Abstract
Objectives
To determine the effect of sea salt on the resistance of Trichoderma harzianum LZDX-32-08 to hygromycin B and speculate the possible mechanisms involved via transcriptome analysis.
Results
Sea salt addition in media to simulate marine environment significantly increased the tolerance of marine-derived fungus Trichoderma harzianum LZDX-32-08 to hygromycin B from 40 to 500 μg/ml. Meanwhile, sea salt addition also elicited the hygromycin B resistance of 5 other marine or terrestrial fungi. Transcriptomic analyses of T. harzianum cultivated on PDA, PDA supplemented with sea salt and PDA with both sea salt and hygromycin B revealed that genes coding for P-type ATPases, multidrug resistance related transporters and acetyltransferases were up-regulated, while genes coding for Ca2+/H+ antiporter and 1,3-glucosidase were down-regulated, indicating probable increased efflux and inactivation of hygromycin B as well as enhanced biofilm formation, which could jointly contribute to the drug resistance.
Conclusions
Marine environment or high ion concentration in the environment could be an importance inducer for antifungal resistance. Possible mechanisms and related key genes were proposed for understanding the molecular basis and overcoming this resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
About 1.5–2 million people die of fungal infection each year (Denning and Bromley 2015). These numbers increased steadily in recent decades (Sekyere and Asante 2018). Worse still, antifungal resistance became a big challenge due to consistent use of limited antimicrobials (Denning and Bromley 2015). Thus, it is extremely urgent to obtain a better understanding of resistance mechanisms in order to reverse the resistance or provide novel targets for drug development. Previous studies revealed three main mechanisms including changes in drug target sites, activation of drug efflux and formation of biofilms (Sekyere and Asante 2018). Hygromycin B (HYG) is an aminoglycoside antibiotic that inhibits protein synthesis by stabilizing the tRNA-ribosomal acceptor site (Kaster et al. 1983). HYB resistance was mainly based on modification of ribosome binding sites (Honore et al. 1995), decreasing intracellular HYG concentrations (Karlowsky et al. 1997), and inactivation of HYG via structure modification by aminoglycoside phosphotransferase, acetyltransferase, or nucleoside adenosine transferase (Rao et al. 1983). Besides, addition of KCl and mutation of pma1 by UV-treatment was found to improve the HYG tolerance of Saccharomyces cerevisiae, which indicated that the HYG transport was associated with the electrochemical proton gradient and cells were unable to uptake HYG, when the H+-ATPase Pma1 was mutated (Perlin et al. 1988). Later, Barreto et al. hypothesized that any mutation resulting in changes of the electrochemical gradient could give rise to anomalous sensitivity to cationic drug independently of its toxicity mechanism. They made a genome-wide screen for mutants that demonstrated altered tolerance to HYG, spermine, and tetramethylammonium in S. cerevisiae and lots of genes related to potassium homeostasis were identified (Barreto et al. 2011). Furthermore, Alao et al. found that KCl can suppress the sensitivity of Schizosaccharomyces pombe to a wide range of antibiotics, which might depend in part on changes to electrochemical gradient of membrane and membrane transport proteins. However, the specific mechanism of ion induced drug resistance remains largely elusive (Alao et al. 2015). In this study, the effects of sea salt on HYG tolerance of Trichoderma harzianum and five other fungi were investigated and the possible mechanisms were proposed thereof.
Materials and methods
Strains, media and growth conditions
Fungal strains used were listed in Supplementary Table 1 and cultivated at 28 °C on PDA (Potato Dextrose Agar, Difco) or PDA with sea salt (3.33%, w/v) or PDA with both sea salt and 100 μg/ml HYG. Sea salt was from Instant Ocean Reef Crystals®. HYG solution (50 mg/ml) was purchased from VWR. Each plate was inoculated with 3 μl spore suspension (105 conidia/ml).
Transcriptome analysis
Trichoderma harzianum were cultivated on PDA supplemented with sea salt (A), PDA supplemented with sea salt and 100 μg/ml HYG (B), and PDA (C) for 6 days at 28 °C. The mycelia were collected respectively and sent to Sangon Biotech (Shanghai) for total RNA extraction, library construction, sequencing and analysis. Total RNA was extracted using Total RNA Extractor (Trizol). Sequencing libraries were generated using VAHTSTM mRNA-seq V2 Library Prep Kit for Illumina® and index codes were added to attribute sequences to each sample. Raw reads were filtered by Trimmomatic (version 0.36) and the remaining clean data were used for further analysis. The direct expression of a gene expression level was quantified by StringTie (version 1.3.3b), which was used to calculate Transcripts Per Million (TPM) of protein-coding genes in each sample. DESeq (version 1.12.4) was used for differential expression analysis and significant DEGs were obtained (p-value < 0.05 and |FoldChange|> 2). TopGO (version 2.24.0) was used for GO (Gene Ontology) enrichment, and the function was thought to be a significant enrichment when the correct p-value (q-value) < 0.05 (Database: https://www.geeontology.org). ClusterProfiler (version 3.0.5) was used for euKaryotic Ortholog Groups (KOG) enrichment analysis (Database: https://www.ncbi.nlm.nih.gov/COG/). The raw sequence data have been submitted to the National Center for Biotechnology information (NCBI) Sequence Read Archive (SRA) with accession number PRJNA623072.
Results
Hygromycin B resistance induced by sea salt
Hygromycin B is commonly used in lab as selection marker for positive transformants. During the genetic manipulation of marine-derived fungus T. harzianum LZDX-32–08, we observed that sea salt addition (3.33%) in PDA could significantly increase its resistance to HYG (Fig. 1a). T. harzianum could only survive 40 μg/ml HYG on PDA, in contrast to 500 μg/ml HYG in the presence of sea salt. The addition of sea salt seemed to be beneficial for the marine-derived fungus to adapt to harsh environment of HYG exposure. To examine whether this effect is limited to T. harzianum LZDX-32-08, other commonly used fungal strains were tested under the same condition, including terrestrial fungus T. harzianum 3.9236, marine-derived Aspergillus tubingensis HSC-1, terrestrial A. tubingensis 3.6402, A. niger AIR-1 and Mucor circinelloides LJC-1. Similar to T. harzianum LZDX-32-08, T. harzianum 3.9236, A. tubingensis HSC-1, A. tubingensis 3.6402 and A. niger AIR-1 only survived 40 μg/ml HYG on PDA, while on PDA supplemented with sea salt, they could even survive 1000 μg/ml HYG (Fig. 1b–e). M. circinelloides LJC-1 was not as sensitive to HYG as other selected fungi and could withstand 1000 μg/ml HYG on both PDA and PDA with sea salt. Even though, the growth diameter of M. circinelloides on PDA decreased with the increased concentration of HYG, while the growth of M. circinelloides LJC-1 with sea salt addition was almost uninhibited (Fig. 1f). Thus, all six fungi demonstrated increased resistance to HYG with sea salt addition in media, no matter it is from marine or terrestrial.
Effect of different ions on hygromycin B resistance
Since the cations in sea salt could be responsible for the increased resistance of fungi to HYG, four cations that have high content in sea salt, K+, Na+, Mg2+, and Ca2+ were individually investigated to figure out which ion might play a major role. 0.57 M CaCl2, KCl, MgCl2, and NaCl could increase the HYG tolerance of T. harzianum up to 1000, 500, 500 and 200 μg/ml, respectively, among which CaCl2 demonstrated the strongest effect (Fig. 2). Both divalent and monovalent cations could increase the tolerance of T. harzianum to HYG, suggesting that this effect might not base on the chelation inactivation of HYG by ions. This suggestion was further proved by the 1H NMR spectrum of a mixture of HYG and sea salt, which was almost identical to that of HYG alone (Supplementary Fig. 1). In addition, different osmotic pressures of PDA media created with sorbitol cannot induce the HYG resistance of T. harzianum (Supplementary Fig. 2). Therefore, the mechanism for the sea salt induced HYG resistance was neither inactivation of HYG by chelation nor osmotic changes, and the remaining possibility would lie on the sea salt-induced transcriptomic changes in T. harzianum.
Transcriptomic changes of T. harzianum in response to sea salt and hygromycin B
To speculate the molecular basis of HYG resistance induced by sea salt, Illumina RNA-sequencing technology (RNA-seq) was used to characterize T. harzianum transcripts under three conditions, i.e. PDA supplemented with sea salt (A), PDA supplemented with sea salt and 100 μg/ml HYG (B) and PDA (C). The numbers of differentially expressed genes (DEGs) were shown in Fig. 3. Most of DEGs were detected when compared B to A, indicating that the expression of many genes under sea salt stress (A) was converse with the extra addition of HYG (Fig. 3).
GO enrichment for DEGs
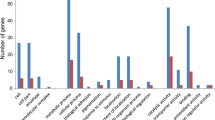
The clustering analysis based on Gene Ontology (GO) categories was performed to analyze the DEGs of T. harzianum response to sea salt and HYG (Fig. 4).
In the presence of sea salt, the most abundant enriched GO terms in “Biological Process” were related to cell wall disassembly (GO:0044277, GO:0071853 and GO:1904541), cell wall organization (GO:0070871), cell septum edging catabolic process (GO:0030995) and extracellular polysaccharide catabolic process (GO:0071999) (Fig. 4a). All of DEGs related to these GO terms were significantly down-regulated for about 13–209.9 times. In the ontological category “Molecular Function”, the DEGs were related to glucosidase activity (GO:0015926, GO:0051118), among which one gene was up-regulated for about eight times and six genes were down-regulated for 4.6–207.9 times (Fig. 4a). These results imply that the cell wall of T. harzianum might experience a great change under sea salt stress due to the extracellular polysaccharide catabolic variation.
Comparing B to C, the most abundant enriched GO terms in the “Biological Process” were also related to cell wall formation, same as the group of A vs C (Fig. 4a and b), which might affect the cell wall phenotypes of T. harzianum. Therefore, the cell walls of T. harzianum cultivated under these 3 conditions (A, B and C) were examined with transmission electron microscope (TEM). Much thicker cell walls were observed in B and A comparing to C, which was in agreement with the transcriptomic changes (Fig. 5). While in the ontological category “Molecular Function”, the result seemed to be a combination of the outcome of A vs C and B vs A, with significantly up-regulated terms as acyltransferase (GO:0016746 GO:0016747 GO:0016410), acetyltransferase (GO:0016407 GO:0008080), and significantly down-regulated term as glucan endo-1,3-alpha-glucosidase (GO:0051118) (Fig. 4b).
Compared between B and A (Fig. 4c), the most abundant enriched GO terms in the “Biological Process” were related to single-organism metabolic process (GO:0044710), followed by small molecule metabolic process (GO:0044281), organic acid metabolic process (GO:0006082, GO:0019752 and GO:0043436), and tRNA aminoacylation (GO:0006418 and GO:0043039), which were among the significantly up-regulated terms. “Cellular Component” related to transmembrane transporter complex (GO:1902495), high-affinity iron permease complex (GO:0033573) and ferroxidase complex (GO:1905862) were significantly up-regulated. In the ontological category “Molecular Function”, DEGs were mostly associated with transferase activity, including N-acyltransferase (GO:0016746, GO:0016747 and GO:0016410), acetyltransferase (GO:0016407 and GO:0008080) and tRNA related ligase (GO:0004812, GO:0016875 and GO:0016876), which were among the significantly up-regulated terms as well (Fig. 4c).
DEGs found in all three groups included ion related transporters as well as genes coding for multidrug resistance protein (MDR), multidrug resistance associated protein (MPR), pleiotropic drug resistance protein (PDR) and other transporters of ATP binding cassette (ABC) superfamily or major facilitator superfamily (MFS) (Supplementary Table 2). The results revealing that T. harzianum might employ diverse transport systems for sea salt and HYG, which were likely involved in the enhanced resistance. Further venn analysis of ion related transporters indicated that 2 DEGs coding for putative Na+/K+-ATPase were considerably up-regulated with sea salt and HYG addition (Fig. 6a A vs C and B vs C), which might be mainly induced by sea salt and independent from HYG (Fig. 6a B vs A). Meanwhile, there were 2 down-regulated overlapping DEGs coding for putative Ca2+/H+ antiporter (M431DRAFT_5034) and Na+/phosphate symporter (M431DRAFT_93229) with HYG addition (Fig. 6a, B vs A and B vs C), indicating that HYG might inhibit the expression of these two genes. 6 DEGs demonstrated that HYG addition with sea salt had adverse effects as that of sole sea salt addition (Fig. 6a, B vs A). Apart from M431DRAFT_495547, the expressions of the other 5 DEGs were inhibited by HYG, including putative solute carrier (SLC), potassium uptake protein, Ca2+/H+ antiporter, and cation transporting ATPases (Fig. 6a). The venn analysis diagram of MDR/MRP-related DEGs showed that the expression of one putative MDR (M431DRAFT_487806) gene was inhibited by sea salt addition (Fig. 6b, A vs C), which can be induced by HYG addition (Fig. 6b, B vs C). Furthermore, there were 6 MRP/PDR genes (M431DRAFT_6304, 77311, 116190, 90674, 477499 and 114027) and 4 MDR genes (M431DRAFT_488109, 70698, 94810 and 88202) elicited by HYG (Fig. 6b). Moreover, the venn analysis diagram of DEGs concerning glucosidase suggested that sea salt addition in the media could remarkedly inhibit the expression of 4 glucosidases (M431DRAFT_479664, 152795, 525334, and 71044), and the extra addition of HYG demonstrated no obvious difference (Fig. 6c). In addition, the expression of 11 acetyltransferases was not affected by sea salt (Fig. 6d, A vs C), but significantly induced by HYG, including M431DRAFT_7382, 276757, 482749, 506859, 511669, 511369, 520260, 511800, 507123, 94097 and 505610 (Fig. 6d, B vs A and B vs C).
Discussion
Ions play a vital role in various living organisms, which have evolved efficient regulatory systems for ions uptake and export to keep ion homeostasis (Han et al. 2017). Halophilic bacteria were found to show natural resistance to antibiotics, whose mechanism have remained loosely defined (Tokunaga et al. 2004). Previous study found that a Na+/ATPase plasma membrane transporter Ena1 in S. cerevisiae mediated toxic cations efflux by hydrolyzing ATP and inhibition of Ena1 dramatically enhanced susceptibility of Cryptococcus neoformans to antifungal drugs, such as amphotericin B (Jung et al. 2012). RNA-seq analysis of T. harzianum LZDX-32-08 revealed two putative Na+/K+ ATPases (coded by M431DRAFT_511629 and 512552), which show 42.64% and 50.05% identity to Ena1, respectively. The significantly increased expression of these two genes with sea salt (Fig. 6a, A vs C) or sea salt and HYG addition (Fig. 6a, B vs C) indicated that they could play a critical role in both ion homeostasis and the induced HYG resistance of T. harzianum. Besides, calcium is a second messenger involved in growth, development, apoptosis, and stress response. Enhanced cytosolic Ca2+ transient was found to increase the azole resistance of A. fumigatus by up-regulating a series of calcineurin-dependent–response-element genes (Li et al. 2020). In our study, calcium addition in PDA displayed the strongest effect to confer the antifungal resistance (Fig. 2). Therefore, Ca2+ transporters were of great interests. Two putative Ca2+/H+ antiporters (coded by M431DRAF_5034 and 72281), shared 48.91% and 33.96% identity to Vcx1 from S. cerevisiae, respectively, were clearly down-regulated with HYG and/or sea salt addition (Fig. 6a), which could contribute to enhanced cytosolic Ca2+ and consequently elicit the HYG resistance (Stathopoulos-Gerontides et al. 1999). In addition, MDR is vital for antifungal resistance. 4 putative MDR transporters of MFS family and 6 MRP/PDR genes of ABC family were considerably up-regulated (log2FoldChange up to 8.70) mainly induced by HYG in the media (Fig. 6b). Among them, M431DRAFT_88202 shows 34.27% identity to Flu1 in Candia albicans. The expression of Flu1 in S. cerevisiae made it resistant to fluconazole and cycloheximide (Calabrese et al. 2000). Besides, putative PDR protein (coded by M431DRAFT_114027) shows 34.65% identity to the Pdr5 of ABC family in S. cerevisiae (Hadiar Rahman et al. 2018). Since these transporters utilize electrochemical potential as energy to transport substrate and require the involvement of cations (Na+ or H+) in the transport of drugs (Fluman et al. 2014), the addition of sea salt might facilitate these transporters and accelerate the efflux of HYG to confer fungal resistance.
In addition to transporters, the formation of biofilms has been shown to be the main reason of drug resistance in Candidia spp. (Silva et al. 2017; Huang et al. 2020). β-1,3-glucan is an important component of biofilm. The expressions of 4 α/β-1,3-glucosidases were significantly down-regulated with sea salt addition, which might reduce the hydrolyzation of β-1,3-glucan and contribute to thicker cell wall and formation of biofilm. Furthermore, microbes could produce enzymes to inactivate antibiotics. For instance, Eis from Mycobacterium smegmatis could acetylate aminoglycoside antibiotics to confer resistance (Pan et al. 2018). In our case, 11 putative N-Acetyltransferase genes were up-regulated mainly induced by HYG, which could inactivate HYG by acetylation (Rao et al. 1983). Among them, a putative N-Acetyltransferase coded by M431DRAFT_482749 shows 37.93% identity to Eis (Pan et al. 2018). Further characterization of these genes might provide novel types of inactivation enzymes.
The aforementioned DEGs are promising candidate genes responsible for the antifungal resistances. Taken together, 3 major mechanisms were proposed for the enhanced HYG resistance of T. harzianum LZDX-32-08 under sea salt stress (Fig. 7). Firstly, sea salt addition induced the expression of P-type ATPases to facilitate ionic transport in order to equilibrium the intracellular osmotic pressure. Along with this process, the electrochemical potential or proton gradient was changed, which might affect the MDR related transporters induced by HYG and promoted the efflux of HYG. Secondly, sea salt addition significantly affected the cell wall phenotype and reduced the expression of 1,3-glucosidase genes, leading to thicker cell wall and forming of biofilms, which could decrease the uptake of HYG. At last, HYG induced the expression of acetyltransferases and resulted in its inactivation. The synergistic effects of the above mechanisms could jointly contribute to the increased resistance of T. harzianum to HYG under sea salt environment.
Further investigation on the relationship between ion transporters and MDR related transporters could provide more detailed information for the resistance mechanism. Meanwhile, functional characterization of the candidate genes in the future could shed light on new targets for overcoming this resistance.
References
Alao JP, Weber AM, Shabro A, Sunnerhagen P (2015) Suppression of sensitivity to drugs and antibiotics by high external cation concentrations in fission yeast. PLoS One 10:0119297
Barreto L, Canadell D, Petrezselyova S et al (2011) A genomewide screen for tolerance to cationic drugs reveals genes important for potassium homeostasis in Saccharomyces cerevisiae. Eukaryot Cell 10:1241–1250
Calabrese D, Bille J, Sanglard D (2000) A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology 146(Pt 11):2743–2754
Denning DW, Bromley MJ (2015) How to bolster the antifungal pipeline. Science 347:1414–1416
Fluman N, Adler J, Rotenberg SA, Brown MH, Bibi E (2014) Export of a single drug molecule in two transport cycles by a multidrug efflux pump. Nat Commun 5:4615
Rahman H, Carneglia J, Lausten M, Robertello M, Choy J, Golin J (2018) Robust, pleiotropic drug resistance 5 (Pdr5)-mediated multidrug resistance is vigorously maintained in Saccharomyces cerevisiae cells during glucose and nitrogen limitation. FEMS Yeast Res 18(4):1–7
Han D, Link H, Liesack W (2017) Response of Methylocystis sp. strain SC2 to salt stress: physiology, global transcriptome, and amino acid profiles. Appl Environ Microbiol 83(20):AEM.00866–17.
Honore N, Marchal G, Cole ST (1995) Novel mutation in 16S rRNA associated with streptomycin dependence in Mycobacterium tuberculosis. Antimicrob Agents Chemother 39:769–770
Huang X, Zheng M, Yi Y, Patel A, Song Z, Li Y (2020) Inhibition of berberine hydrochloride on Candida albicans biofilm formation. Biotechnol Lett. https://doi.org/10.1007/s10529-020-02938-6
Jung KW, Strain AK, Nielsen K, Jung KH, Bahn YS (2012) Two cation transporters Ena1 and Nha1 cooperatively modulate ion homeostasis, antifungal drug resistance, and virulence of Cryptococcus neoformans via the HOG pathway. Fungal Genet Biol 49:332–345
Karlowsky JA, Hoban DJ, Zelenitsky SA, Zhanel GG (1997) Altered denA and anr gene expression in aminoglycoside adaptive resistance in Pseudomonas aeruginosa. J Antimicrob Chemoth 40:371–376
Kaster KR, Burgett SG, Rao RN, Ingolia TD (1983) Analysis of a bacterial hygromycin B resistance gene by transcriptional and translational fusions and by DNA sequencing. Nucleic Acids Res 11:6895–6911
Li Y, Zhang Y, Zhang C, Wang H, Wei X, Chen P et al (2020) Mitochondrial dysfunctions trigger the calcium signaling-dependent fungal multidrug resistance. Proc Natl Acad Sci USA 117:1711–1721
Perlin DS, Brown CL, Haber JE (1988) Membrane potential defect in hygromycin B-resistant pma1 mutants of Saccharomyces cerevisiae. J Biol Chem 263:18118–18122
Pan Q, Zhao F-L, Ye B-C (2018) Eis, a novel family of arylalkylamine N-acetyltransferase (EC 2.3.1.87). Sci Rep 8:1–8
Rao RN, Allen NE, Hobbs JN Jr, Alborn WE Jr, Kirst HA, Paschal JW (1983) Genetic and enzymatic basis of hygromycin B resistance in Escherichia coli. Antimicrob Agents Chemother 24:689–695
Sekyere JO, Asante J (2018) Emerging mechanisms of antimicrobial resistance in bacteria and fungi: advances in the era of genomics. Future Microbiol 13:241–262
Silva S, Rodrigues CF, Araujo D, Rodrigues ME, Henriques M (2017) Candida species biofilms' antifungal resistance. J Fungi (Basel) 3(1):pii: E8
Stathopoulos-Gerontides A, Guo JJ, Cyert MS (1999) Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev 13:798–803
Tokunaga H, Mitsuo K, Ichinose S, Omori A, Ventosa A, Nakae T, Tokunaga M (2004) Salt-inducible multidrug efflux pump protein in the moderately halophilic bacterium Chromohalobacter sp. Appl Environ Microbiol 70:4424–4431
Acknowledgements
This work was supported by the National Key R&D Program of China [Grant Number 2018YFA0902200]; the National Natural Science Foundation of China [Grant Number 21838001, 21525625, and 81803409]; and the Fundamental Research Funds for the Central Universities [Grant Numbers buctrc201810, XK1802-8 and PT1909]. The funding agencies had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Supplementary Information
Supplementary Table 1- Strains used in this study.
Supplementary Table 2- Other transporter-related genes in DEGs of T. harzianum when cultivated at 28 °C for 6 days on PDA supplemented with sea salt (A), PDA supplemented with sea salt and HYG (B) and PDA (C).
Supplementary Fig. 1- NMR spectra of HYG (a) and mixture of HYG and sea salt (b).
Supplementary Fig. 2- Trichoderma harzianum LZDX-32-08 cultivated at 28 °C for 4 days on PDA supplemented with 0, 0.57, 1 or 2 M sorbitol and 0, 10, 50, 100 or 200 μg/mL HYG, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
This article does not contain any studies with human participates or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, X., Wei, H., Liu, X. et al. Enhanced resistance of Trichoderma harzianum LZDX-32-08 to hygromycin B induced by sea salt. Biotechnol Lett 43, 213–222 (2021). https://doi.org/10.1007/s10529-020-02994-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02994-y