the databases and checklists of the national herbarium, and are the most comprehensive and up-to-date datasets available for the study area. To determine if the number of exotics increased over time we fitted linear models using generalized least squares (GLS). These models were based on a restricted maximum likelihood estimation using a first-order autoregressive correlation structure to adjust for temporal autocorrelation (Piepho et al. 2004). Models were fitted using all exotic species, and for annuals and perennials separately.
To test if upper elevational range limits had changed over time we fitted a factorial repeated-measures ANOVA model. For this analysis we used a subset of the exotic species data found in each of the seven consecutive years as the within-subjects factor, and rank of the highest, second highest, and third highest observation as the between-subjects factor. We ensured elevational observations met the requirement of normal distribution at each time point. Since observations did not meet the assumption of sphericity, we adjusted the degrees of freedom downwards using the conservative Greenhouse–Geisser estimate (Mauchly’s test of sphericity; Quinn and Keough 2002). To estimate the slope of trends in upper elevational limits for both lifeforms (annuals and perennials) and for each of the individual species, we fitted similar GLS models as for the richness trend analysis. We also tested a sigmoidal model for both annuals and perennials, which showed a similar pattern and Akaike information criterion value as the linear GLS models. However, we considered linear GLS models as the most appropriate models given the temporal autocorrelation between observations and the spatio-temporal scale at which species dispersal was likely to occur (Wagner and Fortin 2005).
Proximity to dwellings was used as a spatially explicit estimation of local propagule pressure (Kalwij et al. 2008; Gavier-Pizarro et al. 2010). We measured the average Euclidean distance between each highest observation and the location of the nearest group of buildings as potential point of introduction. We then calculated the average Euclidean distance for each life form group, and then tested if observations were spatially associated with dwellings as compared to a sample of 10,000 randomly located points along this road.
To determine if temperature changed over time we used the temperature data from Bishop et al. (2014), supplemented with successive temperature observations to cover the entire sampling period. Temperature data were recorded every 1.0–1.5 h using a pair of Thermocron iButtons (DS1921G/DS1922L, Semiconductor Corporation, Dallas/Maxim, TX, USA) buried 10 mm below the soil surface at 300-m elevational intervals. Summer temperature was calculated as the average temperature for the month January, while winter temperature was calculated as the average temperature for the month September. We then fitted GLS models to the observations for each season and elevational zone separately.
For the GLS analyses we used S-Plus 8.0 (Insightful, Seattle, WA). For all other statistical analyses we used SPSS 20.0 (SPSS, Chicago, IL).
Results
We found a total of 104 exotic plant species over a 7-year period (Appendix B in Supplemental Material). The paired t test showed that, based on the databases of the national herbarium, the average upper elevational range limit of annuals was 432 m higher than previously recorded (t = 5.354, df = 39, P < 0.0001), but not so for the perennials (t = 1.820, df = 45, P = 0.075). Ten exotic species were observed in the 2874–3200 m gradient. This gradient was not monitored annually and these species have, thus, been excluded from the trend analysis. For future reference, however, these observations were retained in Appendix B in Supplemental Material. Although the exotic species composition showed some variation between years, e.g. due to recent road-maintenance activities or erosion, nearly half of the species were consistently recorded in each of the observation years. The number of exotic species observed per year increased significantly for annuals (Fig. 2; slope = 2.6; t = 6.070, P = 0.0018). No significant annual increase in richness was observed for total exotic species richness (P = 0.1355), or for perennials (P = 0.4739).
Exotic species richness for all life forms together (filled triangle), for annuals (filled circle), and for perennials (circle) from 2008 until 2014. The line represents the generalized least squares (GLS) model for annual richness against time, based on a restricted maximum likelihood estimation using a first-order autoregressive correlation structure (slope = 2.6; t = 6.070, P = 0.002). The GLS models for total species richness and perennials were non-significant (both P values >0.05)
The repeated-measures ANOVA indicated a significant effect of time on the upper elevational range limit of annuals (F = 5.831, df = 3.566, P < 0.001), but no interaction between rank of highest observation and time (F = 0.282, df = 7.132, P = 0.962). We found the same trends for perennials: a significant effect of time (F = 3.489, df = 2.949, P = 0.018), but no interaction between rank of highest observation and time (F = 0.277, df = 5.897, P = 0.945). The GLS models showed that, from 2008 to 2014, the average rate of ascent for annuals was 24.5 m/year (Fig. 3a; t = 2.957, P = 0.0081), and for perennials 9.7 m/year (Fig. 3b; t = 2.942, P = 0.0084).
Trends in upper elevational limits (mean ± standard error) against time for annuals (a) and perennials (b). Average elevational limits for the highest (filled circle), second highest (circle), and third highest (filled triangle) observations are displayed separately since these were interdependent observations, and separated along the horizontal axis for display purposes. The lines represent the generalized least squares (GLS) models for observed upper elevational limits against time (annuals: slope = 24.5 m/year, t = 2.957, P = 0.0081; perennials: slope = 9.7 m/year, t = 2.942, P = 0.0084)
We found more ascending annuals than perennials (Table 1; Appendix C in Supplemental Material). Also, the rate at which annuals were moving up in elevation was higher than for perennials. For example, annual species Bidens pilosa, Persicaria lapathifolia and Verbena brasiliensis experienced rates of ascent of 61–124 m per year. In contrast, the fastest ascending perennials were Cotoneaster franchetii, Pennisetum clandestinum and Rubus cuneifolius with rates of ascent of 11–31 m/year. One species was identified as a descending species, namely Acacia decurrens. This descent was caused by a control program on invasive Acacia species in the area between 2013 and 2014.
The repeated-measures ANOVAs showed that the distances between observations and potential points of introduction did not change over time (all P values >0.05). Randomly generated points along the road (as an approximation of an expected distribution of observations) were at an average distance of 1630 m from a dwelling. The t tests showed that, compared to random, both annuals and perennials were spatially associated with potential points of introduction; this pattern showed a higher degree of spatial aggregation for perennials than for annuals (Fig. 4). This result indicates that the upper elevational range limits of exotics were not observed at random locations along the road, but instead relatively close to a potential point of introduction.
Influence of local propagule pressure expressed as the Euclidean distance (mean ± standard error) from nearest potential point of introduction for the highest observation per species against time, and divided into annual (square) and perennial plant life forms (filled square). The horizontal dotted line indicates the expected distance from the nearest potential point of introduction, calculated as the mean of 10,000 randomly distributed points along the road
The temperature data showed no significant trends in average summer or winter temperature in the higher elevational zones (Table 2; 2100–3000 m a.s.l.). In the lower elevational zone (1500–2100 m a.s.l.) significant temperature decreases were observed but not consistently throughout the year. One temperature observation point in the lowland area (at 1200 m a.s.l.) showed a significant warming of the winter temperature. However, this point was at a distance of approx. 68 km from the study area.
Discussion
The observed increase in exotic richness of annuals and the rapid ascent of upper elevational limits exceeded our expectation. We showed that exotics, especially those of annual lifeform, continue to colonize the area and are still significantly expanding their distributional ranges at a rate measurable within a time-span of 7 years. To the best of our knowledge, no other study has reported such rates of change using direct observations over short time periods, rendering a direct comparison with other systems difficult. The upper elevational limits increased by 24.5 m/year for annual species and by 9.7 m/year for perennials, which is in the same range of elevational range expansion as reconstructed from herbarium records in a central European region (16.1 ± 45.3 SD m/year; Pyšek et al. 2011). Commonly reported factors explaining an upward shift of upper elevational limits and an increasing number of exotics are climate change (le Roux and McGeoch 2008), residence time of exotics (Becker et al. 2005), increased propagule pressure at high elevations (le Roux et al. 2013), low biotic resistance (Pauchard et al. 2009; Van Rensburg et al. 2013), facilitation (Richardson et al. 2000), and disturbance (Pyšek et al. 2011). Below we discuss each of these factors in greater detail, and assess their likely role in our study system.
Climate change
There are studies with comprehensive data sets spanning both plants and animals, focusing mainly on native species, that show a general trend of species distribution moving upward in elevation due to broad impacts of climate change (Parmesan 2006; Lenoir et al. 2008). None of these, however, report a rate of ascent in the range that we observed. For example, a re-survey study on the upper elevational limit of indigenous vascular plants on sub-Antarctic Marion Island ascended at a rate of 3.4 m/year, in response to a 1.2 °C warming measured over a 40-year period (le Roux and McGeoch 2008). Although temperature data are available for the current study area (Bishop et al. 2014), we are cautious not to attribute climate to the changes in upper elevational limits of exotic plants over the relatively short time-span of our study of 7 years. Climate is generally measured over a much longer period of time, typically 30 years (McClean et al. 2005; Thuiller et al. 2005). Moreover, the rate of change that we observed would be equivalent to a 1.6 °C warming over a 7-year period (assuming a dry adiabatic lapse rate of 1.0 °C decrease for every 100 m in elevation); such increase is improbable and cannot be ascertained within such short time-span (Kupfer and Cairns 1996). Although a few consecutive warm years could mimic the long-term effects of climate change, especially for annual plants, our own temperature observations did not show a significant temperature increase for any of the elevational bands (Table 2). Therefore, while we cannot exclude climate change as a contributing variable per se knowing that the Drakensberg region is likely to gain climatically suitable areas for many new species based on future climate change scenarios (McClean et al. 2005), climate change is unlikely to be sufficiently fast to be a main driving factor.
Residence time
Residence time of exotics, usually quantified as time since a species was first recorded in its new range, is a well-documented variable influencing the degree to which potential distributional ranges are occupied (Wilson et al. 2007; Wolmarans et al. 2010; Pyšek et al. 2011). An increase in exotic richness and ascending upper elevational limits might thus be attributed to recently introduced species still expanding their distributional ranges (Becker et al. 2005). While the rapid increase in upper elevational limit of some species is a strong indicator that these species have not been in the region long enough to have reached their ecological equilibrium (Table 1), we cannot reliably test minimum residence time as an explanatory variable for the observed trends. Determining a year of introduction is notoriously complicated due to the absence or incompleteness of historical introduction records (Wilson et al. 2007; le Roux et al. 2013). This is especially true for unintentionally introduced species such as pests or inconspicuous weeds. Instead, herbarium records and historical plant checklists are used to determine a year of first observation. Such historical data, however, are often confounded by observer bias and sampling intensity (Wolmarans et al. 2010; Kalwij et al. 2014a), or may be biased towards economically important or ostentatious species, for example “to [..] avoid [..] sending here that which we already have in abundance…” (McGibbon 1858). Such historical observer bias thus results in an irregular pattern of cumulative exotic species richness against time (Wilson et al. 2007), even when considered at the spatial scale of an entire region or country. Consequently, the temporal precision and accuracy of minimum residence time of exotic species in southern Africa does not match that of our study design.
Propagule pressure
The spatial clustering of elevational range limits around dwellings is a strong indication that propagule pressure is a major driver in the range expansion of exotics (Huang et al. 2012; González-Moreno et al. 2014). Indeed, the role of propagule pressure in biological invasions is considered the least contentious aspect responsible for the successful establishment and spread of non-native plants and animals (Richardson et al. 2000; Pyšek et al. 2010). We did not interpret dynamic elevational range limits—where the upper range limit increases in 1 year and then decreases the next—as an indication that species had reached their range limit. Instead, the high variation in exotic species composition between years indicates that not all introductions are successful, while the increase in exotic richness over time shows that colonization events outnumber extinction events. Although traffic density did not increase during the sampling period, propagules are still easily moved across large distances by vehicles, road maintenance vehicles, or even cattle (Richardson et al. 2000; Von der Lippe and Kowarik 2007; Pollnac et al. 2012). Since the study area is a major tourist attraction as well as an important international trade route, vehicle movement is a likely propagule dispersal vector for introducing new species from distant source populations. While livestock movement is another potential vector, animals are usually transported across the area on foot, making it more likely that livestock are a propagule dispersal vector for introducing new species from nearby source populations. We therefore suspect that the introduction of new exotic species originates from both distant and nearby source populations.
Disturbance
Intermittent disturbance events are a likely supplementary driver for the faster rate of ascent of annuals, when compared to perennials, and for the variation between years in establishment success. Disturbance, such as soil disturbance or nutrient enhancement, creates opportunities for new species to establish, but can also hamper species establishment if disturbance interrupts the seed regeneration process. This is especially applicable to annual plants in mountainous road-side verges, which are strongly affected by water run-off and intermit disturbance by erosion or road maintenance activities (Kalwij et al. 2008). Such levels of disturbance imply reduced biotic resistance and little role for facilitation (le Roux et al. 2013; Van Rensburg et al. 2013). Here, fast-growing annual plants may still manage to generate a large number of propagules distributed by long-distance dispersal vectors such as wind, water, animals, or even humans (Richardson et al. 2000; Von der Lippe and Kowarik 2007). Once established, these annuals can create large seedbanks that remain viable for extended periods of time (Fenner 2001). Such traits allow annuals to rapidly colonize and establish seedbanks in newly available habitats, even if these habitats are only temporarily suitable (Richardson et al. 2000; Fenner 2001).
Limitations
A limitation of our study is that observations do not distinguish between self-sustaining populations and stochastic establishments outside of the species’ fundamental niche; such comprehensive sampling was beyond the scope of this study. It is nevertheless important to note that an observation on the outer range limit of species does not necessarily mean that these observations represent the border of a species’ ecological niche (Holt and Keitt 2005). For example, while we have direct observations that many exotics can germinate and grow at much higher elevations than previously reported, we cannot be sure that these species can actually complete their life cycles at those highest elevations. In such cases, observations that do not represent self-sustaining populations are outside of their fundamental niches (Alexander and Edwards 2010). Distribution models should thus be calibrated using records that represent the ecological niche of the species. These records should represent environments where the species is able to maintain a self-sustaining population. In spite of this restriction, observations from the periphery of a species range are widely used to study invasion dynamics in the realized niche or climate change related shifts in the fundamental niche of species (Holt and Keitt 2005; Alexander and Edwards 2010).
Invasion outlook
The high rates of ascent that we observed on the upper elevational range limits are likely to slow down in the long term; either because species reach their potential peripheral range limit, or because of the elevational limit of the region. When an environmental equilibrium will be reached, however, is more difficult to predict. In African ecoregions temperature variability is relatively low. Therefore, the impact of increasing monthly temperature averages will have a relatively large impact in these ecoregions (Beaumont et al. 2011). A consequence of such climate warming is that the potential range limits of exotics will move upwards. It is uncertain which other factors such as local adaptive evolution or phenotypic plasticity will play a role (Kirkpatrick and Barton 1997; Holt and Keitt 2005; Molina-Montenegro et al. 2012). However, if we consider only those species that we have observed in all 7 years, and if the same sampling protocol were to be repeated in the distant future, then we expect that many species will have reached their potential range limits. This means that eventually all exotics will stop increasing in elevation, assuming that climate warming ceases at some point. The number of exotics, however, will continue to increase. Although we observed 104 exotics, which is about 10 % of the currently known exotics in montane regions (Pauchard et al. 2009; McDougall et al. 2011; Kueffer et al. 2013), the species pool of exotic plants in South Africa contains some 8750 taxa (Ilrich et al. 2014). Moreover, our observation that new species successfully colonize the area each year shows that this mountain region is not resistant to invasion by exotics, in spite of its high species richness (Fridley et al. 2007; Van Rensburg et al. 2013). This trend is especially worrying when it shows distributional range expansion of known invasive species such as Cotoneaster spp., Pennesetum clandestinum, R. cuneifolius and Verbena spp. (Carbutt 2012; Wilson et al. 2013). The impact of exotics on the biodiversity of this region is therefore likely to increase for some time to come.
Conclusions
Long-term monitoring programs, i.e. repeated surveys over a 10-year period or more, are notably scarce (Lindenmayer et al. 2012). Although this study does not meet the 10-year time-span of a long-term study sensu Lindenmayer et al., it is the first to implement an annually repeated monitoring of elevational limits for exotics plants in a montane ecosystem. In spite of the relatively short time-span of 7 years in this study, we found unprecedented annual increases in the upper elevational range limits of exotic plants. The rates of increase indicate that many exotics were not yet in equilibrium with the environment and, therefore, had not been in the region long enough to have filled their potential niches. Exotic species could reach much higher elevations than had previously been recorded, indicating that current prediction models are likely an underestimation of potential distributional ranges. The spatial clustering of upper elevational range limits around potential points of introduction indicates ongoing human-mediated propagule pressure. This environmental driver is one of the major causes of rapid exotic range expansion, especially along roads and near dwellings from a local species pool of exotics, some of which are potentially transformer species (Kalwij et al. 2014b). Montane road verges are regularly disturbed by erosion and maintenance, creating unoccupied habitats, while traffic in the form of vehicles and tourists facilitates the introduction of new species. This suggests that easily accessible montane ecosystems are much more susceptible to invasions than previously assumed, due to a combination of anthropogenic disturbance and ongoing propagule pressure (Pauchard et al. 2009; Kueffer et al. 2013; Pollnac and Rew 2014).
References
Alexander JM, Edwards PJ (2010) Limits to the niche and range margins of alien species. Oikos 119:1377–1386
Alexander JM, Kueffer C, Daehler CC, Edwards PJ, Pauchard A, Seipel T (2011) Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proc Natl Acad Sci USA 108:656–661
Beaumont LJ, Pitman A, Perkins S, Zimmermann NE, Yoccoz NG, Thuiller W (2011) Impacts of climate change on the world’s most exceptional ecoregions. Proc Natl Acad Sci USA 108:2306–2311
Beck J, Ballesteros-Mejia L, Nagel P, Kitching IJ (2013) Online solutions and the ‘Wallacean shortfall’: what does GBIF contribute to our knowledge of species’ ranges? Divers Distrib 19:1043–1050
Becker T, Dietz H, Billeter R, Buschmann H, Edwards PJ (2005) Altitudinal distribution of alien plant species in the Swiss Alps. Perspect Plant Ecol Evol Syst 7:173–183
Bishop TR, Robertson MP, van Rensburg BJ, Parr CL (2014) Elevation-diversity patterns through space and time: ant communities of the Maloti-Drakensberg Mountains of southern Africa. J Biogeogr 41:2256–2268
Carbutt C (2012) The emerging invasive alien plants of the Drakensberg Alpine Centre, southern Africa. Bothalia 42:71–85
Convention on Biological Diversity (2002) Decisions adopted by the conference of the parties to the convention on biological diversity at its sixth meeting. COP 6 Decision VI/23. http://www.cbd.int/decisions/cop/. Accessed 3 Nov 2014
Fenner M (2001) Seeds: the ecology of regeneration in plant communities. CABI Publishing, Oxford
Fitzpatrick MC, Preisser EL, Ellison AM, Elkinton JS (2009) Observer bias and the detection of low-density populations. Ecol Appl 19:1673–1679
Fridley JD, Stachowicz JJ, Naeem S, Sax DF, Seabloom EW, Smith MD, Stohlgren TJ, Tilman D, Von Holle B (2007) The invasion paradox: reconciling pattern and process in species invasions. Ecology 88:3–17
Gavier-Pizarro GI, Radeloff VC, Stewart SI, Huebner CD, Keuler NS (2010) Housing is positively associated with invasive exotic plant species richness in New England, USA. Ecol Appl 20:1913–1925
Germishuizen G, Meyer NL (2003) Plants of southern Africa: an annotated checklist. National Botanical Institute, Pretoria
González-Moreno P, Diez JM, Ibáñez I, Font X, Vilà M (2014) Plant invasions are context-dependent: multiscale effects of climate, human activity and habitat. Divers Distrib 20:720–731
Gret-Regamey A, Brunner SH, Kienast F (2012) Mountain ecosystem services: who cares? Mt Res Dev 32:S23–S34
Holt RD, Keitt TH (2005) Species’ borders: a unifying theme in ecology. Oikos 108:3–6
Huang DC, Zhang RZ, Kim KC, Suarez AV (2012) Spatial pattern and determinants of the first detection locations of invasive alien species in mainland China. PLoS One 7:e31734
Ilrich UM, Richardson DM, Davies SJ, Chown SL (2014) Climate change and alien species in South Africa. In: Zikska LH, Dukes JS (eds) Invasive species and global climate change. CABI Publishing, Oxford, pp 129–147
Jarvis A, Reuter HI, Nelson A, Guevara E (2008) Hole-filled seamless SRTM data V4. International Centre for Tropical Agriculture (CIAT). http://srtm.csi.cgiar.org/. Accessed 3 Nov 2014
Kalwij JM, Robertson MP, Van Rensburg BJ (2008) Human activity facilitates altitudinal expansion of exotic plants along a road in montane grassland, South Africa. Appl Veg Sci 11:491–498
Kalwij JM, Robertson MP, Ronk A, Zobel M, Pärtel M (2014a) Spatially-explicit estimation of geographical representation in large-scale species distribution datasets. PLoS One 9:e85306
Kalwij JM, Steyn C, le Roux PC (2014b) Repeated monitoring as an effective early detection means: first records of naturalised Solidago gigantea Aiton (Asteraceae) in southern Africa. S Afr J Bot 93:204–206
Kier G, Kreft H, Lee TM, Jetz W, Ibisch PL, Nowicki C, Mutke J, Barthlott W (2009) A global assessment of endemism and species richness across island and mainland regions. Proc Natl Acad Sci USA 106:9322–9327
Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150:1–23
Kueffer C, McDougall K, Alexander J, Daehler C, Edwards P, Haider S, Milbau A, Parks C, Pauchard A, Reshi ZA, Rew LJ, Schroder M, Seipel T (2013) Plant invasions into mountain protected areas: assessment, prevention and control at multiple spatial scales. In: Foxcroft LC, Pyšek P, Richardson DM, Genovesi P (eds) Plant invasions in protected areas. Patterns, problems and challenges. Springer, Dordrecht, pp 89–116
Kupfer JA, Cairns DM (1996) The suitability of montane ecotones as indicators of global climatic change. Prog Phys Geogr 20:253–272
le Roux PC, McGeoch MA (2008) Rapid range expansion and community reorganization in response to warming. Glob Change Biol 14:2950–2962
le Roux PC, Ramaswiela T, Kalwij JM, Shaw JD, Ryan PG, Treasure AM, McClelland GTW, McGeoch MA, Chown SL (2013) Human activities, propagule pressure and the alien plants in the sub-Antarctic: evidence in support of management. Biol Conserv 161:18–27
Lenoir J, Gégout JC, Marquet PA, de Ruffray P, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771
Lindenmayer DB, Likens GE, Andersen A, Bowman D, Bull CM, Burns E, Dickman CR, Hoffmann AA, Keith DA, Liddell MJ, Lowe AJ, Metcalfe DJ, Phinn SR, Russell-Smith J, Thurgate N, Wardle GM (2012) Value of long-term ecological studies. Austral Ecol 37:745–757
Lomolino MV (2001) Elevation gradients of species-density: historical and prospective views. Glob Ecol Biogeogr 10:3–13
McClean CJ, Lovett JC, Küper W, Hannah L, Sommer JH, Barthlott W, Termansen M, Smith GE, Tokamine S, Taplin JRD (2005) African plant diversity and climate change. Ann Mo Bot Gard 92:139–152
McDougall KL, Alexander JM, Haider S, Pauchard A, Walsh NG, Kueffer C (2011) Alien flora of mountains: global comparisons for the development of local preventive measures against plant invasions. Divers Distrib 17:103–111
McGeoch MA, Butchart SHM, Spear D, Marais E, Kleynhans EJ, Symes A, Chanson J, Hoffmann M (2010) Global indicators of biological invasion: species numbers, biodiversity impact and policy responses. Divers Distrib 16:95–108
McGibbon J (1858) Catalogue of plants in the botanic garden, Cape Town, Cape of Good Hope. Saul Solomon, Cape Town
Millennium Ecosystem Assessment (2003) Ecosystems and human well-being: a framework for assessment. Island Press, Washington, DC
Molina-Montenegro MA, Penuelas J, Munne-Bosch S, Sardans J (2012) Higher plasticity in ecophysiological traits enhances the performance and invasion success of Taraxacum officinale (dandelion) in alpine environments. Biol Invasions 14:21–33
Moore JL, Hauser CE, Bear JL, Williams NSG, McCarthy MA (2011) Estimating detection-effort curves for plants using search experiments. Ecol Appl 21:601–607
Nel W, Sumner P (2008) Rainfall and temperature attributes on the Lesotho-Drakensberg escarpment edge, southern Africa. Geogr Ann Ser A Phys Geogr 90:97–108
Parmesan C (2006) Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669
Pauchard A, Kueffer C, Dietz H, Daehler CC, Alexander J, Edwards PJ, Arévalo JR, Cavieres LA, Guisan A, Haider S, Jakobs G, McDougall K, Millar CI, Naylor BJ, Parks CG, Rew LJ, Seipel T (2009) Ain’t no mountain high enough: plant invasions reaching new elevations. Front Ecol Environ 7:479–486
Piepho HP, Buchse A, Richter C (2004) A mixed modelling approach for randomized experiments with repeated measures. J Agron Crop Sci 190:230–247
Pollnac FW, Rew LJ (2014) Life after establishment: factors structuring the success of a mountain invader away from disturbed roadsides. Biol Invasions 16:1689–1698
Pollnac F, Seipel T, Repath C, Rew LJ (2012) Plant invasion at landscape and local scales along roadways in the mountainous region of the Greater Yellowstone Ecosystem. Biol Invasions 14:1753–1763
Pyšek P, Jarošík V, Hulme PE, Kühn I, Wild J, Arianoutsou M, Bacher S, Chiron F, Didžiulis V, Essl F, Genovesi P, Gherardi F, Hejda M, Kark S, Lambdon PW, Desprez-Loustau ML, Nentwig W, Pergl J, Poboljšaj K, Rabitsch W, Roques A, Roy DB, Shirley S, Solarz W, Vilà M, Winter M (2010) Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci USA 107:12157–12162
Pyšek P, Jarošík V, Pergl J, Wild J (2011) Colonization of high altitudes by alien plants over the last two centuries. Proc Natl Acad Sci USA 108:439–440
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M (2000) Plant invasions—the role of mutualisms. Biol Rev 75:65–93
Seipel T, Kueffer C, Rew LJ, Daehler CC, Pauchard A, Naylor BJ, Alexander JM, Edwards PJ, Parks CG, Arevalo JR, Cavieres LA, Dietz H, Jakobs G, McDougall K, Otto R, Walsh N (2012) Processes at multiple scales affect richness and similarity of non-native plant species in mountains around the world. Glob Ecol Biogeogr 21:236–246
Stewart JR, Lister AM, Barnes I, Dalén L (2010) Refugia revisited: individualistic responses of species in space and time. Proc R Soc B Biol Sci 277:661–671
Steyn C (2014) What makes for a successful alpine alien ascender? Identifying the drivers of upslope range expansion by alien plants. BSc (Hons) thesis, University of Pretoria, Pretoria, ZA
Thuiller W, Lavorel S, Araújo MB, Sykes MT, Prentice IC (2005) Climate change threats to plant diversity in Europe. Proc Natl Acad Sci USA 102:8245–8250
UNESCO (2011) World heritage convention. UNESCO. http://whc.unesco.org/en/list/985. Accessed 3 November 2014
Van Rensburg BJ, Hugo S, Levin N, Kark S (2013) Are environmental transitions more prone to biological invasions? Divers Distrib 19:341–351
Von der Lippe M, Kowarik I (2007) Long-distance dispersal of plants by vehicles as a driver of plant invasions. Conserv Biol 21:986–996
Wagner HH, Fortin MJ (2005) Spatial analysis of landscapes: concepts and statistics. Ecology 86:1975–1987
Walther GR, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarošík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Wilson JRU, Richardson DM, Rouget M, Procheş Ş, Amis MA, Henderson L, Thuiller W (2007) Residence time and potential range: crucial considerations in modelling plant invasions. Divers Distrib 13:11–22
Wilson JRU, Ivey P, Manyama P, Nanni I (2013) A new national unit for invasive species detection, assessment and eradication planning. S Afr J Sci 109:33–45
Wolmarans R, Robertson MP, van Rensburg BJ (2010) Predicting invasive alien plant distributions: how geographical bias in occurrence records influences model performance. J Biogeogr 37:1797–1810
Acknowledgments
This project was supported by the European Union through the European Regional Development Fund (Centre of Excellence FIBIR), the DST-NRF Centre of Excellence for Invasion Biology, and the University of Pretoria (UP). JMK was supported by the long-term research development Project No. RVO 67985939 (The Czech Academy of Sciences). We are indebted to many UP students for field assistance, to the South African National Biodiversity Institute for providing specimen data extracts from the digitized herbarium database PRECIS, to P. N. Msomi (South African Police Services) for traffic data, to Tom Bishop for preparing the temperature data, to the Mazda Wildlife Fund for logistic support, and to two anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kalwij, J.M., Robertson, M.P. & van Rensburg, B.J. Annual monitoring reveals rapid upward movement of exotic plants in a montane ecosystem. Biol Invasions 17, 3517–3529 (2015). https://doi.org/10.1007/s10530-015-0975-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0975-3