Abstract
Objective
To introduce the Cre-loxP system for constructing marker-less multiple-gene deletion mutants in Pectobacterium, overcoming limitations of antibiotic markers and enhancing the understanding of pathogenic mechanisms.
Results
Firstly, a plasmid named pEX18-Cre, containing a sacB sucrose suicide gene, was constructed to express Cre recombinase in Pectobacterium. Secondly, a mutant in which the loxP-Km fragment replaced the target gene was obtained through homologous recombination double-crossover with the chromosome. Finally, pEX18-Cre was introduced into the mutant to excise the DNA between the loxP sites, thereby removing the markers and achieving multiple gene deletions. By utilizing the Cre-loxP system, we successfully constructed multiple marker-less gene deletion mutants in Pectobacterium strains.
Conclusions
The Cre-loxP system efficiently creates marker-less multiple-gene deletion mutants, enhancing the study of Pectobacterium pathogenic mechanisms by overcoming antibiotic marker limitations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The strains from Pectobacterium carotovorum are devastating plant pathogens that affect a variety of crops, vegetables, and ornamentals, causing wilt, rot, and black-leg (Gijsegem et al. 2021). To understand the molecular pathogenic mechanism of Pectobacterium, genetic manipulation is often the initial step, as it is in many other pathogens, and there are usually sorts of genes linked with pathogenicity, but we lack effective techniques available for deleting multiple genes other than homologous recombination with different antibiotic markers in P. carotovorum, which can help us obtain single- or two-gene deletion mutations. However, the antibiotic markers might result in some unexpected effects on our understanding of the pathogenicity. The main challenge is the inability to perform homologous double-crossover without resistance fragments.
Cre recombinase, discovered in phage P1 in 1981, belongs to the λInt supergene family (Sternberg et al. 1981; Hoess et al. 1984; Yarmolinsky & Hoess. 2015). This 38 kDa monomer protein is composed of 343 amino acids and possesses catalytic activity. It can specifically recognize 34 bp loxP sequences to facilitate the deletion or recombination of gene sequences between loxP sites (Yarmolinsky & Hoess. 2015). Cre recombinases can act on DNA substrates with various structures, including linear, circular, and superhelix DNA, without the need for cofactors. The loxP sequence (locus of X-over P1) is composed of two 13 bp inverted repeats and 8 bp spacers, with the spacers determining the direction of loxP. During catalytic DNA strand exchange, Cre covalently binds to DNA, with the 13 bp inverted repeat serving as the binding domain of the Cre enzyme.
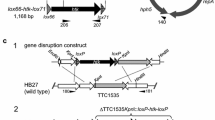
When two loxP sites are present in the cell genome, the presence of Cre recombinase induces sequence recombination between these sites. The outcome of recombination is determined by the orientation of the two loxP sites. If the two loxP sites are located on the same DNA strand, the Cre recombinase can effectively delete the sequence between them, as depicted in Fig. 1. However, after recombination, two loxP sites would still remain that still exhibit high affinity for Cre recombinase. Therefore, in this study, we utilized two mutant loxP sites (lox71 and lox66) to replace the original loxP site. The sequences of these mutant sites are provided in Fig. 1A.
Cre-loxP system application in Pectobacterium. A Sequences of the wild loxP and its mutants. The loxP is original sequence. lox71 and lox66 are loxP variants having 5 bp changed in the left and right elements, respectively. The mutated sequences are underlined. After modification by Cre recombination, the lox71-Km-lox66 fragment will turn to be the lox72 site that contains mutations in both repeats and exhibits reduced affinity for Cre recombinase. B Markerless multiple gene knock out method schematic in Pectobacterium. The recombinant plasmid will be chemically transformed into E. coli S17-1 competent cell using heat shock. After homologous gene fragments double crossover, we will obtain the mutant in which the loxP-Km fragment (lox71-Km-lox66 cassette) has replaced the target genes. pEX18-Cre plasmid then transferred into the mutant strains containing loxP-Km. A positive colony will not be able to survive on the LA (Rif + Km) selective plate because the loxP-Km fragment was deleted by Cre recombination. The above-mentioned strains were inoculated into LB medium without sucrose and cultured for 20 h in a shaker at 28 °C. A 1:100 dilution of the overnight cultured bacteria solution was transferred to fresh LB medium containing 5% sucrose. A sterilized inoculation ring was dipped into the bacterial solution from the previous step and spread on LA (Rif + 10% sucrose) resistant selective medium to facilitate shedding of the vector. The obtained single colony was verified by Gm, and the resistant medium containing both Gm and Km was used for reverse screening. Finally, a mutant strain with the Km resistance fragment deleted and no Cre expression vector was obtained
Previous research has demonstrated the extensive utilization of the Cre-loxP system for multiple gene deletion in various organisms such as mice, plants, yeast, and pathogenic bacteria (Banerjee & Biswas. 2008; Guan et al. 2017; Sauer & Henderson. 1988; Kos. 2004; Chen et al. 2017; Liang et al. 2018). However, its application in Pectobacterium strains has not been reported yet. In this study, we firstly introduced the Cre-loxP system to construct marker-less multiple-gene deletion mutants in Pectobacterium strains.
Materials and methods
Bacterial strains and growth conditions
All the Pectobacterium strains and derivate strains were cultured in Luria–Bertani (LB) medium (Wang et al. 2016) with rifampicin (Rif) at 100 μg·mL−1 at 28 °C, and Escherichia coli containing pEX18 vectors in LB medium with gentamicin (Gm) at 50 μg·mL−1 at 37 °C.
Cre expression plasmid construction
The plasmids and synthetic oligonucleotides utilized in this study are documented in Table 1 and Table 2, respectively. The plasmid pEX18Gm is a reliable vector containing the sacB sucrose suicide gene, while cre is transcribed autonomously by the robust constitutive E. coli promoter Plpp. Subsequently, we obtained the Cre expression plasmid, pEX18Gm-Cre (Fig. 1B), which exhibits potent Cre expression in Pectobacterium and can be eliminated through sucrose reverse selection.
Preparation of Pectobacterium strains genomic DNA
Bacterial DNA genome extraction using the Bacterial Genomic DNA Extraction Kit (TIANGEN, CN), detail steps as below (Wang et al. 2016).
Construction of plasmids for gene deletion containing a lox71-Km-lox66 cassette
Based on homologous recombination, the upstream and downstream fragments of the target genes are supposed to double-crossover with chromosome. We constructed a plasmid that contained both the upstream and downstream fragments of the target genes, as well as a lox71-Km-lox66 cassette. So, we can get a mutant that lox71-Km-lox66 cassette replaced the target gene. The upstream and downstream fragments were amplified from Pectobacterium strains genomic DNA, while the lox71-Km-lox66 cassette was amplified from pET30a. After restriction and ligation, the upstream and downstream fragments, along with the lox71-Km-lox66 cassette, were successfully cloned into pEX18. The resulting ligated product was then chemically transformed into E. coli DH5α competent cells using heat shock. The primers used for this process can be found in Table 2.
lox71-Km-lox66 marker replace target gene
The recombinant plasmid will be chemically transformed into E. coli S17-1 competent cell using heat shock. The LB plates containing X-gal (100 µg·mL−1), Gm (100 µg·mL−1), and Km (100 µg·mL−1) were utilized to select the positive colony. Subsequently, we mixed the wild type strain with E. coli S17-1, and performed conjugation transfer using E. coli S17-1 as the donor to introduce the plasmid into PccS1.
To identify strains with double exchange homologous recombination events, the trans conjugants were plated on LA medium containing 10% sucrose and Km (100 µg·mL−1). In this manner, we obtained the mutant in which the lox71-Km-lox66 cassette has replaced the target genes.
Removal of antibiotic marker
Escherichia coli S17-1 containing pEX18-Cre and the mutant strains were concentrated and mixed at a 1:1 ratio. The mixture was then dropped onto the filter membrane of an LB (Rif + Gm) resistant plate and incubated at 28 °C for 20 h. Next, the concentrated, mixed culture strains were eluted onto an LA (Rif + Gm) medium. Individual colonies on the plate were selected for PCR verification. Using a toothpick, the verified colonies were dipped onto both the LA (Rif + Km) and LA (Rif + Gm) selective plates. A positive colony could not survive on the LA (Rif + Km) selective plate because the loxP-Km-loxP cassette was deleted by Cre recombination.
Eliminating the Cre expression plasmid through sucrose reverse selection
The above-mentioned strains were inoculated into LB medium without sucrose and cultured for 20 h in a shaker at 28 °C. A 1:100 dilution of the overnight cultured bacteria solution was transferred to fresh LB medium containing 5% sucrose and cultured in a shaker at 28 °C. The transfer to fresh LB medium with 5% sucrose was repeated and cultured in a shaker at 28 °C when the bacteria solution showed turbidity. A sterilized inoculation ring was dipped into the bacterial solution from the previous step and spread on LA (Rif + 10% sucrose) resistant selective medium to facilitate shedding of the vector. The obtained single colony was verified by Gm, and the resistant medium containing both Gm and Km antibiotics was used for reverse screening. Finally, a mutant strain with the Km resistance fragment deleted and no Cre expression vector was obtained. Based on this principle, this study modified the strong promoter Plpp of vector pEX18 carrying sacB sucrose sensitive gene, so as to construct Cre expression vector that can be normally expressed in Pectobacterium strains.
Virulence assay
Virulence assay of the bacteria in the host plant was performed as previously described (Wang et al. 2018; Jiang et al. 2017).
Southern blot
Southern blot of the bacteria in the host plant was performed as previously described (Guo et al. 2013). The genomic DNA of wild-type and mutant strains was extracted for testing. Specific digestion sites containing the target gene were selected, the extracted genome was enzymatically digested and electrophoresed. Then, the electrophoretic band was transferred to a nylon membrane. The membrane was stained and photographed for analysis.
Result
Multiple-markerless gene deletion in Pectobacterium carotovorum
In this study, we aimed to investigate the feasibility of using this system for obtaining marker-less gene deletion mutants in Pectobacterium. To achieve this, we modified the strong promoter Plpp into vector pEX18, which carries the sacB fragment, a gene of sucrose sensitive for selection, to construct Cre expression vector that can be normally expressed in PccS1 strain (Fig. 1B).
PccS1 strain harbors a total of 5 homologous vgrGs, and we selected three of them (PccS1_0112, PccS1_0181 and PccS1_3542) as examples for gene deletion using the Cre-loxP system. Initially, we employed the traditional homologous exchange method, where we exchanged kanamycin resistance fragments (Km) and target genes of the homologous genes to obtain mutants containing the lox71-Km-lox66 (loxP-Km) fragment (Fig. 1B). Subsequently, we introduced the pEX18-Cre plasmid into the mutants to delete the loxP-Km fragment and then obtained maker-less mutant with lox72 site. In the later stage of this method, the vector was eliminated by adding sucrose into the medium (5% for LB, 10% for LA) for reverse screening, enabling to obtain marker-less target gene deletion mutants without the pEX18-Cre plasmid.
PCR and southern blot verification demonstrated the successful deletion of the loxP-Km fragment from the mutants (Fig. 2AB), and sequencing analysis further confirmed that the loxP-Km fragment was indeed deleted. These results clearly indicate the specific removal of the antibiotic marker through the Cre-loxP recombination system in vivo. Consequently, we were able to successfully delete three selected genes from the chromosome.
PCR, Southern blot and virulence verification of Pectobacterium carotovorum and the mutants. A PCR analyses of single and double genes deletion in P. carotovorum using the Cre-loxP system. M, the maker; −, negative control; + , positive control. B Genomic southern blot analysis on P. carotovorum wild type strain and mutant Δ0112/0181::lox72. The genomic DNA of wild-type and mutant strains to be tested was extracted, specific digestion sites containing the target gene to be tested were selected, the extracted genome was enzymatically digested and electrophoresed, and then the electrophoretic band was transferred to a nylon membrane. The membrane was stained in the color and photographed for analysis. C Virulence assay. Images present tissue macerations caused by inoculation of P. carotovorum subsp. carotovorum strains PccS1 and the strains with a mutation in vgrGs with or without loxP-Km fragment onto the detached Chinese cabbage. The strains cultured overnight were transferred to fresh LB medium, ddH2O suspension adjusted OD600 = 1.0, and fresh Chinese cabbage (Brassica rapa subsp. pekinensis) petiole parts were inoculated, competitively cultured at 28 °C for 16 h, the lesion sites were measured and photographed. Bar, 0.5 cm. D Bars represent the maceration areas measured 16 HAI (hours after injection), (****P < 0.0001, ns, not statistically significant, versus the wild-type PccS1). Statistical analyses are carried out by GraphPad software and assessed by one-way ANOVA, followed by Dunnett multiple comparisons test post-hoc test
In terms of functional study, whether the lox72 site affects the pathogenicity of the mutant is an important question. Therefore, virulence assay on Chinese cabbage was conducted. The result shows that mutant containing lox72 residue macerate host plants at a level similar to the mutants containing lox71-Km-lox66 cassette, (Fig. 2C and D). Furthermore, when three genes were deleted and virulence was significantly decreased compared to the wild-type strain (Fig. 2C and D), triple-gene deletion mutant containing the lox72 residue shows pathogenicity similar to the mutants containing the lox71-Km-lox66 cassette. All these results indicate that loxP-Km fragment deletion by Cre will not affect pathogenicity.
Multiple-markerless gene deletion in Pectobacterium brasiliense
To further explore whether this method is feasible in other strains of different species in the genus of Pectobacterium, we performed a multi-gene deletion based on the Cre-loxP system in P. brasiliense (Pcb). PCR verification shows that double genes (Pcb_1260 and Pcb_0092) were successfully mutated and then loxP-Km fragment was deleted (Fig. 3A).
PCR and virulence verification of Pectobacterium brasiliense and the mutants. A PCR analyses of single- and double gene deletion strains from the wild type of P. brasiliense using the Cre-loxP markerless gene-deletion system. M, Maker; −, negative control; + , positive control. B Virulence assay. Images present tissue macerations caused by inoculation of P. brasiliense strains and the mutants with or without loxP-Km fragment onto the detached Chinese cabbage. The strains cultured overnight were transferred to fresh LB medium, ddH2O suspension adjusted OD600 = 1.0, and fresh Chinese cabbage (Brassica rapa subsp. pekinensis) petiole parts were inoculated, competitively cultured at 28 °C for 16 h, the lesion sites were measured and photographed. Bar, 0.5 cm. C Bars represent the maceration areas measured 16 HAI (hours after injection), (**P < 0.01, ns, not statistically significant, versus the wild-type PccS1). Statistical analyses are carried out by GraphPad software and assessed by one-way ANOVA, followed by Dunnett multiple comparisons test post-hoc test
On the other hand, the virulence assay also indicated that loxP-Km fragment deletion by Cre did not affect pathogenicity in Pcb (Fig. 3B and C). These results demonstrate that multiple genes deletion method is functional in Pcb.
Discussion
Pectobacterium is a devastating plant pathogen that affects a variety of crops, vegetables, and ornamentals, causing wilt, rot, and black tibia in roots, stems, leaves, and fruit (Gijsegem et al. 2021). However, until recently, there was no effective technique available for deleting multiple genes in P. carotovorum. The main challenge was the inability to perform homologous double-crossover without resistance fragments. In our previous study, we found that the likelihood of obtaining a homologous double-crossover mutant without an antibiotic marker was less than 0.1%. Although substituting target genes with resistance fragments can screen out double-crossover mutants effectively, it is not conducive to the deletion of multiple genes. This problem can be addressed effectively by Cre-loxP system.
The Cre-loxP system has been successfully applied in various bacteria, including Streptococcus and Bacillus, as an efficient method for deleting antibiotic markers (Banerjee & Biswas. 2008; Guan et al. 2017).
While we have developed a multiple-gene deletion system based on the Cre-loxP system and homologous double-crossover, there are still some limitations. Although the Cre-loxP system is user-friendly, it does extend the time required for gene deletion by one week. Another limitation is the use of loxP sites, as directly deleting the target gene between loxP sites results in a loxP site remaining in the mutant after Cre-mediated deletion. If original loxP-Km-loxP is used for gene deletion, there will be two loxP sites in the second gene deletion strain, which may cause confusion for Cre recognition.
To overcome these limitations, in the present study, we recruited variant lox66 and lox71 to replace the original loxP site surrounding the antibiotic marker (Lambert et al. 2007; Albert et al. 1995; Arakawa et al. 2001). Lox66 and lox71 are variant forms of loxP (Fig. 1A), with five bases changed at the right- and left- elements of loxP respectively. By employing Cre recombination to recognize and mediate the lox71 and lox66 sites, we achieved the loxP double mutant in the lox72 site, containing mutations in both repeats and exhibiting reduced affinity for Cre recombinase as previous described (Albert et al. 1995).
Our method solves the most fundamental problem of marker-less homologous double-crossover and lays a good foundation for further functional studies of homologous genes.
This study presents the construction of a Cre-loxP system for marker-less gene deletion in P. carotovorum, which is the first application of this system in Pectobacterium (Fig. 1). Additionally, we have successfully used the Cre-loxP system to remove antibiotic markers in P. brasiliense, resulting in a double mutant (Fig. 2E). Furthermore, whether or not the deleted gene affects pathogenicity, lox72 has no effect on the results of the experiment (Fig. 2C and D; Fig. 3B and C). Additionally, the significant decrease of pathogenicity after triple-gene deletion also reflects the necessity of researching the function of homologous genes (Fig. 2C and D). These findings suggest that the functionality of the Cre-loxP system extends beyond P. carotovorum and can be applied to other species of Pectobacterium. To the best of our knowledge, this is the first study reporting the use of the Cre-loxP system for marker-less gene deletion analysis in Pectobacterium. We believe that this approach will help us make more progress in the study of multi-gene function of Pectobacterium.
References
Albert H, Dale EC, Lee E, Ow DW (1995) Site-specific integration of DNA into wild-type and mutant Lox sites placed in the plant genome. Plant J 7:649–659
Arakawa H, Lodygin D, Buerstedde JM (2001) Mutant loxP vectors for selectable marker recycle and conditional knock-outs. BMC Biotechnol 1:7
Banerjee A, Biswas I (2008) Markerless multiple-gene-deletion system for Streptococcus mutans. Appl Environ Microbiol 74:2037–2042
Chen H, Luo J, Zheng P, Zhang X, Zhang C, Li X et al (2017) Application of Cre-lox gene switch to limit the Cry expression in rice green tissues. Sci Rep 7:14505
Gijsegem FV, Wolf J, Toth IK (2021) Plant diseases caused by dickeya and pectobacterium species. Plant diseases caused by dickeya and pectobacterium species. Springer, Berlin
Guan ZB, Wang KQ, Shui Y, Liao XR (2017) Establishment of a markerless multiple-gene deletion method based on Cre/loxP mutant system for Bacillus pumilus. J Basic Microbiol 57:1065–1068
Guo P, Wang Y, Zhou X, Xie Y, Wu H, Gao X (2013) Expression of soybean lectin in transgenic tobacco results in enhanced resistance to pathogens and pests. Plant Sci 211:17–22
Hoess R, Abremski K, Sternberg N (1984) The nature of the interaction of the P1 recombinase Cre with the recombining site loxP. Cold Spring Harb Symp Quant Biol 49:761–768
Jiang H, Jiang M, Yang L, Yao P, Ma L, Wang C et al (2017) The ribosomal protein RplY is required for Pectobacterium carotovorum virulence and is induced by Zantedeschia elliotiana extract. Phytopathology 107:1322–1330
Kos CH (2004) Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev 62:243–246
Lambert JM, Bongers RS, Kleerebezem M (2007) Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl Environ Microbiol 73:1126–1135
Liang WF, Sun MY, Cui LY, Zhang C, Xing XH (2018) Cre/loxP-mediated multicopy integration of the mevalonate operon into the genome of Methylobacterium extorquens AM1. Appl Biochem Biotechnol 185:565–577
Sauer B, Henderson N (1988) Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA 85:5166–5170
Sternberg N, Hamilton D, Austin S, Yarmolinsky M, Hoess R (1981) Site-specific recombination and its role in the life cycle of bacteriophage P1. Cold Spring Harb Symp Quant Biol 45(Pt 1):297–309
Wang H, Yang ZL, Du S, Ma L, Liao Y, Wang YJ et al (2016) Characterization of Pectobacterium carotovorum proteins differentially expressed during infection of Zantedeschia elliotiana in vivo and in vitro which are essential for virulence. Mol Plant Pathol 19:35–48
Wang H, Yang Z, Du S, Ma L, Liao Y, Wang Y et al (2018) Characterisation of Pectobacterium carotovorum proteins differentially expressed during infection of Zantedeschia elliotiana in vivo and in vitro which are essential for virulence. Mol Plant Pathol 19:35–48
Yarmolinsky M, Hoess R (2015) The legacy of nat sternberg: the genesis of Cre-lox technology. Annu Rev Virol 2:25–40
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2600602); The National Nature Science Foundation of China (32302321). Science and Technology Foundation Project of Suzhou (SNG2020045). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the experimental design, hands on work, discussions, and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Che, S., Zhuo, Y., Yang, L. et al. Multiple genes deletion based on Cre-loxP marker-less gene deletion system for the strains from the genus of Pectobacterium. Biotechnol Lett (2024). https://doi.org/10.1007/s10529-024-03518-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10529-024-03518-8