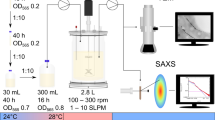
Abstract
Objectives
Developing a simplified flask fermentation strategy utilizing magnetotactic bacterium AMB-1 and optimized iron supplementation for high-yield magnetosome production to address the challenges associated with magnetosome acquisition.
Results
A reliable processing for the pure culture of AMB-1 was established using standard laboratory consumables and equipment. Subsequently, the medium and iron supplementation were optimized to enhance the yield of AMB-1 magnetosomes. The mSLM supported higher biomass accumulation in flask fermentation, reaching an OD565 of ~ 0.7. The premixed solution of ferric quinate and EDTA-Fe (at a ratio of 0.5:0.5 and a concentration of 0.4 mmol/L) stabilized Fe3+ and significantly increased the reductase activity of AMB-1. Flask fermentations with an initial volume of 15 L were then conducted employing the optimized fermentation strategy. After two rounds of iron and nutrient supplementation, the magnetosome yield reached 185.7 ± 9.5 mg/batch (approximately 12 mg/L), representing the highest AMB-1 flask fermentation yield to our knowledge.
Conclusion
A flask fermentation strategy for high-yield magnetsome production was developed, eliminating the need for bioreactors and greatly simplifying the process of magnetosome acquisition.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetotactic bacteria are a distinct type of microorganism that biomineralizes iron within cells to generate magnetic intracellular organelles called magnetosomes. An individual magnetosome consists of a magnetite (Fe3O4) or greigite (Fe3S4) core enveloped by a plasma membrane, and multiple magnetosomes align in chains, clusters, or disperse as single particles within the cells (Schüler 2008). Magnetosomes endow magnetotactic bacteria with a permanent magnetic dipole that enables them to migrate through the geomagnetic field toward the oxic-anoxic transition zone with an optimal oxygen concentration for redox reactions (Li et al. 2021). The magnetic cores, predominantly composed of Fe3O4 nanoparticles, feature a homogeneous size distribution, consistent composition, and single-domain or superparamagnetic crystallization (Faivre and Schüler 2008). These attributes make magnetosomes highly efficient nanobiomaterials for generating heat under high-frequency alternating magnetic fields (HF-AMF) and excellent agent for cancer hyperthermia treatment (Céspedes et al. 2014; Plan Sangnier et al. 2018; Alphandéry 2020a; Gandia et al. 2019). Moreover, the plasma membrane surrounding the magnetic core contains abundant active functional groups and prevents direct exposure of the magnetic nanoparticles to cells and tissues, allowing for various surface modifications and ensuring excellent biocompatibility (Raguraman and Suthindhiran 2020; Nan et al. 2021; Mickoleit et al. 2021). Hence, magnetosomes represent a biologically originated magnetic nanomaterial with promising biotechnological and biomedical applications.
Although magnetotactic bacteria are widely distributed in aquatic environments, constituting up to 30% of the total microbial biovolume in certain habitats, their cultivation presents numerous challenges due to strict requirements for nutrients and cultivation (Spring et al. 1993). Only a limited number of model strains of magnetotactic bacteria are readily available through authoritative culture collections, along with recommended culture media, which facilitates their stable pure culture in laboratory settings (Liu et al. 2008; Alphandéry 2020b). Magnetospirillum magneticum strain AMB-1 (AMB-1) and Magnetospirillum gryphiswaldense strain MSR-1 (MSR-1) stand out as the most widely utilized among these model strains.
Currently, the functional design of magnetosomes in biomedical science is still in its early stages. The fastidious cultivation of magnetotactic bacteria and the associated low yield of magnetosomes pose significant barriers, limiting research and applications in this field (Ren et al. 2023). For instance, in the application of AMB-1-produced magnetosomes in tumor hyperthermia treatment, the intratumoral injection of 1 mg of magnetosomes into murine xenografted breast tumors (approximately 100 mm3) led to a rapid temperature elevation to 43 °C under HF-AMF. After three treatment cycles, magnetosome-mediated hyperthermia significantly reduced tumor volume or completely eliminated tumors in the tested mice (Alphandery et al. 2011). However, expanding biomedical applications to intravenously administered magnetosomes (e.g., 100 µg/g body weight) or assessing their efficacy in larger animal models (rats, rabbits, beagles, etc.) requires greater magnetosome production. Another example is the chemical modification of magnetosomes, which necessitates accurate weighing and chemical identification, demanding at least tens of milligrams of magnetosomes. Previous studies have predominantly focused on optimizing culture medium nutrition, oxygen levels, iron concentrations, etc., in bioreactors equipped with various sensors to facilitate wild or gene-edited bacterial growth and magnetosome synthesis (Alphandéry 2020b; Fernández-Castané et al. 2018). However, the use of bioreactors introduces economic and technical challenges, particularly for material science laboratories embarking on or planning magnetosome-related research. Therefore, the development of a simple and cost-effective fermentation method achieving high-yield magnetosomes is crucial.
In this study, we employed the readily available magnetotactic bacterium AMB-1 as the model strain and conducted a comparative analysis of the effects of modified magnetic spirillum growth medium (mMSGM), whose primary composition aligns with the recommendations of culture collections and closely resembles the suggested medium for MSR-1, and modified sodium lactate medium (mSLM) on the growth density of AMB-1 in common glass bottles. Our findings revealed that mSLM promoted a greater AMB-1 biomass accumulation in flask fermentation. Recognizing the close association between magnetosome synthesis and iron, we adjusted the parameters of the iron source, including its composition and concentration, to simplify iron supplementation and enhance magnetosome biomineralization. This involved a strategic combination of ferric quinate and sodium ferric ethylenediaminetetraacetate (EDTA-Fe), resulting in a stable increase in soluble ferric ions (Fe3+) in the medium. Intriguingly, this refined iron supplementation enhanced the reductase activity of AMB-1, facilitating the biomineralization process. Ultimately, employing the optimized medium and iron source gained from small-scale cultivation, we achieved high-yield magnetosome production in 20 L glass reagent bottles. This fermentation process required only initial inoculation and two feedings, eliminating the need for expensive automated bioreactors and complex operations. With an initial medium volume of 15 L, we achieved a yield of 185.7 ± 9.5 mg/batch (~ 12 mg/L), marking the highest magnetosome productivity reported to our knowledge in the flask fermentation of AMB-1 and comparable to that obtained by bioreactors(de Souza Cabral et al. 2023).
Materials and methods
Chemicals and microbial strain
EDTA-Fe was purchased from Tianjin Bodi Chemical Co., Ltd (Tianjin, China). The cell counting kit-8 (CCK-8) was purchased from MeilunBio Co., Ltd. (Dalian, China). Yeast extract was purchased from Solarbio Science & Technology Co., Ltd. (Beijing, China). Sodium selenite (Na2SeO3) was purchased from Merck KGaA (Darmstadt, Germany). 1,10-Phenanthroline was purchased from Acmec Biochemical Technology Co., Ltd. (Shanghai, China). Other chemicals were purchased from Macklin Biochemical Co., Ltd. (Shanghai, China), unless otherwise specified. The magnetotactic bacterium strain AMB-1 was purchased from the American Type Culture Collection (ATCC No. 70264; Manassas, USA).
Culture medium preparation
The mMSGM and mSLM consisted of a sterilized basal medium, ferric solution, trace mineral element solution, and vitamin solution. The composition and preparation of the media were obtained by slightly modifying the chemical compositions, concentrations, and sterilization processes based on the methods recommended by the strain collections (https://www.atcc.org/; https://www.dsmz.de/) and previous studies (Liu et al. 2008). The basal mMSGM (MSGM-base) comprised the following components per liter: 0.68 g KH2PO4, 0.12 g NaNO3, 0.035 g ascorbic acid, 0.37 g tartaric acid, 0.37 g succinic acid, 0.05 g sodium acetate, and 1.0 L distilled water. The basal mSLM (mSLM-base) comprised the following components per liter: 2.60 g sodium lactate (60% aqueous solution), 0.40 g NH4Cl, 0.50 g KH2PO4, 0.10 g MgSO4·7H2O, 0.10 g yeast extract, 0.05 g sodium thiosulfate and 1.0 L distilled water. The basal mSLM could be prepared as a 15 × concentrated medium (15 × mSLM-base) by dissolving the solutes at 15 times the above amounts in 1.0 L distilled water. The pH of the basal medium was adjusted to 6.8 with NaOH solution and dispensed into 1 L glass reagent bottles. Note that the glass reagent bottles mentioned in this article refer specifically to commonly used laboratory glassware manufactured by DWK Life Sciences (Rockwood, USA) or Shuniu (Chongzhou, China), featuring standard GL45 threading and blue or orange polypropylene caps. The caps were initially screwed on loosely, and several layers of disposable non-woven fabric were used to envelop the caps and threads of the bottle. After autoclaving at 121 °C for 20 min, the caps of the glass reagent bottles were tightened to obtain sterilized basal media. The ferric solutions used in this study were 0.2 mol/L ferric quinate solution and 0.2 mol/L EDTA-Fe solution, respectively. The 0.2 mol/L ferric quinate solution was prepared by dissolving 5.40 g FeCl3·6H2O and 3.80 g quinic acid in 100 mL distilled water. The 0.2 mol/L EDTA-Fe solution was obtained by dissolving 7.35 g EDTA-Fe powder in 100 mL distilled water. The trace mineral element solution contained the following components: 15.0 g nitrilotriacetic acid, 30.0 g MgSO4·7H2O, 5.50 g MnSO4·H2O, 10.0 g NaCl, 1.00 g FeSO4·7H2O, 1.00 g CaCl2·2H2O, 1.80 g ZnSO4·7H2O, 0.10 g CuSO4·5H2O, 0.20 g KAl(SO4)2·12H2O, 0.10 g H3BO3, 0.10 g Na2MoO4·2H2O, 1.80 g CoSO4·7H2O, 0.25 g NiCl2·6H2O, 0.003 g Na2SeO3·5H2O and 2.0 L distilled water. During the preparation of the trace mineral element solution, nitrilotriacetic acid was first added, and the pH value was adjusted to 6.5 with 1 mol/L KOH. Subsequently, the other trace mineral elements were dissolved and the pH of the solution was adjusted to 6.8. The vitamin solution contained the following components: 20 mg biotin, 20 mg folic acid, 100 mg pyridoxine hydrochloride, 50 mg thiamine hydrochloride, 50 mg riboflavin, 50 mg nicotinic acid, 50 mg calcium pantothenate, 1.0 mg cobalamins, 50 mg p-aminobenzoic acid, 50 mg thioctic acid and 1.0 L distilled water. The vitamin solution could be heated to 60 °C to facilitate dissolution. The 0.2 mol/L ferric solutions, trace mineral element solution, and vitamin solution were sterilized through a 0.22 μm sterile filtration system (Bioland #PES90-S1000, Hangzhou, China) under aseptic laminar flow of a clean bench (Medbase #BBS-DDC, Jinan, China). The aforementioned sterile filtrated solutions can be aliquoted into 50 mL sterile centrifuge tubes (Corning #430828, Corning, USA) and stored in the dark. Before use, the mMSGM or mSLM was prepared by mixing sterile syringe filter (Millipore # SLGV033RB, Darmstadt, Germany) filtered solutions, 0.1 mL of ferric solution, 1 mL of trace mineral element solution, and 1 mL of vitamin solution, into each liter of the basal medium.
Resuscitation, subculture and identification of AMB-1
Resuscitation, inoculation, and subculture were performed under aseptic laminar flow to prevent any risk of contamination. The AMB-1 strain, purchased from ATCC, had been securely stored as a frozen stock at -80 °C. Prior to the first inoculation, the frozen AMB-1 stock was thawed, and 0.5 mL of the stock was carefully transferred to a 15 mL sterile centrifuge tube (Corning #430791, Corning, USA). Subsequently, 13 mL of standard mMSGM was added, and the tube was sealed with the screw cap. The mixture was then incubated at room temperature (22–25 °C unless otherwise specified) for 14 days until a turbid AMB-1 culture was successfully obtained. The resuscitated AMB-1 culture was then transferred to a sterilized 250 mL glass reagent bottle supplemented with 190 mL of mMSGM. The bottle was securely sealed with the cap, enveloped with several layers of non-woven fabrics, and incubated at room temperature for an additional 14 days to complete the resuscitation process.
For the first subculture, each 50 mL of the resuscitated AMB-1 culture was combined with 750 mL of mMSGM in a 1 L sterilized glass reagent bottle, followed by a 14-day incubation at room temperature. In subsequent subcultures, the AMB-1 cultures were diluted with mMSGM at a ratio of 1:10 to 1:20 and incubated at room temperature to obtain larger culture volumes. During subsequent subcultures, samples of the AMB-1 culture were collected for magnetic attraction and transmission electron microscope (TEM) observation. Additionally, designated samples were quarterly collected and dispatched to accredited companies (Tsingke Biotechnology Co., Ltd., Beijing, China; RuiBiotech Co., Ltd., Beijing, China) for 16S rRNA gene sequencing. The sequencing results were input into the Basic Local Alignment Search Tool (BLAST) on the National Center for Biotechnology Information website (NCBI, http://www.ncbi.nlm.nih.gov) to identify the cultured AMB-1 strain.
Cell density measurement of AMB-1 in mMSGM and mSLM
Under aseptic laminar flow, 50 mL of AMB-1 culture and 750 mL of sterilized mMSGM or mSLM media were dispensed into sterilized 1 L glass reagent bottles. The caps were loosely screwed, and the bottle openings were fully enveloped by several layers of sterilized non-woven fabric. The cultures were then incubated at room temperature with shaking (60 RPM) in an incubator shaker (Crystal Technology & Industries #IS-RDD3A, Addison, USA) for 14 days. Throughout the cultivation, 25 mL samples were taken on the 1st, 3rd, 5th, 8th, 10th, 12th and 14th days after inoculation. These samples were added to quartz cuvettes (path length = 10 mm; Agilent #6618000100, Santa Clara, USA), and the optical density (OD) at 565 nm (OD565) of the AMB-1 cultures was measured using a UV‒visible spectrophotometer (Agilent #Cary 60 UV‒Vis, Santa Clara, USA), with the corresponding standard culture media as blank samples for baseline correction.
Determination of Fe3+ concentrations in mMSGM and mSLM
The mMSGM-base and mSLM-base were dispensed at 10 mL per tube into 15 mL sterile centrifuge tubes. Specific volumes of 0.2 mol/L ferric quinate or 0.2 mol/L EDTA-Fe were supplemented to obtain media with varying iron concentrations of 0, 0.02, 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, 1.0, 1.2, 1.4 and 1.6 mmol/L. The centrifuge tubes were allowed to stand at room temperature. On the 1st, 2nd, 4th, 6th, and 8th days after iron supplementation, the samples were thoroughly mixed, and 200 µL was collected into sterile microcentrifuge tubes (Axygen #MCT-150-C, Hangzhou, China). The microcentrifuge tubes were centrifuged at 18,000×g for 10 min to separate insoluble iron precipitates. The supernatants were collected and assayed for Fe3+ concentrations using the 1,10-phenanthroline chromogenic reaction (Jain et al. 2008).
For the assay, 10 μL of each sample was dispensed into 96-well plates. With a 10 min interval between each solution, 20 μL of freshly prepared 0.3 mol/L hydroxylamine hydrochloride, 40 μL of freshly prepared 1 mol/L sodium acetate, and 30 μL of freshly prepared 15 mmol/L 1,10-phenanthroline were sequentially added to each well. The absorbance at 510 nm (OD510) of each sample was quantified using a microplate reader (Shanpu #SuPerMax 3100, Shanghai, China). In parallel with the test samples, freshly prepared FeSO4 solutions with known concentrations underwent the same procedure to obtain OD510 values. This process aimed to establish a linear regression equation between the iron concentrations and the OD510 values. The Fe3+ concentrations in the mMSGM-base and mSLM-base were then calculated based on the regression equation and the OD510 values of the samples.
Determination of the relative reductase activity (RRA) of AMB-1 in high-iron mSLM
AMB-1 was cultivated in mSLM for 8 days to reach the late logarithmic growth phase. The culture was thoroughly mixed and dispensed into 50 mL sterile centrifuge tubes at 40 mL per tube (Corning #430828, Corning, USA). Allowing the tubes to stand for 2 h, the bacteria were collected by centrifugation at 4500×g for 15 min. Subsequently, 40 mL of mSLM-base was added to each centrifuge tube to resuspend the bacteria. The bacterial suspensions were combined and supplemented with trace mineral element solution and vitamin solution. The AMB-1 bacterial suspension was then dispensed into 15 mL centrifuge tubes at 10 mL per tube. After these operations, the initial cell density of AMB-1 in each tube was consistent. A premixed 0.2 mol/L iron solution was prepared by mixing 0.2 mol/L ferric quinate and 0.2 mol/L EDTA-Fe solution at ratios of 1:0, 0.75:0.25, 0.5:0.5, 0.25:0.75, and 0:1. Different volumes of the 0.2 mol/L iron solution were added to the tubes to achieve initial iron concentrations of 0.02, 0.1, 0.2, 0.3, 0.4, 0.6, 0.8, and 1.0 mmol/L. The mSLM medium containing 0.02 mmol/L ferric quinate was set as the control group. The blank groups had the same components and treatment as the experimental groups, except for the absence of AMB-1. Incubating all the samples at room temperature for the designated times, 100 μL aliquots were taken from each sample and added to 96-well plates, after which 10 μL of CCK-8 solution was added to each well. The plates were incubated at room temperature for 2–4 h. Absorbance at 450 nm was measured for the samples (OD450), control groups (ODcontrol), and corresponding blank groups (ODblank) using the microplate reader. The OD values of the samples and control groups were corrected by subtracting the blank group values, and the RRA was calculated as follows:
High-yield AMB-1 magnetosome fermentation in 20 L glass bottles and magnetosome extraction
The mSLM of 15 L was prepared in 20 L glass reagent bottles (Shuniu, Chongzhou, China) and autoclaved following aforementioned procedures. Subsequently, 500 mL of AMB-1, cultivated in mMSGM medium for 7 days, was aseptically inoculated into the 20 L glass reagent bottle. The bottle caps were loosely secured, and the bottle openings were enveloped with several layers of sterilized non-woven fabric. The cultures were placed on a horizontal shaker (Crystal Technology & Industries #IS-RDH1, Addison, USA) and incubated for 8 days at 60 RPM and room temperature to promote the biomass accumulation of AMB-1. On the 8th day, aseptically under laminar flow, 30 mL of a 0.2 mol/L premixed ferric solution (0.2 mol/L ferric quinate solution and EDTA-Fe solution mixed at a ratio of 0.5:0.5), along with 15 mL of trace mineral element solution, 15 mL of vitamin solution, and 1 L of 15 × mSLM-base, were added to the reagent bottle. The bottle was then sealed, enveloped, and shaken (60 RPM, room temperature) for the subsequent 6 days. On the 14th day, another round of premixed iron and nutrient supplementations identical to the earlier procedure was performed, and the AMB-1 cultures were incubated for an additional 6 days to complete magnetosome fermentation. Throughout the magnetosome fermentation phase (8–20 days), maintaining aseptic conditions aided by the non-woven fabrics, the bottle caps were rotated a few turns and tightened daily to restore internal pressure to atmospheric levels. A parallel control group was established mirroring the experimental setup, with the only deviation being the addition of 1.5 mL of 0.2 mol/L ferric quinate solution and nutrient supplementations in subsequent feedings on the 8th and 14th days.
Post-fermentation, 2 L of the obtained AMB-1 culture could be aseptically sampled to sterilized containers for subsequent fermentation or other experimental research. Magnetsome extraction and purification were performed by the following steps. The remaining AMB-1 culture was concentrated through the tangential flow filtration (Sartorius, Viva Flow 200, Göttingen, Germany). Subsequently, AMB-1 cells were collected by centrifugation at 15,000×g. The bacterial pellet was then dispersed in 1 mol/L sodium hydroxide solution at 25 mL per gram of wet weight, stirred, and heated to 80 °C for 2 h to thermally hydrolyse AMB-1 cells and separate the magnetosomes. Magnetosomes were magnetically attracted using a neodymium magnet (~ 300 mT) placed at the edge of the container, while the unattracted components (mainly cell debris and lysate) were discarded. The crude magnetosomes underwent an additional round of thermal hydrolysis for further purification. Finally, the purified magnetosomes were subjected to triple washing with distilled water and subsequently lyophilized.
Transmission electron microscope (TEM) observation
A TEM (JEM-1400, JEOL; Tokyo, Japan) was used to observe AMB-1 and intracellular magnetosomes. Samples of 4 mL were collected and centrifuged at 5,000 × g for 5 min at 4 °C. Following the removal of the supernatant, the bacterial pellets underwent three washes with distilled water and were then resuspended in 4 mL of distilled water. Subsequently, 10 μL of the suspension was carefully dropped on a 300-mesh copper grid, which was then left to air-dry at room temperature before being observed via TEM.
Statistical analysis
All experiments were performed in triplicate. The values in figures represent the mean or mean ± standard deviation (SD) of three independent experiments. Significant differences were identified by T-test and indicated by asterisks (P < 0.05).
Results and discussion
Medium preparation, cultivation, and verification of AMB-1
The demanding requirements of magnetotactic bacterium AMB-1 necessitate robust sterility assurance in both media preparation and operational procedures. In response, a dependable and simplified medium preparation method was developed to meet the sterile and nutritional requirements of the magnetotactic bacterium AMB-1. The sterilization process involves the autoclave of the basal medium and the sterile filtration of the ferric solutions, trace element solution, and vitamin solution. The preparation processes for mMSGM-base and mSLM-base were illustrated in Fig. 1a. To enhance sterility assurance, the neck of the container was enveloped with several layers of sterilized non-woven fabrics to effectively isolate the aseptic basal culture medium from the external environment. According to our practical experience, autoclaved basal media could be stored for at least 3 months in a non-aseptic environment without contamination, so ample basal media could be prepared at once without the need for repetitive autoclaving.
Simplified medium preparation and cultivation process of AMB-1. a The main process of preparing sterilized mMSGM-base and mSLM-base using glass reagent bottles and other commonly used laboratory consumables (disposable non-woven fabric, rubber bands, syringes, and sterile needle filters) in a non-aseptic environment. b Sterilized mMSGM and mSLM were obtained through sterile filtering of iron, trace element, and vitamin solutions for AMB-1 resuscitation and subculture. c After cultivating AMB-1 in mMSGM for 14 days, the culture was sampled in a plastic flask, and a circular neodymium magnet (~ 150 mT) was attached to the wall of the flask. After 24 h, the magnet was removed, and the AMB-1 aggregated in the shape of the magnet on the wall. d TEM image of cultured AMB-1; magnetosomes could be observed within the AMB-1 cells. e Growth curves of AMB-1, reflecting the variation in biomass of AMB-1 cultivated in mMSGM and mSLM. Ns indicated no significant difference between the two groups. *** and ****Significant differences with P < 0.0005 and P < 0.0001, respectively. f Identity percentage results of the 16S rRNA gene alignment with the registered AMB-1 sequence in NCBI. Results were obtained from initially resuscitated AMB-1 in July 2021 and subsequently subcultured AMB-1 in 2023
Before cultivation, ferric solutions, trace element solution, and vitamin solution were aseptically filtered again and supplemented to the basal medium to obtain mMSGM. The resuscitation and subculture procedure were illustrated in Fig. 1b. AMB-1 could be inoculated under sterile laminar flow without the need for strict anaerobic inoculation. Magnetosome-producing AMB-1 was obtained through static incubation at room temperature in sealed glass reagent bottles. The AMB-1 cultured in the mMSGM could be attracted by a magnet (Fig. 1c), and the magnetosomes could be observed via TEM (Fig. 1d). Throughout the experiment, specific AMB-1 cultures were designated as sentinel cultures, inoculated, and cultured alongside other AMB-1 cultures. The 16S rRNA gene sequencing results of the initially resuscitated and recently sampled AMB-1 matched the registered AMB-1 sequence in NCBI, with identity percentages exceeding 98% (Fig. 1f). The extended time interval of over 12 months between the first and the latest sampling not only confirmed the axenic state of the AMB-1 culture but also the reliability of the simplified cultivation method.
Cell density measurement of AMB-1 in mMSGM and mSLM
Once the sterility and reliability of the simplified AMB-1 cultivation process using common laboratory glassware and equipment were ensured, the focus shifted to promoting AMB-1 biomass in fermentation as a feasible strategy for enhanced magnetosome yield. The values of OD565 served as indicators of AMB-1 cell density due to their linear correlation (Su et al. 2023). Previous studies conducted AMB-1 cultivation in MSGM medium within bioreactors, achieving a maximum OD565 of 0.8–0.9 after 48 h (Hong et al. 2021). To simplify magnetosome production by reducing reliance on bioreactors and auxiliary equipment, we conducted aerobic incubation of AMB-1 in mMSGM medium using glass reagent bottles, resulting in a peak of OD565 ~ 0.2 (Fig. 1e). To maximize cell density, we explored further cultivation of AMB-1 in mSLM. The OD565 of AMB-1 reached approximately 0.7 in mSLM (Fig. 1e), indicating that in the same volume, the biomass of AMB-1 obtained through this flask-based fermentation progress was comparable to that obtained through bioreactor-based fermentation.
Stability of Fe3+ in mMSGM and mSLM
Having successfully formulated a culture medium supporting high-biomass of AMB-1, our next focus was optimizing the iron source to enhance magnetosome production. This proposition rested on two key considerations. Firstly, the magnetic cores of AMB-1 magnetosomes are composed of Fe3O4 crystals, implying that the total iron concentration in the medium determines the upper limit of magnetosome yield. Secondly, from a mechanistic standpoint of biomineralization, the iron uptake and intracellular enrichment of Fe3+ are intricately linked to magnetosome biosynthesis. Elevated Fe3+ concentrations in the medium might profit iron transport and consequently magnetosome biomineralization, given that the biomineralization process requires a locally supersaturated intracellular Fe3+ concentration of up to 30 mmol/L (Faivre and Schüler 2008).
It is crucial to note that Fe3+ ions are inherently unstable in near-neutral aqueous solutions, prone to forming insoluble iron precipitates. Hence, arbitrarily supplementing iron salt into the culture medium does not directly raise Fe3+ concentration. An effective strategy to prevent iron precipitation involves chelating Fe3+ ions such as ferric quinate and EDTA-Fe.
As a commonly used iron chelator in AMB-1 cultivation, ferric quinate was supplemented into mMSGM-base (Fig. 2a) and mSLM-base (Fig. 2c) ranging initial iron concentrations of 0.1–1.6 mmol/L. In the supplementation of ferric quinate, precipitate phenomenon was observed in high initial iron supplementation groups. The Fe3+ concentration results in the supernatant of the medium aligned with the particulate phenomena and exhibited a consistent trend with the findings of other research teams (Amor et al. 2016). Fe3+ ion concentrations peaked within 0.4 mmol/L and the peak value stabilized within the range of 0.2 mmol/L. Therefore, when ferric quinate was used as the iron source, iron existed in both ionic and precipitate forms, with iron precipitate as the major form. Given the formation of insoluble precipitates in the medium, further elevation of Fe3+ concentration through additional ferric quinate supplementation proved impractical.
Stability of soluble ferric iron (Fe3+) in mMSGM and mSLM. Ferric quinate was supplemented to mMSGM (a) and mSLM (c), and EDTA-Fe was supplemented to mMSGM (b) and mSLM (d), resulting in media with initial iron concentrations ranging from 0.02 to 1.6 mmol/L (vertical axis of the heatmap). Fe3+ concentrations (values in each cell) were determined on the 1st, 2nd, 4th, 6th, and 8th days (horizontal axis of the heatmap) after iron supplementation
In the experimental processes of supplementing mMSGM-base (Fig. 2b) and mSLM-base (Fig. 2d) with EDTA-Fe as the iron source, precipitation was not observed in the experimental process. Results of Fe3+ concentrations affirm that EDTA-Fe exhibits stability in both mMSGM and mSLM, maintaining soluble Fe3+ ion concentrations within predetermined ranges (0.02–1.6 mmol/L) throughout the 8-day experimental period.
Optimized iron source parameters elevated relative reductase activity (RRA) of AMB-1
The culture collection recommended iron concentration of 0.02 mmol/L in the medium restricts the upper limit of magnetosome production. Previous studies have primarily relied on bioreactors for fermentation and continuous supplementation of ferric salt to augment magnetosome yields. However, it is incongruous with our initial intention to develop a low-cost, non-bioreactor fermentation process, as it necessitates not only the bioreactor itself but also peristaltic pumps, iron ion sensors, pH sensors, control software, etc. to facilitate its operation. We sought to establish a simplified and straightforward magnetosome fermentation method by supplying the iron source to a designated concentration in glass flasks via batch feeding, which significantly minimized the dependence on hardware, software and manual operations.
Although elevated Fe3+ concentrations increase the upper limitation of production, excessively high levels of Fe3+ and EDTA could also impose side effects on cellular vitality, which is a factor we need to take into consideration. Meanwhile, it is advisable to determine the optimal parameters of the iron sources for large-scale fermentation. The optimized iron parameters include the ratio between ferric quinate and EDTA-Fe, as well as the iron concentration, which should be determined basing on a quantitative metric indicating enhanced magnetosome synthesis. To address this, CCK-8 assay was firstly introduced as a benchmark for elucidating iron source parameters due to its recent recognition for evaluating microbial vitality (Yang et al. 2021). CCK-8 undergoes reduction by microbial reductases, producing orange formazan whose absorbance is measured at 450 nm. The control group, AMB-1 cultured in mSLM supplemented with 0.02 mmol/L ferric quinate, was established for comparison. The RRA below 100% implies potential adverse impacts on the vitality of AMB-1.
The formation of magnetosomes is an energy-consuming process; thus, heightened RRA correlates with increased energetic metabolism (Fig. 3a). Simultaneously, the formation of magnetosomes involves sequentially interconnected steps. In the latter step of biomineralization, a biological reduction from Fe3+ to Fe2+ occurs, and reductases were presumed to play a critical role in this reduction process (Noguchi et al. 1999; Amor et al. 2020; Singappuli-Arachchige et al. 2022). If the RRA of the sample significantly exceeds 100%, we can infer that under these iron source parameters, the reductase metabolism of AMB-1 is heightened, favourably facilitating the formation of magnetic cores.
Changes in the relative reductase activity (RRA) of AMB-1 with different iron source parameters. a One potential biomineralization process in magnetosome formation, which involved the energy-consuming processes and reduction metabolism. Ferric quinate (b) and EDTA-Fe (f) were the sole iron sources used in AMB-1 fermentation. Additionally, the iron source for AMB-1 fermentation was prepared by premixing ferric quinate and EDTA-Fe at ratios of 0.75:0.25 (c), 0.5:0.5 (d), and 0.25:0.75 (e). The solid blue, purple, mint, and black lines connect the average result values for each iron concentration at days of 2, 4, 6, and 8, respectively. The dashed lines connect the standard deviation ranges of the result values at each concentration
Employing ferric quinate as the sole iron source (Fig. 3b), iron supplementation exceeding 0.02 mmol/L inhibited AMB-1’s RRA on the second day, and nearly complete suppression occurred when iron surpassing 0.6 mmol/L. On the fourth day, inhibitory effects diminished, with concentrations from 0.3 to 0.8 mmol/L resulting significantly higher RRA compared to the control. Concentrations at 1.0 mmol/L sustained strong inhibition. By the sixth to eighth days, the inhibitory effect of iron supplementation ranged from 0.1 to 0.6 mmol/L disappeared, and vitality or RRA reached levels comparable to the control group. These findings are consistent with results obtained by other research teams, supporting the notion that synthesis of magnetosomes was inhibited when ferric quinate was the sole iron source and excess iron was supplemented (Hong et al. 2021). When EDTA-Fe served as the sole iron source, the RRA increased reaching up to 2–4 times that of the control group (Fig. 3f). On the eighth day, the reductase activity at each iron concentration was essentially equivalent to that of the control group.
When ferric quinate and EDTA-Fe were premixed in different ratios and introduced into the cultivation of AMB-1, varying degrees of elevation in the RRA were observed from the second to the sixth day (Fig. 3c–e). The most significant elevation in the RRA was achieved when the ratio of ferric quinate to EDTA-Fe was 0.5:0.5 (Fig. 3d). Establishing the mixing ratio of the two iron sources, 0.4 mmol/L was selected as the optimal initial iron concentration for iron supplementation. On the second day, the RRA of 0.4 mmol/L was the highest among all groups, approximately eight times that of the control group. From the fourth to the sixth days, the RRA decreased but remained significantly higher than that of the control group. The RRA of AMB-1 reached a level essentially consistent with that of the control group until the eighth day. The results above suggest that under this iron source parameter (0.5:0.5, 0.4 mmol/L), AMB-1 was hypothesized to undergo biomineralization at a high level within a cultivation period of six days. Further validation is required through large-scale flask fermentation to confirm this optimized iron supplementation.
Optimized flask fermentation strategy for magnetosome yield
Based on prior experiments, we have identified mSLM as the optimal culture medium for AMB-1 biomass accumulation in flask fermentation. The optimized iron supplementation involves a premixed solution with a 0.5:0.5 ratio of 0.2 mol/L ferric quinate to EDTA-Fe, supplemented at an iron concentration of 0.4 mmol/L relative to the initial fermentation volume of 15 L. Drawing insights from small-scale cultivations, we outlined a 15-L fermentation process aimed at achieving high-yield magnetosomes (Fig. 4a). Extending the cultivation period to 8 days allows AMB-1 to reach the late logarithmic growth phase. Under conditions promoting high RRA, we supplement mSLM medium and iron sources to meet nutritional and iron requirements for biomineralization. Recognizing that total iron content determines the upper limit of magnetosome production, we repeat iron supplementation after 6 days to ensure high-yield magnetosome production.
Fermentation of high-yield magnetosomes by AMB-1. a Fermentation of AMB-1 via a simplified cultivation and an optimized iron supplementation to increase the magnetosome yield. b Photographs of the control group (right) and optimized group (left) after fermentation. c TEM images of AMB-1 in the control group (top) and optimized group (bottom) after fermentation. The arrows in the images represent magnetosome chains that are clearly visible. d Magnetosome yield after fermentation in the control and optimized groups. ****Significant difference with P < 0.0001. Each fermentation in a 20 L glass reagent bottle was considered a batch, and the yield statistics did not include losses from samples taken as seed cultures
Post-fermentation, the culture broth of AMB-1 in the control group, receiving standard medium iron supplementation, exhibited a deep yellow color, while the experimental group, supplemented with optimized iron, displayed a dark brown color (Fig. 4b). TEM observations of magnetosome synthesis in AMB-1 indicated that only a small portion of AMB-1 in the control group synthesized magnetosomes, whereas almost all AMB-1 cells in the optimized group exhibited magnetosome synthesis. The optimized group showed a higher number of magnetosomes in the magnetosome chains and a more uniform size distribution of each magnetosome than the control group (Fig. 4c). Employing the heat-alkali method to extract magnetosomes from AMB-1, the total magnetosome yield in the optimized group was 185.7 ± 9.5 mg/batch, significantly higher than the total yield in the control group, which was 6.9 ± 1.4 mg (Fig. 4d). Therefore, supplementing excess iron with optimized iron source parameters significantly enhanced the synthesis of magnetosomes in AMB-1.
The magnetosome yield of the optimized group is the highest known to us. The magnetosome yield was estimated to be 12.4 mg/L, based on an initial fermentation volume of 15 L. It is essentially comparable to the magnetosome obtained by a bioreactor, which ranges from 3.3 to 21.1 mg/L (Yang et al. 2001; Heyen and Schüler 2003; Hong et al. 2021). The optimized factors would also have beneficial implications for enhancing the yield of magnetosomes in subsequent bioreactor production. Although the fermentation process takes longer compared to bioreactor fermentation, it offers the advantages of low cost, low equipment requirements, and simplicity of manipulation. By simultaneously conducting multiple batches of 15 L fermentation in glass reagent bottles or similar glassware, the magnetosome yield can be exponentially increased.
Conclusions
We purposefully developed a fermentation method using widely available laboratory consumables, glassware, and equipment, breaking free from the dependency on bioreactors, and enabling high-yield magnetosome production. Utilizing mSLM medium and optimized iron supplementation, we achieved a yield of 185.7 ± 9.5 mg/batch, surpassing previous flask fermentation yields and comparable to bioreactor yields. Overall, with the advantages of simplicity, low cost, and low equipment requirements offered by this fermentation method, it significantly reduces the challenges associated with magnetosome acquisition, thus facilitating research and application of magnetosome-related biomaterials.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alphandéry E (2020a) Bio-synthesized iron oxide nanoparticles for cancer treatment. Int J Pharm 586:119472
Alphandéry E (2020b) Applications of magnetotactic bacteria and magnetosome for cancer treatment: a review emphasizing on practical and mechanistic aspects. Drug Discov Today 25:1444–1452
Alphandery E, Faure S, Seksek O et al (2011) Chains of magnetosomes extracted from AMB-1 magnetotactic bacteria for application in alternative magnetic field cancer therapy. ACS Nano 5:6279–6296
Amor M, Busigny V, Louvat P et al (2016) Mass-dependent and -independent signature of Fe isotopes in magnetotactic bacteria. Science 352:705–708
Amor M, Ceballos A, Wan J et al (2020) Magnetotactic bacteria accumulate a large pool of iron distinct from their magnetite crystals. Appl Environ Microbiol 86:e01278-e1320
Céspedes E, Byrne JM, Farrow N et al (2014) Bacterially synthesized ferrite nanoparticles for magnetic hyperthermia applications. Nanoscale 6:12958–12970
de Souza CA, Verdan M, Presciliano R et al (2023) Large-scale cultivation of magnetotactic bacteria and the optimism for sustainable and cheap approaches in nanotechnology. Mar Drugs 21:60
Faivre D, Schüler D (2008) Magnetotactic bacteria and magnetosomes. Chem Rev 108:4875–4898
Fernández-Castané A, Li H, Thomas ORT, Overton TW (2018) Development of a simple intensified fermentation strategy for growth of Magnetospirillum gryphiswaldense MSR-1: physiological responses to changing environmental conditions. New Biotechnol 46:22–30
Gandia D, Gandarias L, Rodrigo I et al (2019) Unlocking the potential of magnetotactic bacteria as magnetic hyperthermia agents. Small 15:1902626
Heyen U, Schüler D (2003) Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl Microbiol Biotechnol 61:536–544
Hong Y, Kankala RK, Yu R et al (2021) Enriched synthesis of magnetosomes by expanding the Magnetospirillum magneticum AMB-1 culture at optimal iron concentration. Magnetochemistry 7:115
Jain TK, Reddy MK, Morales MA et al (2008) Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm 5:316
Li J, Liu P, Tamaxia A et al (2021) Diverse intracellular inclusion types within magnetotactic bacteria: implications for biogeochemical cycling in aquatic environments. J Geophys Res Biogeosciences 126:e2021JG006310
Liu J, Ding Y, Jiang W et al (2008) A mutation upstream of an ATPase gene significantly increases magnetosome production in Magnetospirillum gryphiswaldense. Appl Microbiol Biotechnol 81:551–558
Mickoleit F, Jörke C, Geimer S et al (2021) Biocompatibility, uptake and subcellular localization of bacterial magnetosomes in mammalian cells. Nanoscale Adv 3:3799–3815
Nan X, Teng Y, Tian J et al (2021) A comprehensive assessment of the biocompatibility of Magnetospirillum gryphiswaldense MSR-1 bacterial magnetosomes in vitro and in vivo. Toxicology 462:152949
Noguchi Y, Fujiwara T, Yoshimatsu K, Fukumori Y (1999) Iron reductase for magnetite synthesis in the magnetotactic bacterium Magnetospirillum magnetotacticum. J Bacteriol 181:2142–2147
Plan Sangnier A, Preveral S, Curcio A et al (2018) Targeted thermal therapy with genetically engineered magnetite magnetosomes@RGD: photothermia is far more efficient than magnetic hyperthermia. J Controlled Release 279:271–281
Raguraman V, Suthindhiran K (2020) Comparative ecotoxicity assessment of magnetosomes and magnetite nanoparticles. Int J Environ Health Res 30:13–25
Ren G, Zhou X, Long R et al (2023) Biomedical applications of magnetosomes: state of the art and perspectives. Bioact Mater 28:27–49
Schüler D (2008) Genetics and cell biology of magnetosome formation in magnetotactic bacteria. FEMS Microbiol Rev 32:654–672
Singappuli-Arachchige D, Feng S, Wang L et al (2022) The magnetosome protein, Mms6 from Magnetospirillum magneticum strain AMB-1, is a lipid-activated ferric reductase. Int J Mol Sci 23:10305
Spring S, Amann R, Ludwig W et al (1993) Dominating role of an unusual magnetotactic bacterium in the microaerobic zone of a freshwater sediment. Appl Environ Microbiol 59:2397–2403
Su Q, Andersen HR, Bazylinski DA, Jensen MM (2023) Effect of oxic and anoxic conditions on intracellular storage of polyhydroxyalkanoate and polyphosphate in Magnetospirillum magneticum strain AMB-1. Front Microbiol 14:1203805
Yang C-D, Takeyama H, Tanaka T, Matsunaga T (2001) Effects of growth medium composition, iron sources and atmospheric oxygen concentrations on production of luciferase-bacterial magnetic particle complex by a recombinant Magnetospirillum magneticum AMB-1. Enzym Microb Technol 29:13–19
Yang X, Zhong Y, Wang D, Lu Z (2021) A simple colorimetric method for viable bacteria detection based on cell counting Kit-8. Anal Methods 13:5211–5215
Acknowledgements
We sincerely express our gratitude to Professor Zongjun Du from Shandong University, Weihai, and Professor Jinhua Li from the Institute of Geology and Geophysics, Chinese Academy of Sciences, for generously donating the AMB-1 strain before our AMB-1 purchased from ATCC arrived. This particular strain of AMB-1 played a crucial role in the preliminary experiments exploring the cultivation of magnetotactic bacteria in our laboratory. Special thanks to Professor Jiesheng Tian from China Agricultural University for providing suggestions for the improvement of the SLM. Professor Tian's professional advice greatly assisted us in determining the formulation and preparation process of mSLM.
Funding
This work was supported by Major Science and Technology Innovation Project of Shandong Province (2022CXGC020414) and Special Plan for Major Scientific and Technological Innovation in Qingdao West Coast (ZDKC-2022-04).
Author information
Authors and Affiliations
Contributions
Y.W. and S.Q. designed the experiments. Y.W. conducted the experiments, analyzed the data, wrote the main manuscript, and prepared the figures. Z.L., W.L., and H.C. prepared the figures and reviewed the manuscript. Y.H. validated the experiments. S.Q. contributed to funding and resource acquisition and supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies on human or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Liu, Z., Li, W. et al. High-yield magnetosome production of Magnetospirillum magneticum strain AMB-1 in flask fermentation through simplified processing and optimized iron supplementation. Biotechnol Lett (2024). https://doi.org/10.1007/s10529-024-03507-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10529-024-03507-x