Abstract
Objective
Unlike plant cell suspension culture, the proliferation of callus in bioreactors has received inadequate attention. The magnificent potential of plant callus becomes more appreciated as the research unfolds and promises interesting applications including the production of valuable metabolites, therapeutic antibodies, bioactive extracts with regenerating effects, and the generation of genetically improved plants. Issues such as the lack of 3D-access of the cells to the nutrients, using an interfering gelling substance as the support matrix, and the changes in the medium formulation during the growth phase were discouraging factors for extending research on this topic. Considering the existing drawbacks, a novel open-flow spray bioreactor (OFSB) was configured to circumvent the associated problems with the solid cell culture and promote the applicability of plant callus culture via improving the feeding strategy.
Methods
Applying similar subculture conditions, the proliferation of Arnebia pulchra and Hyoscyamus niger calli as the examples of two important plant families (Boraginaceae and Solanaceae) was studied in the OFSB in comparison with similar calli that grew in Petri dishes and jars.
Results
A. pulchra and H. niger calli obtained the weight gains of (%87.3 and %106.7) in the Petri dishes, (%208.7 and %226) in the jars, and (%288.6 and %320.0) in OFSB, respectively, while no significant changes were observed in the productivity indices of the examined calli.
Conclusion
The simple design of OFSB bypasses most of the notorious problems associated with solid plant callus culture. OFSB technical features allow the bioreactor to be used for growth optimization of various types of plant calli in a cost-effective manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the invaluable potential of plant cell culture (PCC) for producing various natural products and heterologous proteins, the slow growth rate of plant cells, as compared with microbial cultures, has remained a challenge for vast large-scale application of this technology (Ochoa-Villarreal et al. 2016). PCC practically starts from the induction of continuous cell division, upon the effect of phytohormones, on a solid culture medium (callus formation). Then, it proceeds via the proliferation of the cells either on a gel or in a liquid medium. The latter method, suspension culture, has received remarkable attention due to the advantages such as simultaneous access of the cells to the homogeneous nutrients and easy separation of the cells from the medium at the end of the subculture (Yue et al. 2016). Consequently, the engineering of suspension culture has been well investigated via designing different batch, fed-batch, and continuous bioreactors (Sajc et al. 2000; Huang and McDonald 2012). Nonetheless, despite successful examples (Yue et al. 2016), the technology still requires improvement to satisfy the expectations. In addition to the genetic instability of plant cells, there are important challenges to be addressed in the liquid PCC bioreactors such as negative effects of shear stress, foam formation, cell aggregation, incompetent respiration, and problematic gas transport on the doubling rate of the cells and the production yield (Huang and McDonald 2012; Wyma et al. 2018).
Solid culture of plant cells is practically easier. However, during this process, the access of the cells to the nutrients and gases changes as they grow. Moreover, to provide the cells with a solid support, a gelling substance is added to the culture medium, which increases the costs and complexity of the nutrient formulations. The gelling substance affects the concentrations of the mineral nutrients, the physical properties of the medium such as water potential and nutrient availability. Some gelling agents also contain inhibitory compounds that reduce the growth rate of the cells and hinder morphogenesis (Ayenew et al. 2020). Nonetheless, there is magnificent potential in callus culture related to the differentiation-dedifferentiation ability of plant stem cells (Fehér 2016). In addition to the direct use of plant callus for the production of valuable metabolites especially inaccessible ones via chemical synthesis (Yue et al. 2016), production of therapeutic antibodies which are arduously cloned in microbial cells (Corbin et al. 2020; Efferth 2019), and bioactive extracts with regenerating effects (Fehér 2016), plant calli are the basic tools for the preparation of suspension cultures, generation and propagation of improved agricultural and horticultural plants (Efferth 2019).
In view of the above-mentioned premises, a novel spray bioreactor was designed to enhance the applicability of solid PCC by addressing the associated problems with the solid callus culture and to increase biomass production mainly via improving the feeding strategy. Although the research was focused on the proliferation of Arnebia pulchra and Hyoscyamus niger calli, the macroscopic qualities of the cells and their overall biosynthetic potentials were also examined. A. pulchra and H. niger are examples of two important plant families (Boraginaceae and Solanaceae, respectively) with abilities to produce phenolic compounds and tropane alkaloids (Aljibouri et al. 2012; Ezati et al. 2015).
Materials and methods
Gallic acid (GA), ascorbic acid (AsA), and Folin-Ciocalteu reagent were purchased from Merck (Darmstadt, Germany). Potassium peroxodisulfate, 2,2-azinobis-[3-ethylbenzothiazoline-6-sulfonate] (ABTS), kinetin, and 2,4-dichlorophenoxyacetic acid (2,4-D) were purchased from Sigma Chemical and Biochemical Company (St. Louis, MO, USA). The other chemicals, used in this work, were taken from the authentic samples. Linsmaier and Skoog (LS), Murashige and Skoog (MS) (full and half-strength), and Gamborg B5 mediums were prepared according to the literature (Cartwright and Shah 2000). All the spectrophotometric examinations were carried out in conventional quartz cells using a UV–visible ANALYTIK SPECORD-210 (Jenna, Germany) spectrophotometer.
Plant cell culture
Callus induction in A. pulchra and H. niger explants had already been reported (Ezati et al. 2015; Tahmasebi et al. 2021). Prior to the examination of the spray bioreactor, the proliferation of A. pulchra and H. niger cells was studied on solid and in liquid LS, MS (full and half-strength), and B5 mediums containing 2,4-D (1 µM) and kinetin (10 µM) at two levels of sucrose (30 and 50 g L−1) in darkness at 25 ± 2 °C. The liquid cultures were carried out in Erlenmeyer (100 mL) containing 30 mL of the desirable medium. Solid PCC experiments were conducted in Petri dishes (10 cm diameter) and jars (r = 4, h = 9.30 cm) containing 30 mL of the desired mediums. Culture mediums were gelled by agar–agar (8 g L−1). The selected calli were adapted to the desired medium during 2 successive 14-day subcultures. The main experiments were performed in triplicate for 3 successive 30-day subcultures in the desired utensils. To calculate the biomass, the weights of the calli were recorded before and after drying at 37 °C for 48 h.
Bioreactor design
Figure 1A illustrates schematically the design of the spray bioreactor developed in this research. Figure 1B shows the actual growth chamber of the spray bioreactor. The accessories of the bioreactor are introduced in the caption of Fig. 1.
A Schematic design of the spray bioreactor showing 1) tank of liquid medium, 2) Heat resistant silicone tubes, 3) digital timer (Simens, 12/24 RCE), 4) pump (Drop Water pump: Wat 90. 220v), 5) perforated ceramic matrix with 5 mm pores, 6) upper atmosphere of seeding place, 7) gas exchanges, 8) residual outlet, 9) spray nozzle (Lightree, plastic sprinkler, 0.5 mm), 10) digital camera (WiFi endoscope camera, OD:8 mm). B the employed glass proliferation compartment in this study
Sterilization and callus proliferation in the spray bioreactor
The proliferation chamber, medium tank, tubes, and the spray nozzle were autoclaved. To sterilize the pump, a current of NaOCl solution 5% (20 min) followed by alcohol 70% (5 min) and autoclaved double-distilled water (15 min) were passed successively through the pump. The sterilization of the pump was carried out under conventional UV-illumination in a Laminar flow hood. Proliferation of each type of plant callus (1.5 g on day 1) was studied in the spray bioreactor for 3 successive 30-day subculture using MS medium containing 2,4-D (1 µM), kinetin (10 µM) and sucrose (30 g L−1) while all the equipment was in darkness at 25 ± 2 °C. The details of the feeding program and the bioreactor setup is discussed in the following sections.
Analysis of total phenolic content (TPC), total alkaloids content (TAC), and radical scavenging capacity (RSC)
To prepare the crude extracts, the desired sample [wet callus (1.5 g)] was ground in a mortar containing methanol (5 mL). The resulting mixture was centrifuged (1500×g, 15 min) using a Beckman Centrifuge, J-21 model, then filtered through a filtration paper (Grade 42, Whatman® Ashless, Sigma). The filtrate was maintained in the dark at 4 °C. Using Folin-Ciocalteu spectrophotometric method (Khosravi et al. 2019), TPC was calculated in terms of GA Equivalent using GA calibration curve (Fig. S1A, Supplementary document). Using ABTS method (Khosravi et al. 2019), RSC was calculated in terms of AsA Equivalent using AsA calibration curve (Fig. S1B, Supplementary document). TAC was calculated in terms of Atropine Equivalent using the calibration curve of a published method (Fazel et al. 2010).
Results and discussion
Open-flow spray bioreactor (OFSB)
One of the main criticism of the solid PCC is about the gradual changes of the nutrients formulation during a subculture. This problem has been circumvented in the designed spray bioreactor, Fig. 1, as the cells are sprayed by a fresh homogenous nutrient medium throughout the subculture. Gas-phase bioreactors including mist and spray bioreactors were originally developed to support the growing biomass by trouble-free gas exchange and to facilitate overcoming hyperhydration (Towler et al. 2008). But most of the designs were based on using a closed circulation of the medium between the nutrients reservoir and the growth chamber (Sajc et al. 2000; Huang and McDonald 2012). In contrast, there is a one-way flow of the nutrients in the developed open-flow spray bioreactor (OFSB) in this work.
To enhance feeding quality in liquid PCC, continuous bioreactors were developed. They improved the log phase of growth as well as the product yield of the cells in comparison with those of batch and fed-batch bioreactors (Corbin et al. 2020). However, to install an efficient feeding program, online monitoring of all the influencing parameters on the medium quality was necessary (Huang and McDonald 2012; Valdiani et al. 2019). Distinctly in the OFSB, there is no need for online monitoring of the growth chamber as the source of culture medium (Container 1 in Fig. 1) is not in contact with the cells. Therefore, the quality of the medium remains consistent during the whole period of a subculture.
The OFSB feeding method allows the users not only to feed the cells continuously with fresh nutrients, but it also lets them control the nutrients aliquots by applying the proper dilution of the medium and timing of spray (Parts 3 and 4 in Fig. 1). Consequently, this technology is not reliant on sophisticated online equipment while it facilitates setting up a safe and efficient feeding strategy with little chance of contamination during the subculture. In fact, the contamination incident was zero during the successive subcultures of this study.
The other serious criticism of solid callus culture relevant to the use of gelling substances is also addressed in the OFSB because the callus is placed on a solid support (Part 5 in Fig. 1) covered by any type of fluid permeable material such as cotton sheets. This setup helps to reduce the costs as well as to omit the possible undesirable interferences of a gelling substance.
In the conventional solid PCC, the residing cells on the gel surface have direct access to the nutrients whereas the upper layers are fed via the gradient of the substances intervened by the gel and the lower cells. This is unlike to what happens in a suspension culture where most of the cells have direct access to the medium. In the OFSB, the gradient of the nutrients (from top to down) does not face gel diffusion barrier and the descending flow of the materials (in alignment with the gravity direction) is consequently more effortless. Therefore, it improves the rate of the nutrients uptake (Towler et al. 2008). Nonetheless, it cannot be considered as a 3D access. But, it is also worthy of consideration that recent studies question the necessity of equal access of all the cells to the nutrients. Although there is a correlation between the nutrient availability and the plant cell longevity (López-Otín et al. 2016), the incipient results from senescence studies indicate that the pattern of nutrient uptake varies with the age, the plant species, and the type of nutrient (Dijkwel and Lai 2019). Accordingly, as the subculture ages, it is assumed that the need to a 3D access to the food materials varies between the heterogeneous populations of the plant cells in a bioreactor.
Similar to the gas-phase reactors, the OFSB can virtually eliminate any gas-exchange limitation afflicting the growing cells. The gaseous composition of the headspace (space 6 in Fig. 1) is very important to both growth rate and metabolites production (Towler et al. 2008). Although the proliferation of both types of calli was examined in the equilibrium with air in this work, it was essential to show the potential of the OFSB design for altering the gas composition of the proliferation chamber through the additional gas/liquid inlets (inlets 7 in Fig. 1) from top and below the growing biomass that possibly extends the applicability of the OFSB.
Another advantage of the OFSB relates to metabolite excretion phenomenon. The discharged compounds from plant cells can affect the quality of a suspension culture in different ways. For instance; the excreted substances may change the viscosity of the medium, participate in foam formation (Valdiani et al. 2019), reduce the solubility capacity of the medium for the gases that are necessary for both respiration and proper function of the cells, interfere positively or negatively in the signaling cascades associated with the cell division (Ramirez-Estrada et al. 2016), etc. To minimize the side effects of the discharged compounds, some various successful designs such as the perfusion bioreactors have been developed but at the cost of applying more apparatuses (Sajc et al. 2000; Huang and McDonald 2012; Corbin et al. 2020). In the OFSB, it is possible to control the abundance of the excreted compounds on the cell surface. In fact, in case of need, it is possible to wash down these compounds from the cells into the lower part of the growth compartment where they can be collected, sampled, or removed (outlet 8, Fig. 1). This means that, in contrast to the developed continuous bioreactors for liquid PCC, there is no need for agitation or circulation of the medium through the separating columns since the liquid culture medium is kept in a different container from the proliferation vessel. As a result, the chance of foam formation and mechanical shear stress to the cells approach zero while the OFSB permits the cells to have access freely to the surrounding gases for an efficient respiration.
Callus proliferation
The literature is not consistent regarding the proliferation conditions for the selected calli (Ghorbanpour et al. 2013; Parray et al. 2018), therefore, the proliferation of A. pulchra and H. niger cells was first studied on solid and liquid LS, MS (full and half-strength), and B5 mediums containing two levels of sucrose (30 and 50 g L−1) under identical hormonal treatments. The results showed no significant growth rates for both types of plant cells in the liquid cultures. In addition, the high ratio of C/N was not beneficial to the cells (Dong et al. 2016) as most of them developed browning and were not friable (data not shown here).
Since the results were in favor of solid MS medium containing 30 g L−1 of sucrose, the experiments were repeated but in two different utensils (Petri dish and jar) because of their different aeration capacities. Figure 2 shows that, under similar conditions, A. pulchra callus (1.5 g on day 1) gained extra weights of 1.31 ± 0.06 (87.3%) and 3.13 ± 0.03 g (208.7%) by day 30 in Petri dishes (Fig. 2A) and jars (Fig. 2B), while H. niger callus gained extra weights of 1.6 ± 0.14 (106.7%) and 3.4 ± 0.19 g (226.7%) in Petri dishes (Fig. 2C) and jars (Fig. 2D), respectively. See the Supplementary Document for the raw data. This simply means that both types of the plant cells achieved the highest growth on MS medium in jars. The average dry biomass of A. pulchra and H. niger calli were 4.0 and 3.7%, respectively. Consequently, the plant calli (1.5 g) were placed on the perforated ceramic (part 5, Fig. 1) covered by 3 layers of wet cotton sheets (part 5 in Fig. 1). Sterile double-distilled water (30 mL) was poured into the bottom of the growth chamber to support the humidity maintenance. As it was the main objective of this study to compare the proliferation of the calli in Petri dish, jar, and the OFSB under similar conditions, the same selected nutrient medium was applied to the seeded calli in the growth chamber. Therefore, liquid MS medium was diluted and sprayed continuously at a rate of 1 mL per 30 min from Tank 1 (Fig. 1) to keep the total nutrient amount equal to those of the Petri and the jar dishes. The spray nozzle (part 9, Fig. 1) was adjusted to cover a dispersion diameter equal to 90% of part 5 (Fig. 1). Then, the proliferation of the calli in the OFSB was examined during 3 successive subcultures while the process was being monitored by an online camera (part 10, Fig. 1). This setup kept the cell surfaces fresh while it avoided saturation of the cells’ surfaces by the medium droplets.
Normalized (with regard to the highest data of each type of callus) weight gains of the fresh calli of A. pulchra on the selected mediums in A Petri dishes, B jars and H. niger in C Petri dishes, and D jars at different time intervals [day 1 (diagonal), day 17 (light color), day 21 (dashed), and day 30 (dark) columns] of the subculture in darkness at 25 ± 2 °C. All the mediums were supplemented by 2,4-D (1 µM), kinetin (10 µM), sucrose (30 g L−1) and solidified by agar–agar (8 g L−1). See the raw data in Tables S1 and S2 in the Supplementary document
At the end of these experiments, the average weight gains of 4.33 ± 0.16 and 4.8 ± 0.08 g (equal to 388.6 and 420% growth) were obtained for the A. pulchra and H. niger callus, respectively. This data indicates that the A. pulchra and H. niger calli had 80.0 and 93.3%, respectively, higher growth than their corresponding counterparts that grew in the jars.
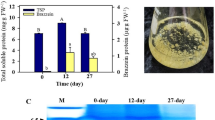
Callus productivity indices
To examine the biosynthetic abilities of the plant cells proliferated in the bioreactor, TPC and RSC of both types of the plant calli as well as TAC of H. niger callus were also measured. Table 1 discloses high capacity of A. Pulchra cells for production of phenolic compounds as compared with H. niger cells. This is in agreement with the other reports about Boraginaceae plants (Dresler et al. 2017). TAC of H. niger callus was close to the results reported by Aljibouri et al. (2012). They reached the highest amount of hyoscyamine (1.7 mg L−1) in the cell culture of H. niger in MS medium in darkness under the effect of proline (100 mg L−1) which was much lower than the results obtained from H. niger hairy root cultures in a gas bioreactor (33.5 mg g−1 dry root) (Jaremicz et al. 2014). These findings support the assumption that tropane alkaloids production might be a tissue-dependent process, similar to was observed for pyrrolizidine alkaloids (Khosravi et al. 2019).
More importantly, Table 1 shows little difference between the TPC and TAC data of the proliferated calli in the OFSB and those that grew in Petri dishes and jars. Comparison of the RSC data also results in similar conclusion (Table 1). These results indicate that the proliferation of the examined calli in the OFSB has improved the growth rates of the plant cells without afflicting their biosynthetic indices.
Conclusion
In the simple design of OFSB, most of the notorious problems of solid plant callus culture are bypassed. Running OFSB does not involve using sophisticated accessories while it provides the cells with fresh and intact nutrients as well as botherless access to the gases continuously during the subcultures. The technical features of OFSB allow it to be used for the growth optimization of various types of plant calli in a cost-effective manner.
Data availability
The supplementary data of this paper is available at.
References
Aljibouri AM, Al-samarraei KW, Abd AS, Mageed DM, Ali AJ (2012) Alkaloids production from callus of Hyoscyamus niger L. in vitro. J Life Sci 6(8):874. Corpus ID: 83444221
Ayenew B, Mengesha A, Tadesse T, GebreMariam E (2020) Ensete ventricosum (Welw.) Cheesman: a cheap and alternative gelling agent for pineapple (Ananas comosus var. smooth cayenne) in vitro propagation. J Microbiol Biotechnol Food Sci 6:640–52. Corpus ID: 84041804
Cartwright T, Shah GP (2000) Culture media, in basic cell culture: a practical approach. Ed. by J M Davis. The Quart Rev Biol 77:318–318. https://doi.org/10.1086/345171
Corbin JM, McNulty MJ, Macharoen K, McDonald KA, Nandi S (2020) Technoeconomic analysis of semicontinuous bioreactor production of biopharmaceuticals in transgenic rice cell suspension cultures. Biotechnol Bioeng 117:3053–3065. https://doi.org/10.1002/bit.27475
Dijkwel PP, Lai AG (2019) Hypothesis: plant stem cells hold the key to extreme longevity. Transl Med Aging 3:14–16. https://doi.org/10.1016/j.tma.2018.12.002
Dong YS, Fu CH, Su P, Xu XP, Yuan J, Wang S, Zhang M, Zhao CF, Yu LJ (2016) Mechanisms and effective control of physiological browning phenomena in plant cell cultures. Physiol Plant 156:13–28. https://doi.org/10.1111/ppl.12382
Dresler S, Szymczak G, Wójcik M (2017) Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm Biol 55:691–695. https://doi.org/10.1080/13880209.2016.1265986
Efferth T (2019) Biotechnology applications of plant callus cultures. Eng J 5:50–59. https://doi.org/10.1016/j.eng.2018.11.006
Ezati T, Marefatjoo MJ, Haghbeen K, Ahmadkhaniha R (2015) Successful Indirect Regeneration of Arnebia pulchra (Roemer and Schultes) as Medicinal Plant. J Med Plants by Prod 4: 233–242. Corpus ID: 89222972
Fazel S, Hamidreza M, Rouhollah G, Verdian-rizi M (2010) Spectrophotometric determination of total alkaloids in some Iranian medicinal plants. J Appl Hortic 12:69–70
Fehér A (2016) Callus, dedifferentiation, totipotency, somatic embryogenesis: what these terms mean in the era of molecular plant biology? Front Plant Sci 26:536. https://doi.org/10.3389/fpls.2019.00536
Ghorbanpour M, Omidi M, Etminan A, Hatami M, Shooshtari L (2013) In vitro hyoscyamine and scopolamine production of black henbane (Hyoscyamus niger) from shoot tip culture under various plant growth regulators and culture media. Trakia J Sci (TJS) 11:126. ISSN 1313–3551 (online)
Huang TK, McDonald KA (2012) Bioreactor systems for in vitro production of foreign proteins using plant cell cultures. Biotechnol Adv 30:398–409. https://doi.org/10.1016/j.biotechadv.2011.07.016
Jaremicz Z, Luczkiewicz M, Kokotkiewicz A, Krolicka A, Sowinski P (2014) Production of tropane alkaloids in Hyoscyamus niger (black henbane) hairy roots grown in bubble-column and spray bioreactors. Biotechnol Lett 36:843–853. https://doi.org/10.1007/s10529-013-1426-9
Khosravi E, Mousavi A, Farhadpour M, Ghashghaie J, Ghanati F, Haghbeen K (2019) Pyrrolizidine alkaloids-free extract from the cell culture of Lithospermum officinale with high antioxidant capacity. Appl Biochem Biotechnol 187:744–752. https://doi.org/10.1007/s12010-018-2830-3
López-Otín C, Galluzzi L, Freije JM, Madeo F, Kroemer G (2016) Metabolic control of longevity. Cell 166:802–821. https://doi.org/10.1016/j.cell.2016.07.031
Ochoa-Villarreal M, Howat S, Hong S, Jang MO, Jin YW, Lee EK, Loake GJ (2016) Plant cell culture strategies for the production of natural products. BMB Rep 49:149. https://doi.org/10.5483/BMBRep.2016.49.3.264
Parray JA, Kamili AN, Jan S, Mir MY, Shameem N, Ganai BA, AbdAllah EF, Hashem A, Alqarawi AA (2018) Manipulation of plant growth regulators on phytochemical constituents and DNA protection potential of the medicinal plant Arnebia benthamii. Biomed Res Int 4:2018. https://doi.org/10.1155/2018/6870139
Ramirez-Estrada K, Vidal-Limon H, Hidalgo D, Moyano E, Golenioswki M, Cusidó RM, Palazon J (2016) Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 21:182. https://doi.org/10.3390/molecules21020182
Sajc L, Grubisic D, Vunjak-Novakovic G (2000) Bioreactors for plant engineering: an outlook for further research. Biochem Eng J 4:89–99. https://doi.org/10.1016/S1369-703X(99)00035-2
Tahmasebi Goojgi S, Haghbeen K, Mousavi M, Piri K (2021) The effect of subculture on somaclonal variation in callus culture of Hyoscyamus niger using ISSR markers. Iran J Rangelands for Plant Breed Genet Res 28:203–211
Towler MJ, Kim Y, Wyslouzil BE, Correll MJ, Weathers PJ (2008) Design, development, and applications of mist bioreactors for micropropagation and hairy root culture. Plant Tissue Cult Biotechnol. https://doi.org/10.1007/978-1-4020-3694-1_7
Valdiani A, Hansen OK, Nielsen UB, Johannsen VK, Shariat M, Georgiev MI, Omidvar V, Ebrahimi M, Tavakoli Dinanai E, Abiri R (2019) Bioreactor-based advances in plant tissue and cell culture: challenges and prospects. Crit Rev Biotechnol 39:20–34. https://doi.org/10.1080/07388551.2018.1489778
Wyma A, Martin-Alarcon L, Walsh T, Schmidt TA, Gates ID, Kallos MS (2018) Non-Newtonian rheology in suspension cell cultures significantly impacts bioreactor shear stress quantification. Biotechnol Bioeng 115:2101–2113. https://doi.org/10.1002/bit.26723
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2016) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 36:215–232. https://doi.org/10.3109/07388551.2014.923986
Acknowledgements
It is acknowledged that S. Tahmasebi and M. Tavakoli had equal contributions in this work.
Funding
This study was supported by the National Institute for Genetic Engineering and Biotechnology of Iran (Project 582).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by STG and MT. The first draft of the manuscript was written by KH and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest of any kind.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goojgi, S.T., Tavakoli, M., Haghbeen, K. et al. A novel spray bioreactor for the proliferation of plant callus; Hyoscyamus niger and Arnebia pulchra. Biotechnol Lett 44, 333–340 (2022). https://doi.org/10.1007/s10529-022-03235-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-022-03235-0