Abstract
Invasive species and environmental change often occur simultaneously across a habitat and therefore our understanding of their relative roles in the decline of native species is often poor. Here, the environmental mediation of a critical interspecific interaction, intraguild predation (IGP), was examined between invasive (Gammarus pulex) and native (G. d. celticus) freshwater amphipods. In the laboratory, IGP asymmetries (males preying on congeneric females) were examined in river water sourced from zones where: (1) the invader has completely displaced the native; (2) the two species currently co-exist, and (3) the native currently persists uninvaded. The invader was always a more effective IG predator, but this asymmetry was significantly weaker moving from ‘invader-only water’ through ‘co-existence water’ to ‘native-only water’. The constituent of the water that drives this mediation of IGP was not identified. However, balancing the rigour of laboratory experiments with field derived ‘environment’ has advanced understanding of known patterns in a native species decline, and its co-existence and persistence in the face of an invader.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Disentangling the respective roles of environment and interspecific interactions in determining the success of invasive species over natives is a difficult but necessary task if we are to predict the spread and impacts of the former (Kelly et al. 2006; Lockwood et al. 2007). Field surveys often cannot identify cause and effect, for example, when environmental disturbance and invasive species occur simultaneously alongside declines in native species, which has occurred, for example, in many European rivers (Leppäkoski et al. 2002). On the other hand, laboratory studies may lack realism as they cannot take account of all potential factors, such as the myriad of environmental variables and potential interactions among species. However, we have a well characterised invasion scenario (see Dick 2008) that allows us to test the hypothesis that an interspecific interaction between an invader and a native, an interaction which is known to be causal in the decline of the latter, is mediated by environmental factors and this may explain observed spatio-temporal patterns of exclusion, co-existence and native persistence.
The freshwater amphipod Gammarus pulex invades the lower stretches of Irish rivers and rapidly replaces the native G. duebeni celticus (Dick et al. 1993; MacNeil et al. 2004; Dick 2008). Intraguild predation (IGP) is mutual but asymmetric in favour of the invader and this explains the extirpation of natives (Dick et al. 1993). However, there are short reaches of co-existence upstream and, in the most upper reaches, G. pulex ceases to spread, or does so only very slowly, and the native G. d. celticus may persist long-term (MacNeil et al. 2004). This suggests a gradient of strength of the IGP interaction, but we have little evidence as to what environmental feature would mediate this. However, as IGP is most severe towards moulting females of both species, we suspect some feature of the water chemistry. To test this hypothesis, we examined female survival and moult timing and the asymmetry of mutual IGP between the native and invader in laboratory experiments using water collected directly from the field zones of current exclusion, co-existence and native persistence.
Materials and methods
Specimen collection and maintenance
In July/August 2006, Gammarus spp. were collected by kick sampling the River Lissan, Co. Tyrone, N. Ireland. G. pulex (invader) was collected from immediately upstream of the weir that hindered the upstream invasion of this species until 2003 but is now entirely colonized by the invader (UK Grid Ref. H828804; see ‘Mixed site’ in Kelly et al. 2003, 2006). G. d. celticus (native) was collected from 8 km upstream (H773850), a site that has not as yet been invaded by G. pulex. This site was chosen because, typically, G. pulex does not appear able to successfully invade such higher reaches of Irish rivers, or does so only very gradually (see Dick et al. 1993, 1994; MacNeil et al. 2001). The animals from each site were kept in tanks (120 × 60 × 25 cm deep) with source water, gravel, vegetation and fauna. Water was collected from the two sites detailed above and also from an intermediate site where the two species currently co-occur (‘mixed Gammarus spp.’ site; H806806) corresponding to the ‘GDC’ site in Kelly et al. (2003, 2006). Point measurements of temperature, pH, conductivity and dissolved oxygen were taken at each site.
Experimental procedures
For all experiments, precopula pairs were removed from holding tanks with males in the range of body lengths 14–16 mm and females 8–10 mm for both species, thus controlling for any effects of body size. Pairs were separated by placement on tissue paper.
Experiment 1: For both species, females were placed individually in 5 cm diameter plastic cups with 30 ml of each of the three water sources (n = 15 each group), and monitored for deaths and moulting for 3 weeks at 16°C and 12:12 h Light:Dark (see Table 1).
Experiment 2: Plastic containers of 10 cm diameter were supplied with washed stones, decaying leaf material and 250 ml of source water in temperature and light conditions as above. For both species, five females were placed with two male congenerics, this for each of the three source waters (n = 10 per group). We counted surviving females after 3 weeks and noted evidence of predatory interactions (see Table 1).
For both experiments, the animals were fed with catfish pellets and their water replaced every 2 days.
We used χ² on frequency data and ANOVA (SuperAnova) on continuous data (proportional data were arcsine transformed; Sokal and Rohlf 1995). Least Squares Means contrast tests were used, in Experiment 2, for planned comparisons among means across factors (see Abacus Concepts 1989).
Results
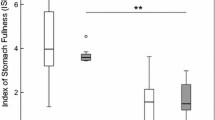
Gross measures of site physico-chemistry (Table 2) differed little among years (2003 data from Kelly et al. 2006) and there was little indication of differences among sites on the day of collection (Table 2). In experiment 1, there were no significant differences in female survival among water types for G. d. celticus (χ² = 0.034; NS) or G. pulex (χ² = 0.034; NS). Survival was very high, only three Gammarus dying, two G. pulex and one G. d. celticus in their own source waters. There was no significant difference in mean time to moult between the species (F 1,84 = 0.9, NS; Fig. 1) or among the water sources (F 2,84 = 1.1, NS; Fig. 1) and there was no significant ‘species’ × ‘water source’ interaction effect (F 2,84 = 1.93, NS; Fig. 1). In experiment 2, all males survived and their predation of females was actually observed, and apart from that the dishes were littered with female body parts. There was no significant difference in overall mean percentage survival of female Gammarus spp. among the three water sources (F 2,54 = 0.24, NS; Fig. 2), however, overall survival of G. d. celticus was significantly lower than that of G. pulex (F 1,54 = 42.1, P < 0.001; Fig. 2) and the interaction term was close to significance (F 2,54 = 2.2, P = 0.1; Fig. 2). Indeed, our planned comparisons show that there was significantly lower survival of G. d. celticus females in the G. pulex source water as compared to females in both the ‘mixed species’ source water (P < 0.05; Fig. 2) and the G. d. celticus source water (P < 0.02; Fig. 2).
Mean (± SE) percentage survival of female G. d. celticus and G. pulex in the presence of male congenerics in the three source waters (GPW = water from the G. pulex site; MX = water from the mixed Gammarus spp. site; and GDCW = water from the G. d. celticus site). P-values correspond to between-species comparisons by the Least Squares Means test
Discussion
Invasive species and habitat modification are separately regarded as two of the most important drivers of biodiversity change (Sala et al. 2000). However, invaders often arrive coincidentally with environmental disturbances, confounding cause and effect interpretations of native species declines and losses (Leppäkoski et al. 2002). Previously, it was identified that mutual but asymmetrical IGP drives the replacement of the native G. d. celticus by invading G. pulex but that, upstream, there remain reaches of rivers that are populated only by the native and these populations appear to ‘resist’ invasion in the long term, or are only gradually invaded (Dick et al. 1993; MacNeil et al. 2004; Dick 2008). In the present study, females of the two species survived and moulted normally in water collected from regions of invader-driven native exclusion, and current co-existence and native persistence. Superficially, the water chemistries of these zones appear similar, shown in this and previous studies (Kelly et al. 2003, 2006) and the level of agricultural impact is low, with low levels of suspended solids (5 mg L−1) and nutrients (2.2 mgN L−1 of nitrate; 0.06 mgN L−1 of ammonium and 0.04 mgN L−1 of phosphate; Water Management Unit, Northern Ireland Environment and Heritage Service, pers. com). Indeed, the catchment area of the River Lissan is free from industrial plants and other major pollution discharges. The lack of difference of response among water sources in terms of female survival and moulting could thus be interpreted as indicating little difference in water chemistry, or any differences that do occur do not directly disadvantage one species over the other.
In a second experiment, however, the effect of the three water sources on the predatory interaction of males with moulting females was examined, such predation being a large determinant of the exclusion of G. d. celticus by G pulex (Dick et al. 1993). G. pulex males were always more effective predators of G. d. celticus females than in the reciprocal interaction, however, the strength of this asymmetry was clearly influenced by some feature of the water chemistry. Female G. d. celticus were significantly more often killed and consumed by male G. pulex in the water originating from where the native has been replaced. However, female natives increasingly resisted invader predation moving ‘upstream’, that is, in water from the zone of current co-existence to the zone currently only populated by G. d. celticus. This demonstrates an environmental mediation of a critical interspecific interaction, the experimental data being entirely congruent with the repeatedly observed field spatio-temporal patterns of displacement, co-existence and persistence (Dick et al. 1994; MacNeil et al. 2001, 2004). Indeed, we can predict that, while G. d. celticus populations in the upper stretches of this river have so far not been invaded, the lesser magnitude of the asymmetry in IGP may only slow this process, with the eventual invasion of these areas likely since IGP is still in favour of G. pulex in such water. By similar argument, we predict the zone of current co-existence will become G. pulex only in future. We will test these predictions in future years.
The specific constituent(s) of the water that could be responsible for the observed mediation of predation were not identified. G. pulex is more tolerant than G. d. celticus to low dissolved oxygen concentration, organic pollution and chemically degraded water (MacNeil et al. 2004, Piscart et al. 2007), but the large differences in these parameters that drive large scale patterns of distribution between two species (see Kelly and Dick 2005) are not evident in the present study. Sub-lethal effects of chemical parameters are likely, such as concentrations of ions that affect the moulting process. For example, moulting might be less efficient and/or lead to softer cuticles for longer in G. d. celticus in response to small differences in available ions, such as Ca, Mg and Na (e.g. see Wright 1980). Elucidating the causal agents requires systematic exploration of a huge number of chemical constituents both in isolation and combination. This is outside the scope of the present study, however, the current laboratory experimental approach using field derived water is an advance in disentangling the role of environment in mediating interspecific interactions and helping explain invader/native distribution patterns in real systems.
References
Abacus Concepts (1989) SuperAnova. Abacus Concepts, Berkeley
Dick JTA (2008) Role of behavior in biological invasions and species distributions; lessons from interactions between the invasive Gammarus pulex and the native G. duebeni (Crustacea: Amphipoda). Contrib Zool 77:91–98
Dick JTA, Montgomery I, Elwood RW (1993) Replacement of the indigenous amphipod Gammarus duebeni celticus by the introduced G. pulex: differential cannibalism and mutual predation. J Anim Ecol 62:79–88
Dick JTA, Elwood RW, Montgomery WI (1994) Range expansion of the alien amphipod Gammarus pulex in the River Lagan, Co. Down. Irish Nat J 24:403–404
Kelly DW, Dick JTA (2005) Effects of environment and an introduced invertebrate species on the structure of benthic macroinvertebrate species at the catchment level. Archiv Hydrobiol 164:69–88
Kelly DW, Dick JTA, Montgomery WI et al (2003) Differences in composition of macroinvertebrate communities with invasive and native Gammarus spp. (Crustacea: Amphipoda). Freshwat Biol 48:306–315
Kelly DW, Bailey RJ, MacNeil C et al (2006) Invasion by the amphipod Gammarus pulex alters community composition of native freshwater macroinvertebrates. Div Distrib 12:525–534
Leppäkoski E, Gollasch S, Olenin S (eds) (2002) Invasive aquatic species of Europe: distribution, impacts and management. Kluwer Academic Publishers, Dordrecht
Lockwood JL, Hoopes MF, Marchetti MP (2007) Invasion ecology. Blackwell, Oxford
MacNeil C, Montgomery WI, Dick JTA, Elwood RW (2001) Factors influencing the distribution of native and introduced Gammarus spp. in Irish river systems. Arch Hydrobiol 151:353–368
MacNeil C, Prenter J, Briffa M et al (2004) The replacement of a native freshwater amphipod by an invader: roles for environmental degradation and intraguild predation. Can J Fish Aquat Sci 61:1627–1635
Piscart C, Manach A, Copp GH et al (2007) Distribution and microhabitats of native and non-native gammarids (Amphipoda, Crustacea) in Brittany, with particular reference to the endangered endemic sub-species Gammarus duebeni celticus. J Biogeogr 34:524–533
Sala OE, Chapin FS, Armesto JJ et al (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sokal RR, Rohlf FJ (1995) Biometry. W.H. Freeman, San Francisco
Wright DA (1980) Calcium balance in pre-moult and post-moult Gammarus pulex (Amphipoda). Freshw Biol 10:571–579
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piscart, C., Dick, J.T.A., McCrisken, D. et al. Environmental mediation of intraguild predation between the freshwater invader Gammarus pulex and the native G. duebeni celticus . Biol Invasions 11, 2141–2145 (2009). https://doi.org/10.1007/s10530-009-9497-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-009-9497-1