Abstract
The successful establishment and spread of invasive species may be determined by intrinsic traits of the invader, characteristics of the invaded community, and interactions between the two. The red filamentous macroalga Heterosiphonia japonica has recently invaded and expanded its range in the northwestern Atlantic. We surveyed and compared macroalgal communities at sites with different levels of invasion in Nahant, Massachusetts, and examined potential factors contributing to Heterosiphonia’s successful invasion by assessing its growth rates and rates of herbivory by native grazers in the laboratory. We evaluated growth and herbivory for isolated individuals and for individuals within a macroalgal assemblage comprised of the most abundant species in the native community. We also measured macroalgal nitrate uptake rates to examine a potential mechanism underlying observed differences in growth rate. High abundances of Heterosiphonia were associated with lower native macroalgal species richness, evenness, and diversity and with differences in species composition in the field. Experiments showed that rates of growth and grazing were context dependent. In isolation, Heterosiphonia grew comparably and was grazed similarly to the two native filamentous macroalgae. Within the context of the native macroalgal assemblage, it grew much faster than all common native species, experienced reduced herbivory from one native grazer, and increased herbivory from another. Consistent with its faster growth, Heterosiphonia was the most efficient at nitrate uptake. Our results suggest that multiple interactions between intrinsic traits (e.g., competitiveness for nutrients) and extrinsic factors (e.g., presence of native macroalgal species, identity and relative abundances of native grazers) contribute to Heterosiphonia’s invasion success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increases in the scale, frequency, and numbers of species invasions, as well as in the negative impacts of invasive species (Vitousek et al. 1997; Pimentel et al. 2001), have led to sustained interest in understanding why certain species successfully invade communities. Some, but not all, species introduced to new regions manage to overcome barriers to survival, reproduction, and dispersal into new areas to become invasive (Blackburn et al. 2011). A clearer understanding of the factors that contribute to successful invasions and of the effects of invaders on their new communities is needed to predict and manage future species invasions.
Many studies of invasion success have focused on intrinsic, species-specific characteristics that may predispose a species to become invasive. For example, several r-selected life-history traits, such as fast growth, high fecundity, and asexual reproductive strategies (Rejmanek and Richardson 1996; Nyberg and Wallentinus 2005), have been found to correlate with invasiveness within some groups of species. Some of these intrinsic species traits have been successfully used to develop models for predicting the invasive potential of non-native species in different biogeographic regions (Tucker and Richardson 1995; Reichard and Hamilton 1997; Pheloung et al. 1999). In addition, extrinsic factors such as community disturbance (Olyarnik et al. 2009), native species diversity (Stachowicz and Tilman 2005), and native species traits and interactions (Richardson et al. 2000) can affect the inherent invasibility of the native community and therefore the success of potential invaders. Finally, intrinsic and extrinsic factors may interact within complex ecological communities to influence the success of a species’ invasion. For example, a non-native species with fast growth rates may be able to capitalize on native consumers’ preferential consumption of native competitors to increase its abundance and distribution (Keane and Crawley 2002). Because of such complex interactions between factors, the intrinsic species traits that contribute to successful invasion may vary across different habitats and disturbance regimes (Thuiller et al. 2006; Jauni and Hyvonen 2012), and between different invaded communities (Shea et al. 2005).
In marine systems, invasions are widespread and have increased exponentially over the last 200 years (Ruiz et al. 2000). Recent meta-analyses have highlighted the negative impacts of marine invaders on native communities (Sorte et al. 2010; Thomsen et al. 2009). In particular, macroalgae represent a substantial fraction of invasive species in marine systems (Schaffelke and Hewitt 2007) and are of particular concern due to their ability to alter ecosystem structure and function by dominating primary space (Thresher 1999). In the Gulf of Maine, at least 17 of 64 known invasive species are macroalgae (Pappal 2010), some of which have undergone recent range expansions (Mathieson et al. 2008).
A recent algal invader in the Gulf of Maine is the filamentous red macroalga Heterosiphonia japonica Yendo (hereafter, Heterosiphonia). Native to Asia, it became widespread on European coastlines following its initial discovery in France in 1984 (Sjøtun et al. 2008). [The higher taxonomy of this species is uncertain, as molecular studies suggest that it is a member of the Dasyaceae family, rather than a Heterosiphonia (Choi et al. 2002; Schneider 2010)]. Heterosiphonia was first seen on the Atlantic coast of North America in 2009 in collections of drift algae in Rhode Island (Schneider 2010). In 2010, we found it growing attached to subtidal rocky substrata in Nahant and Rockport, Massachusetts. It has subsequently been observed southward to Waterford, Connecticut, and northward to Cape Elizabeth, Maine and Mahone Bay, Nova Scotia (Newton et al. 2013; Savoie and Saunders 2013).
Studies of Heterosiphonia in Europe indicate that the species is characterized by broad temperature and salinity tolerances, high growth rates in laboratory cultures, and asexual reproduction through fragmentation (Bjærke and Rueness 2004), intrinsic traits that are often associated with high invasive potential (Nyberg and Wallentinus 2005). However, Heterosiphonia’s ecological interactions have yet to be studied in either its native or invasive ranges. In its native East Asian waters, Heterosiphonia occurs only sporadically throughout the year and typically comprises less than 1 % of macroalgal biomass (Kang et al. 2008; Kim et al. 2008; Choi et al. 2010). In contrast, Heterosiphonia can be highly abundant in its invasive eastern Atlantic range (Bárbara et al. 2004; Husa et al. 2004), and our initial observations suggested a similarly high abundance in its newly-discovered northwestern Atlantic range. As with most invasive species, virtually nothing is known about the interactions between Heterosiphonia’s intrinsic traits and the characteristics of its native and invaded communities, and their effects on invasion success. Therefore, this recent invasion of Heterosiphonia represents a novel opportunity to study the potential intrinsic and extrinsic factors contributing to the successful invasion of a new, rapidly-spreading macroalgal species.
In this study, we conducted a field survey of Heterosiphonia-invaded communities in Nahant, Massachusetts (USA), to characterize communities with different levels of Heterosiphonia invasion. We then used laboratory experiments to examine macroalgal growth rates and rates of herbivory by native grazers as potential ecological mechanisms contributing to Heterosiphonia’s successful invasion. We studied these processes in Heterosiphonia both by itself and within a representative community assemblage of common native macroalgae to investigate potential interactions between intrinsic and extrinsic factors underlying invasion success. In particular, we were interested in evaluating how growth and grazer control of Heterosiphonia might be altered within the context of the native macroalgal community in Nahant. Finally, we examined macroalgal nitrate uptake rates in the laboratory as a potential physiological mechanism underlying the patterns observed in the laboratory experiments and field survey.
Materials and methods
Diversity and composition of invaded macroalgal communities
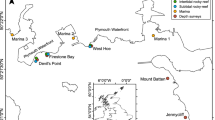
To characterize the macroalgal communities invaded by Heterosiphonia, we conducted quantitative SCUBA surveys of subtidal macroalgal communities (4–6 m below mean lower-low water [MLLW]) at Canoe Beach (42.4210°N, 70.9061°W) in Nahant, Massachusetts, in the summer of 2011. We collected all non-crustose algae from rocky substrata in randomly-placed quadrats (25 cm × 25 cm) at two sites, Canoe Beach Chimneys (CBC) and Canoe Beach West (CBW), separated by ca. 100 m and characterized by different degrees of invasion by Heterosiphonia. The sites were classified as highly invaded and less invaded, respectively, based on the amount of Heterosiphonia observed (see Results). A total of 33 quadrats were sampled, 20 from CBC and 13 from CBW. Algae from each quadrat were sorted by species, oven dried to constant mass at 65 °C, and weighed to estimate the abundance (in biomass) of each species present. Identifications of all filamentous species, including Heterosiphonia, were confirmed under the microscope. We verified that the sites differed with respect to the degree of Heterosiphonia invasion (Mann–Whitney U Tests on total Heterosiphonia biomass: W = 260, P < 0.001; on Heterosiphonia’s percentage contribution to total macroalgal biomass: W = 258, P < 0.001) but not with respect to total algal biomass (Mann–Whitney U test: W = 155, P = 0.37). We also calculated macroalgal species richness (S), evenness (Pielou’s J), and diversity (H′) for each plot and assessed differences in these diversity metrics between the highly-invaded and the less-invaded site using t tests. To assess differences in macroalgal community assemblages between the two sites, we conducted a permutational multivariate analysis of variance (PERMANOVA, Anderson 2001; McArdle and Anderson 2001) using PRIMER 6 with PERMANOV+. Species that contributed the most to differences between assemblages were identified using SIMPER (Clarke 1993).
Algal growth and grazing rates
We experimentally examined algal growth rates and rates of herbivory by the two most common subtidal grazers in Nahant [the gastropod Lacuna vincta Montagu (hereafter, Lacuna) and the isopod Idotea balthica Pallas (hereafter, Idotea)] for Heterosiphonia and other macroalgal species in the New England subtidal community. From our surveys of the subtidal macroalgal species in Nahant, we identified the six most common species present: the branching Chondrus crispus Stackhouse, the articulated coralline Corallina officinalis L., the filamentous Heterosiphonia, Polysiphonia fucoides (Hudson) Greville, and Polysiphonia stricta (Dillwyn), and the kelp Saccharina latissima (L.) J.V. Lamouroux (referred to hereafter by genus, with the exception of the two Polysiphonia species). Heterosiphonia was the only common non-native species. Based on abundance, these six species were deemed to be important ecological players and competitors for primary space in the community, and we therefore used them in our experiments. To explore how community context, which we define as the presence of other species in the community that are potential direct or indirect interactors, might influence rates of herbivory and growth in individual species, we conducted these experiments both with isolated single species (individual-species treatments) and in a more realistic assemblage of all six common species (macroalgal assemblage treatments). Experiments for each grazer species were conducted separately in the late summer of 2011, with individual-species and macroalgal assemblage treatments running simultaneously in each experiment.
We collected Lacuna and Idotea individuals haphazardly from local subtidal macroalgal assemblages at Canoe Beach and maintained them, unfed, in running seawater tanks for 48 h before use in the experiments. All six species of macroalgae were also collected from rocky substrata at Canoe Beach at depths of 4–6 m below MLLW on the day of the experiment. Algae were cleaned of epiphytes, spun in a salad spinner to eliminate excess water, and weighed prior to the start of each experiment. Experiments were conducted using plastic containers divided in half by a fine plastic mesh to create paired grazer and no-grazer (growth only) compartments. Each experiment consisted of six individual-species treatments and one macroalgal assemblage treatment, to give a total of seven treatments and 10 replicates per treatment (70 experimental chambers per experiment) for each grazer species. Experimental chambers were submerged in an outdoor tank supplied with running unfiltered seawater, such that experimental algae were exposed to ambient seawater nutrients and temperatures. Light intensity was not measured directly during the experiment, but based on daily mean values for photosynthetically active radiation measured at the nearby Boston Logan Airport (42.3631°N, 71.0064°W; Wilcox 2012) and a 20 % shading effect previously measured in these experimental chambers, we estimate that algae were exposed to between 563 and 734 μmol photons m−2 s−1 during our experimental trials, which are slightly higher than those measured in the field (280–400 μmol photons m−2 s−1 at about 5 m below MLLW in July 2012; LI-COR quantom sensor [LI-193] and meter [LI-250], LI-COR Biosciences, Lincoln, Nebraska, USA).
To examine grazing rates by Lacuna, we used experimental chambers constructed from 1.0-L perforated plastic containers divided by plastic mesh barriers with 1.1 × 2.0 mm openings to create two compartments. We placed 30 Lacuna of 4- to 5-mm shell diameter into one half of each experimental chamber (“grazer compartment”) along with 6 g of fresh, pre-weighed algae. The other half of each chamber was stocked with the same amount of algae but without grazers to measure growth alone (“no-grazer compartment”). For each of the six individual-species treatments, the 6 g of algal wet mass consisted of a single piece of Chondrus, Corallina, Heterosiphonia, P. fucoides, P. stricta, or Saccharina. For the macroalgal community treatment, one 1-g piece of each of the six algal species was placed in random arrangement in each compartment, for a total of 6 g of algal wet mass. We attached a weight to the base of the thallus of each experimental alga, such that all algae were anchored to the bottom of the chamber and floated upright throughout the experiment. Whole individual macroalgae were used for all species except the large kelp Saccharina, for which pieces of the blade were used. Densities of Lacuna were maintained at five individual snails per gram of algal wet mass for all treatments, which is within the range of field densities found on these macroalgal species in Nahant (0.6–7.6 snails per gram of algal mass; A. Drouin and N. Low, unpublished data).
To examine grazing rates by Idotea, we used experimental chambers constructed from 8.5-cm diameter Petri dish bases with fiberglass windowscreen mesh covers divided by fiberglass windowscreen barriers (1.4 × 1.5 mm openings) to create two adjacent compartments. Three Idotea of 8–10 mm length were placed into the grazer compartment of each Petri dish. All grazer and no-grazer compartments were stocked with 0.9 g of fresh, pre-weighed algae to maintain 3 Idotea per gram of algal wet mass, a density within the range observed in the field (0.0–5.9 isopods per gram of algal mass; A. Drouin and N. Low, unpublished data). Similar to the design for the Lacuna experiment, each compartment in the macroalgal community treatment contained one 0.15 g piece of each algal species in random order (a total of 0.9 g wet mass), while each compartment in the individual-species treatments contained 0.9 g of a single algal species.
Each growth and grazing experiment ran for 3 days. At the end of each experiment, we removed all grazers and algae from the experimental chambers. Grazers were carefully separated from the algae and re-counted. We spun each algal sample in a salad spinner, determined its final wet mass, and calculated biomass-specific growth as (final wet mass − intial wet mass)/initial wet mass, g g−1. This value was then divided by the duration of the experiment to obtain a daily biomass-specific growth rate, g g−1 d−1. To calculate the mass of algae consumed by the grazers, we subtracted the daily change in wet mass in no-grazer compartments (total growth) from the daily change in wet mass in grazer compartments (growth and herbivory) for each species. This value was divided by the initial wet mass of algae in the grazer compartment to obtain the daily biomass-specific consumption by grazers (g g−1 d−1).
We examined between-species differences in specific growth and herbivory in both individual-species and macroalgal assemblage treatments using nonparametric tests due to heterogeneity in the variances that could not be resolved by transformation. For individual-species treatments, between-species differences in growth and herbivory were assessed using Kruskal–Wallis tests. For macroalgal assemblage treatments, differences in growth and herbivory between species were assessed using Friedman tests to account for the fact that macroalgal samples from the same experimental chamber were not statistically independent (Lockwood 1998). To compare differences between individual species, we conducted post hoc Tukey HSD tests on the rank-transformed data from the Kruskal–Wallis and Friedman tests. We also estimated how species were likely to perform when growth rates and combined losses to both native grazers were considered simultaneously. For each species, we subtracted mean specific grazing rates by both Lacuna and Idotea from the mean specific growth rate in the macroalgal assemblage treatments in order to estimate an expected rate of biomass change.
To test if the rates of macroalgal growth and herbivory were influenced by the presence of the other common species in the community, we assessed within-species differences in biomass-specific growth and grazing between individual-species and macroalgal assemblage treatments. Because variances in the growth and grazing data did not conform to a normal distribution, we used a generalized linear model (GLM) with a gamma distribution and a log link. GLMs are extensions of general linear models that model data with probability distributions other than the Gaussian, and can be used to assess both continuous and categorical predictor variables (Ramsey and Schafer 2013). In general, differences in process rates between individual- species and macroalgal assemblage treatments indicate that rates of growth or herbivory rates are altered when that species co-occurs with the other common species in the community. In the case of herbivory, such differences represent non-random consumption patterns, which reflect grazers’ preferences for or against particular species in the community (Underwood and Clarke 2005). Statistical analyses were conducted using the R software package (R Development Core Team 2013).
Nitrate uptake rates of macroalgal species
Because nitrogen is a growth-limiting nutrient for algae in coastal waters (Ryther and Dunstan 1971), especially in summertime nutrient-depleted waters around Nahant (<5 µmol NO3 − L−1; Perini and Bracken 2014), we investigated the role of nitrate uptake in mediating Heterosiphonia’s success within the context of the Nahant macroalgal community. We measured nitrate uptake rates of the six macroalgal species in 2-h uptake trials using artificial seawater (35 psu) with four different nitrate concentrations: 2, 15, 30, and 50 μmol L−1. Trials were conducted in 1.0-L cylindrical acrylic chambers within a chilled, recirculating water bath, which maintained water temperatures between 13 and 15 °C, which is within the range of field water temperatures in Nahant measured during the late summer and early fall of 2011 (11–21 °C; TMP36 sensor, Analog Devices Inc., Norwood, Massachusetts, USA). Each chamber was fitted with a pump (March Mfg., model LC-2-CP-MD, Glenville, Illinois, USA), which generated turbulent flow within the chambers. Chambers were placed under 129-cm 54W T5 HO light fixtures (Model 1123, Current, Inc., Vista, California, USA) equipped with four SlimPaq 10,000 K daylight and 4,460-nm actinic fluorescent bulbs that were shaded so that experimental macroalgae were exposed to light intensities (350.5 ± 25 μmol photons m−2 s−1) within the range measured in the field.
Nitrate uptake trials were run in the late summer and early fall of 2011 for all species. For each trial, we collected experimental macroalgae from Canoe Beach no more than 18 h in advance. We ran two replicate trials per nitrate concentration for each species. One 6-g piece of macroalgae (wet weight) was placed in each chamber. Algae were allowed to acclimate for 10 min before the seawater was spiked with a NaNO3 stock solution. Water samples were collected from each chamber at 15-min intervals for 2 h and analyzed for nitrate concentration using a QuickChem 8500 Series FIA+ analyzer (Lachat Instruments, Loveland, Colorado, USA). At the end of each trial, experimental algae were oven-dried to constant mass and weighed to determine exact dry tissue biomass.
We calculated biomass-specific nitrate uptake rates for each species (μmol h−1 g−1) as a function of initial nitrate concentration. These data were fitted to Michaelis–Menten models:
where V (μmol h−1 g−1) was the uptake rate, V max (μmol h−1 g−1) was a parameter that defined the maximum uptake rate of the species or assemblage, S (μmol L−1) was the initial nitrate concentration, and K s (μmol L−1) was a parameter that defined the initial concentration at V max/2. Data were also fitted to linear models:
where m was the slope of the linear relationship and b was the intercept. Michaelis–Menten and linear fits described saturating and non-saturating relationships, respectively, between nitrate uptake and nitrate concentration and were fit using the R software package (R Development Core Team 2013). The Akaike Information Criterion (AIC) was used to determine goodness of fit and select the most appropriate model for each species. We used the parameters V max and K s (Michaelis–Menten fit) and b (linear fit) to calculate the nitrate uptake coefficient, α, representing the slope of the relationship between nitrate concentration and uptake at low ambient nitrate concentrations. In species with saturating (i.e., Michaelis–Menten) relationships between concentration and uptake, α represents the initial, non-saturating portion of the curve and was defined as
In species where a linear relationship was fitted,
These nitrate uptake efficiency coefficients (α) describe the effectiveness of those species at taking up nitrate at low nitrate concentrations, with greater values indicating more efficient uptake (Bracken et al. 2011; Bracken and Williams 2013).
Results
Diversity and composition of invaded macroalgal communities
Heterosiphonia biomass was lower in all quadrats sampled at the less invaded site (CBW; 0–11.7 g m−2) compared to quadrats sampled at the highly invaded site (CBC; 26.9–345.4 g m−2; Fig. 1). On average, Heterosiphonia had 32 times more biomass and made up 49.4 % of the total macroalgal biomass at the highly-invaded site, compared to 2 % at the less-invaded CBW site.
Relationships between H. japonica biomass (g m−2) and a species richness S, b species evenness J, and c species diversity H′ of macroalgal communities Data are presented by quadrat, with open circles representing quadrats from the highly-invaded Canoe Beach Chimneys (CBC) and filled circles representing quadrats from the less-invaded Canoe Beach West (CBW)
Heterosiphonia abundance was negatively correlated with macroalgal species richness S (R 2 = 0.15, P = 0.015; Fig. 1a), evenness J (R 2 = 0.16, P = 0.013; Fig. 1b), and diversity H′ (R 2 = 0.35, P < 0.001; Fig. 1c). Both species richness (t 31 = −5.55, P < 0.001) and species diversity (t 31 = −3.22, P = 0.003) were significantly lower at the highly-invaded site. On average, this was a difference of 3.6 species between the two sites. Species evenness did not differ significantly between sites (t 31 = −0.64, P = 0.528). The sites also differed in terms of macroalgal species assemblages [Pseudo-F 1,31 = 7.25, P(perm) = 0.001]. In addition to the difference in Heterosiphonia biomass, five species accounted for greater than 65 % of the remaining dissimilarity between the two sites. According to the SIMPER analysis, Corallina, P. fucoides, Chondrus, and Bonnemaisonia hamifera were all found to be more abundant at the less invaded site, while Ahnfeltia plicata was more abundant at the highly invaded site.
Algal growth rates
In the absence of grazers, all macroalgae showed positive growth. Biomass-specific growth rates differed significantly among species grown in isolation (individual-species treatments; Kruskal–Wallis test: χ2 = 21.4, P < 0.001). Heterosiphonia grew significantly faster than the native Chondrus, Corallina, and Saccharina (Fig. 2, open bars). The native filamentous species P. fucoides and P. stricta had intermediate growth rates that did not differ significantly from that of Heterosiphonia.
Biomass-specific growth (g g−1 d−1) ±SE of H. japonica and the five most abundant native macroalgal species in individual-species (open bars) and macroalgal assemblage (filled bars) treatments over 3 days. Capital letters (individual-species treatment) and small letters (macroalgal community treatment) indicate between-species differences in growth based on Tukey HSD tests on ranks. Asterisks (*) indicate species for which growth rates in the macroalgal assemblage treatment differed significantly from that expected based on individual growth rates from the individual-species treatment
Growth rates also differed between species when they were grown together in an assemblage characteristic of the local New England community (macroalgal assemblage treatment; Friedman test: χ2 = 32.7, P < 0.001). Heterosiphonia grew significantly faster than all native species, showing a 25 % gain in wet mass per day (Fig. 2, filled bars), which was more than twice that of P. stricta and P. fucoides, the fastest growing native species, and more than 3.5 times the average percentage growth of all native species.
Comparisons within species showed that Heterosiphonia, Chondrus and the two Polysiphonia species grew significantly faster in the macroalgal assemblage treatment than they did in the individual-species treatment (Fig. 2). Heterosiphonia showed the greatest difference between treatments, growing 300 % faster in the macroalgal assemblage treatment than expected from its individual specific growth rate (GLM: χ2 = 28.0, P < 0.001) whereas Chondrus grew 81 % faster (GLM: χ2 = 10.0, P = 0.002), P. fucoides grew 52 % faster (GLM: χ2 = 5.8, P = 0.016), and P. stricta grew 137 % faster (GLM: χ2 = 5.3 P = 0.021).
Grazing rates
When the native snail Lacuna was given isolated macroalgal species (i.e., in the individual-species treatments), it grazed the different algal species at different rates (Kruskal–Wallis test: χ2 = 30.2, P < 0.001). Lacuna readily grazed the invasive Heterosiphonia at a similar rate to the native P. fucoides, P. stricta, and Saccharina, and at a greater rate than Chondrus and Corallina (Fig. 3a, open bars).
Biomass-specific herbivory (g g−1 d−1) ±SE in H. japonica and the five most abundant native macroalgal species by the native grazers a L. vincta, and b I. balthica in individual-species (open bars) and macroalgal assemblage (filled bars and right-hand y-axes) treatments over 3 days. Capital letters (individual-species treatment) and small letters (macroalgal assemblage treatment) indicate between-species differences in growth based on Tukey HSD tests on ranks. Asterisks (*) indicate species for which biomass-specific herbivory rates in the macroalgal assemblage treatment differed significantly from those in the individual-species treatment
When Lacuna encountered the six-species assemblage of macroalgae typical of the local New England subtidal community, it also grazed different algae at different rates (Friedman test: χ2 = 20.6, P = 0.001). Snails consumed significantly more native P. stricta than invasive Heterosiphonia and native Chondrus, Corallina, P. fucoides, and Saccharina (Fig. 3a, filled bars).
Comparisons of Lacuna grazing rates between treatments revealed that Lacuna consumed the same macroalgal species at different rates when they encountered the algae in isolation compared to when they encountered the algae in the six-species assemblage. Snails consumed 81 % less Heterosiphonia when it was in the macroalgal assemblage than when it was in isolation (GLM: χ2 = 4.3, P = 0.039). In contrast, they grazed 84 % more Saccharina (GLM: χ2 = 3.8, P = 0.05) and 94 % more P. stricta (GLM: χ2 = 4.0, P = 0.046) in the macroalgal assemblage, compared to the individual species treatments.
We found no significant differences in consumption of algal species by the native isopod Idotea in the individual-species treatments (Kruskal–Wallis test: χ2 = 4.0, P = 0.55), but species were consumed at different rates in the macroalgal assemblage treatment (Friedman test: χ2 = 11.8, P = 0.038) (Fig. 3b). Within-species comparisons showed that Idotea grazed the kelp Saccharina almost four times more when they encountered it in the six-species assemblage than when they encountered it on its own (GLM: χ2 = 7.3, P = 0.0069). Specific grazing rates did not vary significantly between individual-species treatments and macroalgal assemblage treatments for the other five macroalgal species, though the difference was only marginally insignificant for Heterosiphonia (GLM: χ2 = 3.4, P = 0.067). Grazing rates on Heterosiphonia were highly variable, but on average, isopods consumed 13 times more Heterosiphonia biomass within the six-species assemblage compared to the individual species treatment.
When we simultaneously considered both mean growth rates and mean grazing rates by both grazer species, we estimated that Heterosiphonia would experience a 15 % daily increase in biomass, compared to a 3–5 % daily increase for Chondrus, Corallina, and P. fucoides. In contrast, P. stricta and Saccharina were estimated to experience net biomass losses of about 6 and 10 %, respectively.
Nutrient uptake
Relationships between nitrate uptake and nitrate concentrations of Chondrus and Heterosiphonia were best fit by Michaelis–Menten models, whereas nitrate uptake relationships of Corallina, P. fucoides, P. stricta, and Saccharina were best fit by linear models. Heterosiphonia exhibited the greatest nitrate use efficiency (α) of all tested species, with an uptake coefficient that was, on average, an order of magnitude higher than those of common native species (one-sample t 4 = 22.0, P < 0.001; Fig. 4).
Discussion
Our field surveys indicate that in Nahant, invasion of the subtidal community by Heterosiphonia is associated with lower macroalgal species richness, evenness, and diversity. This trend reflects the larger-scale pattern across invaded sites in the western Atlantic; higher amounts of Heterosiphonia are correlated with lower species richness in plots across 700 km of coastline (Newton et al. 2013). In Nahant, Heterosiphonia invasion is also associated with significant differences in the species composition of the rest of the community. Direct field experiments (e.g., removal of Heterosiphonia from invaded communities) will be required to determine if these patterns represent invader impacts, invasion susceptibility, or some environmental correlate affecting the invasion process (Didham et al. 2005). In contrast, studies of invaded Norwegian macroalgal communities indicate that though Heterosiphonia is a successful competitor for space and represents up to 65 % of the total macroalgal biomass (Husa et al. 2004), it is not associated with differences in the species richness or composition of native macroalgae based on surveys conducted before and after invasion (Husa et al. 2008).
Our laboratory work experimentally evaluated potential mechanisms—growth rates and herbivory rates by two different native grazers—that might contribute to invasion success in Heterosiphonia. The results suggest that within the context of the typical subtidal macroalgal assemblage in Nahant, rapid growth rates may facilitate Heterosiphonia’s invasion success, but different native grazer species have different levels of impact on the invader. Within a representative assemblage comprised of the six most common subtidal macroalgal species from Nahant, Heterosiphonia grew much faster than all five native species. This high growth rate corresponds to Heterosiphonia’s high capacity for the uptake of nitrate, a known limiting nutrient (Perini and Bracken 2014); it had the highest nitrate uptake coefficient among all species in our nitrate uptake experiments.
Because macroalgae with simpler morphologies (e.g., sheets and filaments) generally exhibit higher nutrient uptake capacity (Rosenberg and Ramus 1984) and greater productivity (Littler and Littler 1980; 1983), functional form differences may partly account for the differences between the filamentous Heterosiphonia and some of the common native species (Chondrus, Corallina, and Saccharina) with more complex morphologies. However, Heterosiphonia also took up nitrate more efficiently and grew significantly faster than the two native species with similar filamentous functional forms, P. stricta and P. fucoides, indicating that with regard to nitrate uptake and growth, it was more competitive than the dominant native species both between and within functional growth forms.
Within the same assemblage of common native species, the native snail Lacuna consumed Heterosiphonia significantly less than P. stricta, the fastest-growing common native species. P. fucoides experienced an intermediate level of grazing between Heterosiphonia and P. stricta. On the other hand, the isopod Idotea appeared to have preferences for Heterosiphonia, P. stricta, and Saccharina, although due to high variability in grazing rates and the lower power of the nonparametric tests, these differences were not statistically significant. Some of the observed differences in grazing may also stem from differences in functional form; simpler forms like filaments are thought to be more palatable to grazers compared to more complex growth forms (Littler and Littler 1980, 1983), and the need to cut the large-bladed Saccharina for the experiment may have increased its susceptibility to isopod grazing due to release of phototannins (Jormalainen et al. 2005; Halm et al. 2011). However, as with growth rates, the rates of native herbivory also differed between Heterosiphonia and the native filamentous species, suggesting that grazer preferences are only partly driven by differences in functional form. Indeed, Heterosiphonia grew faster than all its native competitors for space and was preferred by fewer native grazers than the next fastest-growing species, the filamentous P. stricta.
Lower impacts of native grazers on non-native species, relative to their native competitors, has often been thought to be a key factor in invasion success (Keane and Crawley 2002). However, the relative level of grazer impact on a non-native species often interacts with the species’ inherent competitiveness to determine if it can compensate for losses to grazers and/or capitalize on reduced grazer impacts in order to increase in abundance (Keane and Crawley 2002). Many invasive species for which low relative impacts from native grazers have been reported (Gollan and Wright 2006; Monteiro et al. 2009) are also known to have high growth rates (Boudouresque et al. 1995; Pedersen et al. 2005), and not all invasive species experience lower impacts from native grazers relative to their native competitors (Parker and Hay 2005; Strong et al. 2009). Nejrup et al. (2012) also noted that in Denmark, lower grazer impacts on the invasive Gracilaria vermiculophylla meant that it was likely better at compensating for herbivory losses than its native competitors Ulva spp. and Ceramium virgatum, even though the native species grew several times faster. Here, Heterosiphonia does not experience reduced grazing pressure from all native grazers, but its extremely high growth likely allows it to compensate for grazing losses, and also capitalize on the relatively higher losses suffered by P. stricta. When its biomass-specific growth rates and combined losses to both native grazers are considered simultaneously, Heterosiphonia’s estimated biomass accumulation rate would be at least 2.5 times greater than any common native species, placing it in a good position to compete for primary space in the Nahant subtidal community. Although correlative and spatially limited, our field survey supports this hypothesis: the native species Chondrus, Corallina, and P. fucoides were less abundant at the highly-invaded site, even when Heterosiphonia was not included in the analysis. Together with its broad physiological tolerance and ability to reproduce asexually through fragmentation (Bjærke and Rueness 2004; Husa and Sjøtun 2006), this advantage over native species likely contributed to Heterosiphonia’s successful establishment within the Nahant community.
Our results also highlight that some of the processes that contribute to the successful invasion of a non-native species may be highly dependent on the community context in which they occur. When we assessed growth and herbivory on isolated Heterosiphonia individuals, the invader grew comparably and was grazed similarly to the two native species with similar functional forms (P. fucoides and P. stricta). However, when we explicitly incorporated community context into the assessment, i.e., the presence of common native species, we observed important differences in both growth and losses to native grazers. Heterosiphonia experienced significantly faster specific growth rates when it co-occurred with native macroalgal species than it did when it occurred on its own, even though the total biomass of macroalgae remained the same. Several other species also experienced greater growth in the macroalgal assemblage treatment, suggesting that this trend may partially arise from greater biomass-specific growth in smaller macroalgal individuals, when compared to larger individuals in the single-species treatments. This phenomenon could arise due to reduced self-shading or greater surface-area-to-volume ratios and potential for mass exchange (e.g., Hein et al. 1995). However, when included in a diverse assemblage, Heterosiphonia still showed a greater enhancement in growth rate than all native species, which may indicate that on a per-gram basis, Heterosiphonia experiences less competition for limiting resources (e.g., nitrate) from native species than from conspecifics, allowing it to grow faster in the Nahant macroalgal assemblage. Indeed, Heterosiphonia was four times better at taking up nitrate than the next most efficient species. Its high nitrate uptake efficiency could allow it to access more of this limiting nutrient, and thus to grow faster, in an assemblage of native species compared to a monoculture of itself. Heterosiphonia’s biomass-specific growth rates in the individual-species treatment were much lower than the daily 30–41 % biomass-specific growth measured at comparable temperatures and salinities in the Norwegian population (Bjærke and Rueness 2004). However, the measurements of Heterosiphonia growth in Norway were conducted in high-nitrate medium (IMR 1/2, ~250 μmol L−1 NO3 −), whereas our measurements were conducted under ambient, limiting nitrate conditions (<5 μmol L−1 NO3 −, Perini and Bracken 2014). The growth rates we measured for Heterosiphonia in the macroalgal assemblage treatment approached those recorded in the Norwegian study, suggesting that Heterosiphonia had greater access to nitrate in the assemblage treatment, compared to the individual-species treatment.
Interestingly, the effect of considering community context on invader losses to herbivory was strongly dependent on the identity of the native grazer: Lacuna grazed Heterosiphonia at lower rates when it was presented alongside common native macroalgae, while Idotea appeared to consume Heterosiphonia at greater rates when Heterosiphonia was included in an assemblage of native species. Underwood and Clarke (2005) suggest that differences in consumption rates of prey species available in isolation, versus those of prey species presented together, represent prey choice by the consumer. Therefore, Lacuna appears to show a preference for P. stricta and an aversion to the invasive Heterosiphonia, while Idotea may prefer both of these filamentous species, along with Saccharina, over other native macroalgae.
Native grazer preferences are a known mechanism for context-dependence in invader losses to herbivory. For example, Lyons and Scheibling (2008) found that the presence of preferred and/or unpalatable native macroalgae altered the survival rates of the invasive Codium fragile ssp. tomentosoides when it encountered grazing fronts of the native urchin Strongylocentrotus droebachiensis. Additionally, studies on widespread invaders indicate that within different native communities (grazers and macroalgal competitors), the same invasive species can experience opposite patterns of relative grazing pressure due to different grazer preferences: the invasive brown macroalga Sargassum muticum benefits from reduced herbivory by native grazers in invaded Portuguese communities (Monteiro et al. 2009), but experiences greater herbivory rates than native macroalgae in invaded communities from Northern Ireland (Strong et al. 2009). The species-specific grazing preferences observed in our experiment indicate that such opposing patterns of relative grazing pressure can occur even within a single benthic community with different grazers, and suggests that the overall effect of grazing on invader success may be determined by the relative abundances of the different native grazers in the community. At the same time, the invasion of a species like Heterosiphonia, with different palatability to different grazers, may influence the relative abundance of these grazers by altering the availability of preferred food. Overall, the context-dependent rates of growth and herbivory in Heterosiphonia suggest that there are multiple interactions between intrinsic traits (e.g., functional form, nitrogen uptake efficiency, growth rates) and extrinsic factors (e.g., co-occuring native competitors, preferences of different native grazers) that have contributed to Heterosiphonia’s invasion success.
The scope of our inferences is somewhat limited by the need to conduct our experiments within the laboratory and our surveys over a relatively short time period. Firstly, our laboratory experiments could not account for differences in flow velocities and wave exposure experienced in the field. Studies of Heterosiphonia in its European invaded range have noted that it tends to favor environments with low or medium wave exposure (Husa et al. 2004; Moore and Harries 2009), and both small- and large-scale patterns of water flow may have effects on macroalgal access to nutrients, and therefore their growth (Hurd 2000). Therefore, the relatively homogeneous, slow flow conditions within the experimental chambers may have influenced absolute and relative rates of macroalgal growth. Secondly, our surveys and experiments were conducted during a limited time period: the mid to late summer. Seasonal variation is known to occur both in mesograzer abundance and activity (Arrontes and Anadón 1990; Krumhansl and Scheibling 2011) and in Heterosiphonia field abundances (Newton et al. 2013), and such seasonal dynamics may drive seasonal changes in the rates of growth and herbivory, and therefore in the relative abundance of species in the community. Examining the cumulative growth and herbivory of the macroalgal species over the year would provide a better understanding of mechanisms of longer-term patterns of invasion.
Finally, our experimental setup did not consider differences in abundances of the six macroalgal species or the potential for non-random associations between macroalgal species (e.g., epiphytic relationships). We used equal masses of each species and arranged them randomly in our macroalgal assemblage treatments, but the six species were not equally abundant in the field. Generally, the order of abundances was Chondrus > Heterosiphonia > Corallina > P. fucoides > Saccharina > P. stricta. In addition, the filamentous Heterosiphonia and P. stricta are found both on primary substrate and as epiphytes on Chondrus and Corallina. For generalist consumers, food preferences may interact with the availability of the different foods to determine the realized rates of consumption of each type of food (Chesson 1983), such that field grazing rates on the more abundant species might be higher than those measured in the experiment. At the same time, the presence of macroepiphytes can potentially reduce host growth and fitness through shading or increased drag (D’Antonio 1985; Cebrián et al. 1999), and alter rates of grazing on their hosts (Karez et al. 2000). As a result, the additional effects of consumer behavior and macroalgal associations may further influence the rates of growth and relative losses to native grazers that Heterosiphonia experiences in the field.
Our study helps to elucidate some of the mechanisms contributing to the successful invasion of Heterosiphonia in Nahant, and potentially at other invaded sites within the same biogeographic region, which contain similar native macroalgal species (Newton et al. 2013). Our work suggests that the species traits and species interactions that influence an invader’s success can be strongly influenced by the context of the invaded community, including the characteristics of co-occurring native competitors and the preferences and relative abundances of different enemies in the community. As a widespread invader that has both a long invasion history in European waters, and a more recent history in the northwestern Atlantic, Heterosiphonia provides opportunities to study the extent to which community context matters in invasion, both in space and in time. Additional assessments of algal growth rates and herbivory by suites of native grazers in Europe, as well as in Heterosiphonia’s native North Pacific range could allow for comparisons of the relative importance of different aspects of community context and the length of invasion history in contributing to invasion success.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Arrontes J, Anadón R (1990) Seasonal variation and population dynamics of isopods inhabiting intertidal macroalgae. Sci Mar 54:231–240
Bárbara I, Cremades J, Veiga AJ (2004) Floristic study of a maërl and gravel subtidal bed in the “Ría de Arousa” (Galicia, Spain). Bot Complut 28:27–37
Bjærke MR, Rueness J (2004) Effects of temperature and salinity on growth, reproduction and survival in the introduced red alga Heterosiphonia japonica (Ceramiales, Rhodophyta). Bot Mar 47:373–380
Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan RP, Jarosĭk V, Wilson JRU, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339
Boudouresque C, Meinesz A, Ribera M, Ballesteros E (1995) Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: possible consequences of a major ecological event. Sci Mar 59:21–29
Bracken MES, Williams SL (2013) Realistic changes in seaweed biodiversity affect multiple ecosystem functions on a rocky shore. Ecology 94:1944–1954
Bracken MES, Jones E, Williams SL (2011) Herbivores, tidal elevations, and species richness simultaneously mediate nitrate uptake by seaweed assemblages. Ecology 92:1083–1093
Cebrián J, Enríquez S, Fortes M, Agawin N, Vermaat JE, Duarte CM (1999) Epiphyte accrual on Posidonia oceanica (L.) Delile leaves: implications for light absorption. Bot Mar 42:123–128
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecology 64:1297–1304
Choi H, Kraft G, Lee I, Saunders G (2002) Phylogenetic analyses of anatomical and nuclear SSU rDNA sequence data indicate that the Dasyaceae and Delesseriaceae (Ceramiales, Rhodophyta) are polyphyletic. Eur J Phycol 37:551–569
Choi CG, Oh SJ, Kang IJ (2010) A study on the community structure of subtidal marine algae in Kijang, Korea. J Fac Agric Kyushu Univ 55:39–45
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
D’Antonio C (1985) Epiphytes on the rocky intertidal red alga Rhodomela larix (Turner) C. Agardh: negative effects on the host and food for herbivores? J Exp Mar Biol Ecol 86:197–218
Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ (2005) Are invasive species the drivers of ecological change? Trends Ecol Evol 20:470–474
Gollan JR, Wright JT (2006) Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar Freshw Res 57:685–694
Halm H, Lüder UH, Wiencke C (2011) Induction of phlorotannins through mechanical wounding and radiation conditions in the brown macroalga Laminaria hyperborea. Eur J Phycol 46:16–26
Hein M, Pedersen MF, Sand-Jensen K (1995) Size-dependent nitrogen uptake in micro- and macroalgae. Mar Ecol Prog Ser 118:247–253
Hurd CL (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36:453–472
Husa V, Sjøtun K (2006) Vegetative reproduction in “Heterosiphonia japonica” (Dasyaceae, Ceramiales, Rhodophyta), an introduced red alga on European coasts. Bot Mar 49:191–199
Husa V, Sjøtun K, Lein TE (2004) The newly introduced species Heterosiphonia japonica Yendo (Dasyaceae, Rhodophyta); geographical distribution and abundance at the Norwegian southwest coast. Sarsia 89:211–217
Husa V, Sjøtun K, Brattenborg N, Eiliv Lein T (2008) Changes of macroalgal biodiversity in sublittoral sites in southwest Norway: impact of an introduced species or higher temperature? Mar Biol Res 4:414–428
Jauni M, Hyvonen T (2012) Interactions between alien plant species traits and habitat characteristics in agricultural landscapes in Finland. Biol Invasions 14:47–63
Jormalainen V, Honkanen T, Vesakoski O, Koivikko R (2005) Polar extracts of the brown alga Fucus vesiculosus (L.) reduce assimilation efficiency but do not deter the herbivorous isopod Idotea balthica (Pallas). J Exp Mar Biol Ecol 317:143–157
Kang C-K, Choy EJ, Son Y, Lee J-Y, Kim JK, Kim Y, Lee K-S (2008) Food web structure of a restored macroalgal bed in the eastern Korean peninsula determined by C and N stable isotope analyses. Mar Biol 153:1181–1198
Karez R, Engelbert S, Sommer U (2000) ‘Co-consumption’ and ‘protective coating’: two new proposed effects of epiphytes on their macroalgal hosts in mesograzer-epiphyte-host interactions. Mar Ecol Prog Ser 205:85–93
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Kim YS, Choi HG, Nam KW (2008) Seasonal variations of marine algal community in the vicinity of Uljin nuclear power plant, Korea. J Environ Biol 29:493–499
Krumhansl KA, Scheibling RE (2011) Spatial and temporal variation in grazing damage by the gastropod Lacuna vincta in Nova Scotian kelp beds. Aquat Biol 13:163–173
Littler MM, Littler DS (1980) The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Am Nat 116:25–44
Littler MM, Littler DS (1983) Evolutionary strategies in a tropical barrier reef system: functional-form groups of marine macroalgae. J Phycol 19:229–237
Lockwood JR III (1998) On the statistical analysis of multiple-choice feeding preference experiments. Oecologia 116:475–481
Lyons DA, Scheibling RE (2008) Context-dependent survival of the invasive seaweed Codium fragile ssp. tomentosoides in kelp bed and urchin barren habitats off Nova Scotia. Aquat Biol 2:17–27
Mathieson AC, Pederson JR, Neefus CD, Dawes CJ, Bray TL (2008) Multiple assessments of introduced seaweeds in the Northwest Atlantic. ICES J Mar Sci 65:730–741
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Monteiro CA, Engelen AH, Santos ROP (2009) Macro- and mesoherbivores prefer native seaweeds over the invasive brown seaweed Sargassum muticum: a potential regulating role on invasions. Mar Biol 156:2505–2515
Moore CG, Harries DB (2009) Appearance of Heterosiphonia japonica (Ceramiales: Rhodophyceae) on the west coast of Scotland, with notes on Sargassum muticum (Fucales: Heterokontophyta). Mar Biodiv Rec 2:e131
Nejrup LB, Pedersen MF, Vinzent J (2012) Grazer avoidance may explain the invasiveness of the red alga Gracilaria vermiculophylla in Scandinavian waters. Mar Biol 159:1703–1712
Newton C, Bracken MES, McConville M, Rodrigue K, Thornber CS (2013) Invasion of the red seaweed Heterosiphonia japonica spans biogeographic provinces in the western North Atlantic Ocean. PLoS ONE 8:e62261
Nyberg CD, Wallentinus I (2005) Can species traits be used to predict marine macroalgal introductions? Biol Invasions 7:265–279
Olyarnik SV, Bracken MES, Byrnes JE, Hughes AR, Hultgren KM, Stachowicz JJ (2009) Ecological factors affecting community invasibility. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems: ecological, management, and geographic perspectives. Springer, Heidelberg, pp 215–240
Pappal A (2010) Marine invasive species: state of the Gulf of Maine report. Gulf of Maine Council on the Marine Environment, Dartmouth
Parker JD, Hay ME (2005) Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol Lett 8:959–967
Pedersen MF, Stæhr PA, Wernberg T, Thomsen MS (2005) Biomass dynamics of exotic Sargassum muticum and native Halidrys siliquosa in Limfjorden, Denmark—implications of species replacements on turnover rates. Aquat Bot 83:31–47
Perini VC, Bracken MES (2014) Nitrogen availability limits phosphorus uptake in an intertidal macroalga. Oecologia 175:667–676
Pheloung P, Williams P, Halloy S (1999) A weed risk assessment model for use as a biosecurity tool evaluating plant introductions. J Environ Manag 57:239–251
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agr Ecosyst Environ 84:1–20
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramsey FL, Schafer DW (2013) The statistical sleuth: a course in methods of data analysis. Brooks/Cole, Cengage Learning, Boston
Reichard SH, Hamilton CW (1997) Predicting invasions of woody plants introduced into North America. Conserv Biol 11:193–203
Rejmanek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655
Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M (2000) Plant invasions—the role of mutualisms. Biol Rev Camb Philos Soc 75:65–93
Rosenberg G, Ramus J (1984) Uptake of inorganic nitrogen and seaweed surface area: volume ratios. Aquat Bot 19:65–72
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Ryther JH, Dunstan WM (1971) Nitrogen, phosphorus, and eutrophication in the coastal marine environment. Science 171:1008–1013
Savoie AM, Saunders GW (2013) First record of the invasive red alga Heterosiphonia japonica (Ceramiales, Rhodophyta) in Canada. BioInvasions Rec 2:21–32
Schaffelke B, Hewitt CL (2007) Impacts of introduced seaweeds. Bot Mar 50:397
Schneider CW (2010) Report of a new invasive alga in the Atlantic United States: “Heterosiphonia” japonica in Rhode Island. J Phycol 46:653–657
Shea K, Kelly D, Sheppard DW, Woodburn TL (2005) Context-dependent biological control of an invasive thistle. Ecology 86:3174–3181
Sjøtun K, Husa V, Peña V (2008) Present distribution and possible vectors of introductions of the alga Heterosiphonia japonica (Ceramiales, Rhodophyta) in Europe. Aquat Invasions 3:377–394
Sorte CJB, Williams SL, Carlton JT (2010) Marine range shifts and species introductions: comparative spread rates and community impacts. Glob Ecol Biogeogr 19:303–316
Stachowicz JJ, Tilman D (2005) Species invasions and the relationships between species diversity, community saturation, and ecosystem functioning. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography. Sinauer, Sunderland, pp 41–64
Strong JA, Maggs CA, Johnson MP (2009) The extent of grazing release from epiphytism for Sargassum muticum (Phaeophyceae) within the invaded range. J Mar Biol Assoc UK 89:303–314
Thomsen MS, Wernberg T, Tuya F, Silliman BR (2009) Evidence for impacts of nonindigenous macroalgae: a meta-analysis of experimental field studies. J Phycol 45:812–819
Thresher R (1999) Key threats from marine bioinvasions: a review of current and future issues. In: Pederson J (ed) Marine bioinvasions: proceedings of the first national conference. MIT Sea Grant, Boston, pp 24–34
Thuiller W, Richardson DM, Rouget M, Procheş S, Wilson JRU (2006) Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 87:1755–1769
Tucker K, Richardson DM (1995) An expert system for screening potentially invasive alien plants in South African fynbos. J Environ Manag 44:309–338
Underwood A, Clarke K (2005) Solving some statistical problems in analyses of experiments on choices of food and on associations with habitat. J Exp Mar Biol Ecol 318:227–237
Vitousek P, D’Antonio C, Loope L, Rejmánek M, Westbrooks R (1997) Introduced species: a significant component of human-caused global change. NZ J Ecol 21:1–16
Wilcox S (2012) National Solar Radiation Database 1991–2010 update. National Renewable Energy Laboratory, U.S. Department of Energy
Acknowledgments
We thank C. Schneider, D. Cheney, and E. Jones for their help with species identification and K. Benes, J. Foertch, S. Genovese, C. Newton, V. Perini, M. Roberts, C. Rudd, J. Selwyn, and S. Smith for field and laboratory assistance. C. Sorte provided valuable comments on the manuscript. This work was supported by the National Science Foundation (OCE-0961364 to MESB and G. Trussell and 0963010 to G. Trussell et al. as part of the Academic Research Infrastructure Recovery and Reinvestment Program) and Woods Hole Sea Grant (Subaward A100923 to MESB and C. Thornber).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Low, N.H.N., Drouin, A., Marks, C.J. et al. Invader traits and community context contribute to the recent invasion success of the macroalga Heterosiphonia japonica on New England rocky reefs. Biol Invasions 17, 257–271 (2015). https://doi.org/10.1007/s10530-014-0724-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-014-0724-z