Abstract
Very few studies have addressed how the invasive kelp Undaria pinnatifida (Harvey) Suringar spreads beyond initial founding populations in harbours. Surveys of the harbours and accessible areas of open coast throughout southern New Zealand were conducted to determine how far U. pinnatifida populations had extended since initial incursions. Our findings clearly demonstrate that U. pinnatifida is capable of invading native communities and can establish reproductive populations in locations subjected to significant and consistent wave action. The extent of spread from source populations differs between harbours in which it has established. Dispersal is greatest in harbours with long established populations, those where populations have not been strategically managed, harbours with high water exchange with surrounding coastal waters, and where prevailing currents allow establishment of U. pinnatifida on suitable substrata close to harbour entrances. Dispersal along the open coast is primarily achieved by drifting adult sporophytes that are washed up in the rocky intertidal zone. Founding populations are most often found in the intertidal zone, primarily within rockpools. Subtidal transects and observations indicate that U. pinnatifida is well adapted to invade exposed coastlines and can establish within a broad range of niches in wave-exposed areas including rockpools, the low intertidal, shallow subtidal, Macrocystis pyrifera kelp forests, and in low light areas beyond the vertical extent of large native macroalgae. The current range of U. pinnatifida is much greater than expected and appears to be expanding. Due to its ability to grow in a broad range of environments and to form dense monospecific stands, U. pinnatifida has the potential to strongly modify almost all rocky subtidal and intertidal communities in temperate locations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globalisation of the marine environment as a result of long-distance shipping activities is markedly homogenising the flora and fauna of marine ecosystems near to ports. The Asian kelp Undaria pinnatifida (Harvey) Suringar is fast becoming a cosmopolitan species worldwide and is a key example of this process. Undaria pinnatifida is a native of cold temperate regions of Japan, China and Korea, however, in recent decades this species range has expanded via anthropogenic modes of dispersal to include Argentina, Australia (and Tasmania), Britain, France, Italy, the Netherlands, New Zealand, Spain, and most recently Mexico and California (Wallentinus 1999; Sinner et al. 2000; Silva et al. 2002; Aguilar-Rosas et al. 2004; Casas et al. 2004; Uwai et al. 2006). Its bi-phasic life history has been a key factor in facilitating this transoceanic dispersal between hemispheres via the transportation of the microscopic gametophytic stage in ship ballast.
Undaria pinnatifida has proven to be highly adaptable after establishment in new localities where it is found in a broad range of habitats, primarily in areas without significant wave action (Brown and Lamare 1994; Floc’h et al. 1996). The ability of U. pinnatifida to attach and grow on almost any natural (e.g. rock, shells, other macroalgae) and artificial substrata (e.g. wood, steel, glass, plastic) aids in its establishment in new locations. After its initial introduction into an area by anthropogenic activity, natural dispersal occurs primarily through dislodged fragments or whole sporophytes that drift over distances of hundreds of metres to kilometres (Forrest et al. 2000). In contrast, spore dispersal occurs over a much smaller spatial scale, less than 100 m, and is likely to be of greater importance in the establishment phase of new incursions (Forrest et al. 2000).
In its native range Undaria pinnatifida displays an annual life cycle, recruitment of the macroscopic sporophyte occurs during autumn, while zoospore release occurs in early to mid spring and senescence occurs in mid-summer (Stuart et al. 1999). The microscopic gametophytic stage persists on a suitable substratum until the following winter (Hewitt et al. 2005). This well-defined seasonality however, is not consistently displayed throughout its introduced range and provides further evidence of this kelp’s ability to adapt to new environments. In both Tasmania and New Zealand there is evidence to suggest that this species displays overlapping generations, with sporophytes present year round, a change attributed to less extreme seasonal fluctuations in ocean temperatures in these regions compared to the native range of U. pinnatifida (Brown 1999; Thornber et al. 2004; Schaffelke et al. 2005).
Undaria pinnatifida was first recorded in New Zealand in Wellington Harbour in 1987 (Hay and Luckens 1987). Gametophytes were transported to New Zealand in the ballast of foreign fishing vessels. Since then it has expanded its geographic range to include all of the major ports throughout New Zealand and a number of secondary ports (Fig. 1). Most recently it has been found in the northern harbours of Taranaki, Auckland (Waitemata) and Tauranga (Fig. 1). Presently in New Zealand, U. pinnatifida is classified as an ‘Unwanted Organism’ under the Biosecurity Act 1993 (Wotton et al. 2004). This classification is ascribed to organisms capable of causing unwanted harm to any natural and physical resource or human health. Various control strategies to eradicate or minimise the spread of this species have been trialed with varying degrees of success, including physical removal, hot water treatment, complete covering of localised incursion sites with black polythene and widespread public awareness and vessel management campaigns (Stuart 2004; Hewitt et al. 2005; Wotton et al. 2004).
Locations of Undaria pinnatifida populations in major and secondary ports in New Zealand as of July 2006. The year U. pinnatifida was first observed in each port is included in brackets (note U. pinnatifida has been eradicated from the Chatham Islands since June 2001 refer to Wotton et al. 2004)
The establishment of founding populations of Undaria pinnatifida within New Zealand ports has been well documented, but subsequent range expansion beyond these founding populations is poorly documented. The prevailing perception is that U. pinnatifida is unable to invade wave impacted open coastlines. This study was undertaken to document the distribution and spread of U. pinnatifida from six commercial and small fishing ports about the southern coast of New Zealand’s South Island, in order to identify which mechanisms are most important to the spread of founding populations and identify which habitats are most susceptible to invasion by this species (Fig. 1). The objectives of this study were threefold, to: (1) document the range expansion of established populations of U. pinnatifida within the sheltered environment of Otago Harbour over the last 16 years; (2) determine if populations of U. pinnatifida are expanding from initial areas of introduction within Otago Harbour to surrounding inlets and wave-exposed open coasts; (3) document the current geographic range of U. pinnatifida on the coastline of the southern South Island of New Zealand.
Methods
Range expansion of Undaria pinnatifida in Otago Harbour from 1990–2004
The spread of Undaria pinnatifida within Otago Harbour (Figs. 1, 2), where it was first discovered in 1990 (Hay and Villouta 1993), was conducted via shore and subtidal surveys of mature sporophytes around the perimeter of the harbour during the periods July to August (1997–1999) and September to October (2000, 2004), during periods of extreme low water. The change in sampling period reflects a change in the seasonal pattern of sporophyte presence within the harbour since the initial surveys were conducted by Hay and Villouta (1993), during which there was a clearly defined summer hiatus of sporophytes. In recent years, large standing crops of remain within the harbour during summer months and mature sporophytes are still present in February in some regions (L. Russell pers. obs.). Quantitative surveys were conducted in 1998–2000 and 2004 during which the density of U. pinnatifida sporophytes was recorded. Density categories followed Curiel et al. (1998): <2 sporophytes per m2, 2–10 sporophytes per m2, >10 sporophytes per m2.
Otago Harbour is a flooded river valley that extends 21 km from its head at Dunedin city to its entrance at Harington Point. A narrow shipping channel (10–35 m deep) runs along its northwest side and is regularly dredged. Winter and summer isotherms range between 9–14°C.
Distribution of Undaria pinnatifida on wave-sheltered and exposed shores surrounding Otago Harbour
The spread of Undaria pinnatifida from Otago Harbour into surrounding inlets and the open coast was followed via intensive intertidal and subtidal surveys conducted during September and October 2004. The survey region extended 100 km along the coast from Karitane in the north to Akatore in the south. In addition, during 2004 quantitative vertical shore profiles were compiled for a population of U. pinnatifida at a wave-exposed coastal site at Mapoutahi, north of Otago Harbour (Fig. 2). Six randomly positioned contiguous transects (0.25 m wide) were sampled, extending from the lower intertidal zone to the maximum depth of U. pinnatifida. The vertical extent and density of U. pinnatifida sporophytes and other prominent species was recorded. A further qualitative vertical shore profile was compiled for a wave sheltered population at Papanui inlet on the Otago Peninsula (Fig. 2).
Geographic extent of Undaria pinnatifida around the southern South Island
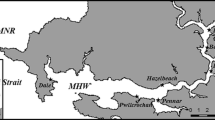
The geographic extent of Undaria pinnatifida around southern New Zealand was based on shore and subtidal surveys conducted during September–November 2005. The survey region extended from Smithfield (44°22′S) immediately north of Timaru Harbour on the east coast of the South Island to Te Waewae Bay (46°17′S), western Southland (Fig. 3). Additional surveys were also conducted at a small fishing port at Jackson Bay in Westland (43°58′), and both Milford and Doubtful Sounds in Fiordland, which are subjected to high levels of local and international shipping activity primarily due to the tourism industry. Inaccessible regions of the lower South Island, such as a number of fiords in Fiordland and also Stewart Island, that has established populations in both Halfmoon and Big Glory Bays, were not included in these surveys. Information from more remote parts of Fiordland was provided by Dr. S. Wing (Department of Marine Science, University of Otago) who conducts annual subtidal surveys throughout Fiordland. In order to assess the range expansion of this species on to the open coast, particular emphasis was focused in geographic regions adjacent to the primary incursion sites at the ports of Bluff, Moeraki, Oamaru and Timaru (Fig. 1).
Results
Range expansion of Undaria pinnatifida in Otago Harbour from 1990–2004
The expansion of founding populations of Undaria pinnatifida in Otago Harbour has been monitored at various time intervals over the past 16 years since its initial introduction in 1990. The initial sites of invasion were within the Port Chalmers container terminal at the timber storage area and adjacent to the oil terminal within the main Dunedin city wharf (Fig. 4; C. Hay pers. com). There was only limited range expansion observed between 1990 and 1993, where a discrete population established on jetty piles in Careys Bay immediately to the north of the Port Chalmers container terminal (Fig. 4). Individuals in all populations in 1993 were confined to artificial substrata associated with the shipping ports. Four years later in 1997 U. pinnatifida was present at all of the main jetties within the upper harbour and extending as far north as Deborah Bay. Populations were predominantly on the western side of the harbour, adjacent to the main shipping channel (Fig. 4). Additional populations were also present on the jetty at Vauxhall and at Aquarium Point on the east side of the harbour (Fig. 4). At Aquarium Point U. pinnatifida was also found on natural substrata within Macrocystis pyrifera beds. Observed population densities were greatest on jetty piles and artificial substrata, where U. pinnatifida formed a dense fringe, compared to surrounding natural habitats, where solitary or patches comprising of a few individuals were present.
In 1998 a quantitative survey was undertaken in order to ascertain the density distribution of Undaria pinnatifida around the harbour. Greatest densities were observed at all of the sites occupied by U. pinnatifida in the previous year’s survey, in particular on jetties and wharves (>10 sporophytes per m2; Fig. 5). Natural substrata immediately adjacent to these jetties supported between 2 and 10 sporophytes per m2. The distribution of U. pinnatifida within Otago Harbour increased substantially between the surveys in 1997 and 1998 with populations of U. pinnatifida establishing at the harbour entrance on the Aramoana mole and at Harington Point (Fig. 5). Sporophytes were also recorded in 1998 at the jetty on the east side of Quarantine Island (Fig. 5).
Over the subsequent 5 years populations of Undaria pinnatifida extended throughout the harbour (Fig. 5). Presently most locations around the perimeter of the harbour support patches comprising of at least 2–10 sporophytes per m2. The highest densities of U. pinnatifida are generally on or surrounding artificial structures and on rocky headlands (>10 sporophytes per m2; Fig. 5). The most significant range expansion was observed on the east side of the harbour between 2000 and 2004. Only a few sparsely distributed populations were present in 2000, in contrast to an almost continuous band present within the upper harbour in 2004 (Fig. 5). Regions where U. pinnatifida was absent during the 2004 survey are all shallow areas of the harbour predominantly comprised of fine silt sediment (Fig. 5).
Distribution of Undaria pinnatifida on wave-sheltered and exposed shores surrounding Otago Harbour
Populations of Undaria pinnatifida have become established on the wave-exposed coastline external to Otago Harbour. The distribution of U. pinnatifida is extending north with prevailing currents along the open coast from the entrance to Otago Harbour. Over the 6 years between the initial discovery of U. pinnatifida at the harbour entrance and the survey in 2005 it has spread 12 km northwards (Fig. 2). Populations between the harbour and Warrington are small and intermittent, restricted to shallow wave-exposed bays and headlands. From Warrington northwards a continuous band of U. pinnatifida is present in low intertidal rockpools and in the subtidal as far north as Omimi Creek (Fig. 2). Over this period U. pinnatifida has not been found on the exposed coastline immediately to the south of Otago Harbour. Populations have however, established within sheltered inlets close to the harbour entrance (Papanui and Hoopers Inlets) and in wave exposed habitats at their entrances (Fig. 2).
The typical vertical distribution of Undaria pinnatifida at shallow wave-exposed habitats is displayed in Fig. 6A. Undaria pinnatifida was found between depths of 4–5 m (or as deep as suitable substrate for attachment allowed) extending up into the low intertidal, where it forms a dense canopy in some intertidal rockpools. Undaria pinnatifida is also establishing new populations within nearshore and offshore Macrocystis pyrifera kelp forests in deeper water between Warrington and Seacliff (Fig. 2). Our observations suggest invasion occurs initially in the shallow subtidal zone on rocks that extend to just below or above mean low water.
Quantitative subtidal transects at Mapoutahi indicate that the vertical range of Undaria pinnatifida sporophytes was greater than any of the other large, prominent macroalgae observed (Fig. 7). Sporophytes were present at depths greater than 3 m beneath the native kelp canopy, an area commonly colonized by Demarestia ligulata, diminutive red macroalgae, and sessile animals including sponges and ascidians. Sporophytes of U. pinnatifida were also present within and directly above the zone (0–3 m) colonized by the native kelp Lessonia variegata, and fucoids Marginariella boryana and Durvillaea spp., extending into the intertidal zone (Figs. 6A, 7).
At wave-sheltered sites, such as Papanui and Hoopers Inlets, Undaria pinnatifida was found at mean low water and extending down to 4 m, typically the extent of suitable substrata (Fig. 6B). Within these sites, U. pinnatifida formed a distinct, dense band above and below kelps such as Macrocystis pyrifera, and the fucoids Carpophyllum flexuosum and Sargassum sinclarii. Undaria pinnatifida also acted as a subcanopy species below continuous M. pyrifera canopies in dense patches and intermixed with the aforementioned kelp and fucoid species.
Geographic extent of Undaria pinnatifida around the southern South Island
The documented distribution of Undaria pinnatifida around the southern South Island is displayed in Fig. 3. Significant expansion of the distribution of U. pinnatifida has been primarily limited to the north east coast and is derived primarily from Otago (described above) and Moeraki Harbours. Two small populations were also identified at Kakanui and Orore Points approximately 12 km from the nearest established U. pinnatifida population at Oamaru Harbour in the north and 20 km from a population in Moeraki to the south (Fig. 1). At Kakanui U. pinnatifida sporophytes were restricted to a single low intertidal rockpool from which 76 juvenile thalli and 33 adult sporophytes were removed. Extensive intertidal and subtidal surveys of the surrounding region failed to find any other U. pinnatifida thalli. Further south at Orore Point a single sporophyte was observed and subsequently removed.
Undaria pinnatifida is notably absent from significant stretches of coastline around the south of the South Island. It has yet to be found along more than 800 km of coastline on the west coast of the South Island from Golden Bay in the north down to Bluff in the south (Figs. 1, 3). Another significant area apparently free from U. pinnatifida extends along 225 km of coastline from Otago Peninsula in the north to Bluff in the south and includes the Catlins coastal region (Fig. 3).
The range of Undaria pinnatifida has expanded to surrounding wave-exposed coasts in four of the five ports we surveyed that supported established populations (Fig. 8). The exception to this trend was Bluff Harbour where this species is only found within the harbour confines, despite its presence in the harbour since 1988 (Fig. 8D). The extent of range expansion varied among the other ports. In Timaru Harbour the U. pinnatifida population has extended less than 1 km north of the harbour despite being present in this region for almost 20 years (Fig. 8A). Dense monospecific stands of U. pinnatifida are present within low intertidal rockpools and amongst Durvillaea willana at wave-exposed sites north of the harbour. Towards the south, U. pinnatifida was observed on the open coastal side of the rock wall at the southern end of the harbour entrance. No drift or attached individuals were observed further south, although low visibility did hamper subtidal surveys of this area. At Oamaru Harbour U. pinnatifida was observed as a dense fringe on all the port structures (Fig. 8B). U. pinnatifida individuals are also present on the open coastal side of the rock walls surrounding the harbour. No populations or drift U. pinnatifida sporophytes were found on exposed gravel beaches to the north of the harbour, and small isolated patches extended approximately 1 km immediately to the south of the harbour entrance (Fig. 8B). Undaria pinnatifida is now present along a considerable length of the coastline surrounding the initial incursion site at the small fishing port at Moeraki (Fig. 8C). This population has expanded approximately 10 km south along the rocky shore and is present in dense patches amongst native kelp and fucoid canopies (Fig. 8C). At Bluff Harbour, U. pinnatifida has not spread far from initial points of invasion and is primarily found on artificial substrata surrounding the main commercial wharf (Fig. 8D). There were no sporophytes observed immediately to the south of these jetties towards the harbour entrance on natural or artificial substrata despite extensive subtidal and intertidal surveys of suitable substrata in this area.
Discussion
Our surveys show for the first time in New Zealand that Undaria pinnatifida is capable of establishing reproductive populations in locations subject to significant and consistent wave action. Undaria pinnatifida exhibits a much wider vertical and ecological range than previously anticipated (Brown and Lamare 1994; Floc’h et al. 1996; Stuart 2004). An unexpected finding was the presence of U. pinnatifida sporophytes in relatively high densities at some localities amongst the large bull kelp species Durvillaea antarctica and D. willana. These species characterise strongly wave-impacted shores (Adams 1994), and therefore these zones have previously been thought to be prohibitive to the establishment of U. pinnatifida (Stuart 2004). Sites in shallow water to the north of Otago Harbour are subject to consistent wave action and an annual average wave height of 1.07 m (±95% C.I. 2.01 m; Dr P. McComb, MetOceans Solutions Ltd, pers. com.). At one site (Mapoutahi) U. pinnatifida was observed at the front of rock platforms in areas exposed to the full force of the wave battery. In these areas U. pinnatifida avoided the more extreme regions of water motion and the whiplash action of Durvillaea spp. fronds by growing dense patches in deeper water below the Durvillaea spp. zone. In less turbid sites it is proposed that U. pinnatifida could survive below zones subject to extreme wave surge in even the most wave-exposed sites due to their capacity for growth in low light environments. Our observations suggest U. pinnatifida is equally capable of resisting the affects of wave action as native kelps common on wave-exposed shores such as Lessonia variegata and Marginariella. Established stands of U. pinnatifida are now present on the open coastline external to four of the five harbours we surveyed.
The extent of Undaria pinnatifida’s distribution on the open coastline differs between harbours in which it has established. At present the most extensive expansion has occurred from Otago Harbour. The temporal pattern of spread within this harbour reflects that previously published overseas, that is minimal population expansion in the few years following the incursion event, with individuals confined to artificial substrata associated with shipping, followed by significant spatial expansion throughout the harbour on all suitable substrate types. In less than a decade the population extended throughout the harbour to the entrance, and in the subsequent 7 years has extended along 15 km of wave-dominated coastline. This rate of spread is comparable with reported population expansion observed in Tasmania (Sanderson 1997). The Moeraki population appears to be spreading at a much faster rate, extending greater than 10 km in less than 11 years. This difference may be attributed to the topography of the harbour, Moeraki Harbour is located on an open bay and is likely to have higher degrees of dislodgment of sporophytes during storm events and water exchange with the surrounding coastal waters than the other harbours which have more confined entrances.
Populations at Oamaru and Timaru Harbours have not undergone a similar spatial or temporal pattern of expansion, despite nearly 20 years of occupancy by this invasive. In both harbours populations of Undaria pinnatifida have extended less than 1 km either side of the entrance. This is most likely due to the lack of suitable substrate available at the entrances to both harbours.
The clear exception to this pattern of establishment is the population of Undaria pinnatifida at Bluff Harbour. Populations are presently confined to artificial surfaces associated with the port, with only a few individual sporophytes observed on natural substrata below jetty piles. The lack of expansion is most likely the result of a successful control programme carried out by the Department of Conservation that included monthly manual eradication of sporophytes from port structures. This programme was halted in 2004 due to the removal of government funding. Expansion of populations within Bluff Harbour and out on to the open coast now appears inevitable if the evidence from other harbours in southern New Zealand is taken into account. This is of great concern as Bluff is an important stopping point for vessels enroute to the Sub-Antarctic Islands and Fiordland, which includes both world heritage sites and marine parks of high conservation and biodiversity value (Hewitt et al. 2005).
The primary vector facilitating the spread of Undaria pinnatifida on to the open coast appears to be via the drifting of reproductively mature sporophytes from founding populations in sheltered harbours and inlets (Forrest et al. 2000). Our observations indicate that shallow subtidal areas that have gently sloping rocky intertidal regions are more susceptible to colonisation by U. pinnatifida than surrounding areas of shallow reef not connected to dry land. From observed distributions of U. pinnatifida in newly colonized sites we suggest that U. pinnatifida establishes in intertidal (primarily in rockpools or in sheltered locations behind large rocks) or shallow subtidal locations first, before spreading into other niches beneath the large kelp species of exposed coastlines. As patches of U. pinnatifida increase in size and density at new sites there is an associated increase in spore production that allows the colonization of deeper subtidal areas in the zone below dominant kelp and fucoid species (Durvillaea spp., Lessonia variegata, Marginariella boryana; Fig. 6A). This deep zone is generally characterised by patchy populations of Desmerestia ligulata, Zonaria, bladed and filamentous red macroalgae, geniculate and encrusting coralline species, and a range of sessile animals. Over time U. pinnatifida populations become dominant within these deeper subtidal zones replacing or covering D. ligulata and the understorey species.
The final stage in this sequence on wave-dominated shores is the invasion of the kelp and fucoid zone. These deep subtidal populations of Undaria pinnatifida extend upwards into the Lessonia variegata and Marginariella boryana zones, eventually growing directly below Durvillaea spp. stands. In deeper (>3–10 m) more sheltered subtidal areas U. pinnatifida invades Macrocystis pyrifera forests competing with sub-canopy species like Ecklonia radiata and Landsburgia quercifolia. Concurrently the intertidal and shallow subtidal populations also increase in size and begin to extend down amongst the Durvillaea spp. stands on less exposed shores. All stages of invasion proposed here, were observed along the Otago coastline.
The rate of range expansion of Undaria pinnatifida at a new location is likely to be closely related to where initial establishment occurs. If establishment occurs within a rockpool this spread could be limited as most spores produced by a founding population could initially remain within the confines of the pool. If establishment occurs in the low subtidal zone water motion may facilitate greater spore dispersal resulting in a more widely scattered population.
Few studies have investigated the resistance of native New Zealand seaweed assemblages to colonisation by Undaria pinnatifida (Forrest and Taylor 2002; Thompson 2004). In Tasmania disturbance events that result in canopy removal were identified as key factors in facilitating the invasion of native seaweed assemblages (Valentine and Johnson 2003). While the role of disturbance was not directly assessed in this study, our observations suggest that the invasion of native macroalgal assemblages in wave-exposed environments by U. pinnatifida can occur independently of disturbance. In southern New Zealand U. pinnatifida invades inherently patchy habitats either lacking kelp canopies such as in the intertidal or in sites without contiguous kelp canopies in shallow subtidal zones locations. These patches may be a result of disturbance but other contributing factors such as topography, low light and resource limitation (e.g. nutrients in rockpools) are also likely to be important. The remarkable ability of Undaria pinnatifida to attach to a wide range of substrata also implies that disturbance events are not necessary in facilitating invasion events. We observed U. pinnatifida growing in a wide spectrum of habitat types ranging from below dense multilayered canopies to growing in wave-exposed environments attached epiphytically to sponges, ascidians, bivalves and macroalgae.
Conclusion
The ultimate assessment of the impacts of Undaria pinnatifida on native communities depends on the ability of populations to move out of harbours and colonise natural low intertidal and subtidal substrata where native macroalgal assemblages occur (Thornber et al. 2004). This process has clearly occurred in the southern South Island of New Zealand, as populations of U. pinnatifida are now present on wave-dominated shores external to the majority of ports in this region. These findings have direct implications not only for coastal managers in New Zealand, but also those throughout the temperate region, particularly in regions where U. pinnatifida is currently resident. Management and research programmes need to focus on controlling harbour populations and reducing the risk of transport to new ports. It seems apparent that the full ecological and economic costs of this invasive species are yet to be fully realised.
References
Adams N (1994) Seaweeds of New Zealand. Canterbury University Press, Christchurch, New Zealand
Aguilar-Rosas R, Aguilar-Rosas L, Avila-Serrano G, Marcos-Ramirez R (2004) First record of Undaria pinnatifida (Harvey) Suringar (Laminariales, Phaeophyta) on the Pacific coast of Mexico. Bot Mar 47:255–258
Brown S (1999) Dispersal characteristics of the adventive seaweed Undaria pinnatifida in New Zealand. MSc thesis. University of Otago, New Zealand
Brown M, Lamare M (1994) The distribution of Undaria pinnatifida (Harvey) Suringar within Timaru Harbour, New Zealand. Jap J Phycol: 42:63–70
Casas G, Scrosati R, Piriz M (2004) The invasive kelp Undaria pinnatifida (Phaeophyceae, Laminariales) reduces native seaweed diversity in Nuevo Gulf (Patagonia, Argentina). Biol Invasions 6:411–416
Curiel D, Bellemo G, Marzocchi M, Scattolin M, Parisi G (1998) Distribution of introduced Japanese macroalgae Undaria pinnatifida, Sargassum muticum (Phaeophyta) and Anthithamnion pectinatum (Rhodophyta) in the Lagoon of Venice. Hydrobiologia 385:17–22
Floc’h J, Pajot R, Mouret V (1996) Undaria pinnatifida (Laminariales, Phaeophyta) 12 years after its introduction into the Atlantic Ocean. Hydrobiologia 326/327:217–222
Forrest B, Brown S, Taylor M, Hurd C, Hay C (2000) The role of natural dispersal mechanisms in the spread of Undaria pinnatifida (Laminariales, Phaeophyceae). Phycologia 39:547–553
Forrest B, Taylor M (2002) Assessing invasion impact: survey design considerations and implications for management of an invasive marine plant. Biol Invasions 4:375–386
Hay C, Luckens P (1987) The Asian kelp Undaria pinnatifida (Phaeophyta: Laminariales) found in a New Zealand harbour. NZ J Bot 25:364–366
Hay C, Villouta E (1993) Seasonality of the adventive Asian kelp Undaria pinnatifida in New Zealand. Bot Mar 36:461–476
Hewitt C, Campbell M, McEnnulty F, Moore K, Murfet N, Robertson B, Schaffelke B (2005) Efficacy of physical removal of a marine pest: the introduced kelp Undaria pinnatifida in a Tasmanian Marine Reserve. Biol Invasions 7:251–263
Sanderson J (1997) Survey of Undaria pinnatifida in Tasmanian coastal waters, January–February 1997. Report to Tasmanian Department of Marine Resources
Schaffelke B, Campbell M, Hewitt C (2005) Reproductive phenology of the introduced kelp Undaria pinnatifida (Phaeophyceae, Laminariales) in Tasmania, Australia. Phycol 44(1):84–94
Silva P, Woodfield R, Cohen A, Harris L, Goddard J (2002) First report of the Asian kelp Undaria pinnatifida in the northeastern Pacific Ocean. Biol Invasions 4:333–338
Sinner J, Forrest B, Taylor M (2000) A strategy for managing the Asian kelp Undaria: final report. Cawthron Report No. 578. Cawthron Institute, New Zealand
Stuart M (2004) Review of research on Undaria pinnatifida in New Zealand and its potential impacts on the eastern coast of the South Island. Department of Conservation Internal Series 166, New Zealand
Stuart M, Hurd C, Brown M (1999) Effects of seasonal growth rate on morphological variation of Undaria pinnatifida (Alariaceae, Phaeophyceae). Hydrobiologia 398/399:191–199
Thompson G (2004) Mechanisms of invasion and persistence of the invasive kelp Undaria pinnatifida (Harvey) Suringar within intertidal areas of southern New Zealand. PhD Dissertation, University of Canterbury, New Zealand
Thornber C, Kinlan B, Graham M, Stachowicz J (2004) Population ecology of the invasive kelp Undaria pinnatifida in California: environmental and biological controls on demography. Mar Ecol Prog Ser 268:69–80
Uwai S, Nelson W, Neill K, Wang WD, Aguilar-Rosas L, Boo SM, Kitayama T, Kawai H (2006) Genetic diversity in Undaria pinnatifida (Laminariales, Phaeophyceae) deduced from mitochondria genes–origins and succession of introduced populations. Phycol 45:687–695
Valentine J, Johnson C (2003) Establishment of the introduced kelp Undaria pinnatifida in Tasmania depends on disturbance to native algal assemblages. J Exp Mar Biol Ecol 295:63–90
Wallentinus I (1999) Undaria pinnatifida (Harvey) Suringar. In: Gollasch S, Minchin D, Rosenthal H, Voight M (eds) Case histories on introduced species: their general biology, distribution, range expansion and general impact, pp. 13–19. Department of Fishery Biology, Institute for Marine Science, University of Kiel, Germany
Wotton D, O’Brien C, Stuart M, Fergus D (2004) Eradication success down under: heat treatment of a sunken trawler to kill the invasive seaweed Undaria pinnatifida. Mar Pollut Bull 49:844–849
Acknowledgements
We thank all of the divers and field assistants involved in this project over the years, in particular Louise Kregting, Daniel Pritchard, Stewart Bell and Wallace Russell. Thanks also to Peter Bannister and Daphne Lee for constructive advice on experimental design and manuscript preparation. We also thank MetOceans Solutions Ltd for access to wave data. This research was supported by the J.S. Watson Conservation Award from the Royal Forest and Bird Protection Society of New Zealand and the Tennant Bequest Fund, Department of Botany, University of Otago.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russell, L.K., Hepburn, C.D., Hurd, C.L. et al. The expanding range of Undaria pinnatifida in southern New Zealand: distribution, dispersal mechanisms and the invasion of wave-exposed environments. Biol Invasions 10, 103–115 (2008). https://doi.org/10.1007/s10530-007-9113-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-007-9113-1