Abstract
Objective
To effectively and conveniently detect pathogenic bacteria, this study aimed to develop label-free biosensors fabricated affinity peptides that can recognize targeted bacteria strains and enable precise quantitative detections.
Results
A 12-mer peptide with high binding affinity toward Escherichia coli O157:H7 was discovered by biopanning of phage-displayed peptide library. The peptide modified with glycine residues (G3) and one cysteine (C) residue at C-terminal, could self-assemble on gold electrodes, enabling electrochemical impedance spectroscopy (EIS) analysis for quantitative detection of E. coli O157:H7. This method showed a low detection limit of 20 CFU/mL and a liner range from 2 × 102 to 2 × 106 CFU/mL.
Conclusion
It appears that, by designing and optimizing the structures of peptides, such a strategy can be greatly promising in developing quick, sensitive and quantitative biosensor of pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli O157:H7 (E. coli O157:H7) is the predominant virulent serotype in a pathogenic subset of Enterohemorrhagic E. coli (EHEC), and is especially founded responsible for serious foodborne infection outbreaks. The control of E. coli O157:H7 infection outbreaks requires rapid and sensitive detections of microbial contamination. However, it is often a daunting task to isolate and concentrate a small number of bacterial cells from large volume of samples. Currently available methods for qualitative microbial detection generally require a tedious processing procedure of the bacteria in a sample. Therefore, people have been very interested in seeking alternative real-time fast detections in recent years. In particular, a variety of sensors has been developed based on the antigenicity of the target bacterial cells.
Technologies including fluorescence (Kulpakko et al. 2015), surface plasmon resonance (SPR) (Vaisocherova-Lisalova et al. 2016), electrochemical impedance spectroscopy (EIS) (Ruan et al. 2002) were combined with antibody–antigen detection, resulting sensitive biosensors. Electrochemical method is fast response, label-free, and easy to integrate into miniaturized microdevices like portable biosensors. EIS is a convenient method to measure molecular interactions with electrodes and therefore can afford sensors high sensitivity (Bogomolova et al. 2009). The bacteria was detected by using EIS with a lower limit of detection (2 CFU/mL) (Santos et al. 2013). Nevertheless, these methods relied on antibodies are relatively expensive and the instability of the protein structure often lead deactivation of biosensors.
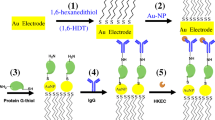
In comparison to the size of antibody, peptides are relatively small fragment of functional protein, and it can be cost-effectively synthesized. Biosensors modified by antimicrobial peptides (AMPs) had the advantages of good stability and high binding affinity, greatly appealing for selective bacterial detection (Li et al. 2014; Liu et al. 2016). To avoid toxic of AMPs to the bacterial cells, affinity peptides need selection though another method. In recent years, phage display has been widely used to screen affinity peptides against biomolecule (Kim et al. 2005) and whole cells (Rao et al. 2013). It has been regarded as an effective tool for the discovering peptides binding to surface epitopes on pathogenic microorganism (Fang et al. 2006), which have been used in developing biosensor for microbial detections (Hwang et al. 2017). In this work, we examined the use of a Ph.D.12 library for discovery of peptides that can specifically bind to E. coli O157:H7, and further studied biosensors fabricated with affinity peptides for bacteria detection by EIS (Scheme 1).
Materials and methods
Bacterial strains, bacteriophage, and reagents
Escherichia coli O157:H7 (ATCC700728) strain was purchased from China Center of Industrial Culture Collection (CICC, China). Ph.D.-12 Library kit (E8110S) was obtained from NEB including E. coli ER2738. HRP/Anti-M13 Monoclonal conjugate antibody (GE Healthcare, 27–9421-01) and 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, A1888) were purchased from Sigma-Aldrich (Inc., USA). Ultrapure water (18.2 MΩcm) was prepared using a Millipore simplicity system and used throughout the experiments.
Biopanning of phages binding to E. coli O157:H7
We used the Ph.D.-12 library in this study following the phage display instruction manual and the biopanning as described earlier (Katz 1997). Briefly, 10 μL phage library (~ 1013 pfu/mL) were added into 1 mL E. coli O157:H7 cell suspensions (OD600 = 0.5) and incubated for 1 h at room temperature with gentle agitation. Bacteria with bound phages were precipitated by spinning for 5 min at 16,000×g, and separated from unbound phages in solution by a series of 10 washing and centrifugation steps (16,000×g, 5 min) with 1 mL TBST buffer (50 mM Tris–HCl, 0.05% (v/v) Tween 20) each time. After washing, bound phages with E. coli O157:H7 were suspended in 200 μL elution buffer (0.2 M glycine–HCl, pH 2.2) with gentle shaking at room temperature for 10 min. The eluted phages were neutralized with 150 μL, 1 M Tris–HCl (pH 9.1).
The titer of the phage was determined by plating them on LB X-gal/IPTG plates; amplified by infecting E. coli ER2738; purified with PEG/NaCl (20%(w/v) polyethylene glycol-8000, 2.5 M NaCl) double precipitation; and used as input for next round biopanning against fresh E. coli O157:H7 cells.
DNA sequencing and peptide synthesis
After four rounds affinity selection, the plaques of phage single clones were singled out for preparing phage stocks to isolate genomic DNA for nucleotide sequencing by M13 DNA isolation Kit (Biomiga. Inc). DNA sequences were translated into amino acid residues of peptides. The affinity peptides (APs) modified with a G3C were synthesized by Top-peptide Biotechnology (Shanghai, Co. Ltd).
Enzyme-linked Immunosorbent Assays (ELISA) detection of affinity phages binding to E. coli O157:H7 cells
Log-phase cultures of bacteria were centrifuged and washed by phosphate buffer saline (PBS, pH7.4) for three times. Bacterial cell suspension at a concentration of 105 CFU/mL was coated to the wells of 96-well microplate and incubated overnight at 4 °C. Subsequently, cells were fixed with ethanol and blocked with 5% not-fat dry milk in PBS for 1 h at RT with gentle shaking, rinsed with PBST (PBS with 0.05% Tween 20) and then phage clones suspended in PBS with 10 serial dilutions were added to all the wells and incubated for 2 h at RT with shaking. After being washed, wells were further incubated with 200 μL of HRP/Anti-M13 Monoclonal conjugate antibody at a dilution of 1:5000 in blocking buffer for 1 h at RT and washed again. ABTS substrate (200 μL) was used to detect amount of Anti-M13 antibodies, which reflect the phage display peptides binding. The color development was recorded using a microplate reader (Thermo Fisher, Multiskan™ GO) by monitoring absorbance at 405 nm. E. coli O157:H7 and all the control bacteria were incubated separately in the wells of 96-well plate, and the plastic plate was used as blank control.
Preparation of the affinity peptide (AP) based biosensor
Gold electrode (3 mm diameter) was first polished with 0.5 μm alumina slurry, sonicated with ultrapure water and ethanol for 10 min separately, and dried under nitrogen gas flow before being further processed. To immobilize the APs, the clean gold electrode immersed in peptide solution (20 mM Tris–HCl buffer, containing 0.1 mg/mL peptide) for 0.5 h. After incubation, the electrode was washed using Tris–HCl buffer to remove unbounded peptide.
Electrochemical measurements
The electrochemical measurements were conducted with a CHI 760D electrochemical station (Shanghai CH Instruments Co., Ltd), using a three-electrode system in an enclosed faraday cage with the gold working electrode, an Ag/AgCl/KCl (3.0 M) reference electrode, and a platinum wire counter electrode. Cyclic voltammetry (CV) and EIS were performed in 5 mM K3[Fe(CN)6]/0.1 M KCl solution after the solution was bubbled for 15 min with nitrogen. The electrochemical properties of the electrodes prepared at different stages were examined by CV using FeII(CN)64−/FeIII(CN)63− reversible redox system. The potential of the working electrode was controlled between −0.1 and 0.6 V at a scan rate of 50 mV/s.
Bacteria detection was conducted by contacting the working electrode with bacteria solutions with different concentrations prepared in PBS (pH 7.4) for 30 min. The electrode was then washed twice using PBS buffer to remove unbounded cells. EIS experiments were implemented in the frequency range from 100 kHz to 0.1 Hz with disturbance amplitude of 5 mV. All measurements were repeated at least three times. The Origin 8.0 and Adobe illustrator CC software were used for data analyze and treatment.
Results and discussion
Biopanning and sequence analysis of affinity binding peptides to E. coli O157:H7
NEB phage displayed random peptide library was applied to identify peptide candidates that can recognize cell surface of E. coli O157:H7 via affinity binding. We performed a selection procedure in which phage clones were allowed to bind with E. coli O157:H7 cells in suspension first and were then separated by centrifugation. Cell-bounded phages isolated by acid elution were then amplified in an E. coli ER2738 host strain and applied for subsequent rounds of screening. After each round of selection, the phage stocks were tittered prior to amplification. During the selection, the recovery rate of E. coli O157:H7-binding phages showed an increase (data shown in Supplementary Table 1) as procedure proceeded, indicating an effective selection of bacterial binders.
Totally 23 monoclonal phages with peptide inserted were eventually identified from the third and fourth round and were isolated, from which genomic DNA was extracted and sequenced. The peptide sequences obtained from last two rounds were summarized in Supplementary Table 2. There were some insertless phage clones (no peptide display on the capsid protein pIII) in each round, which can be attributed to the inherent rate of insertless phages in the NEB’s Ph.D.-12 library (~ 5.8%).
The frequency distribution of amino acid residues of the totally 23 peptides selected is shown in Fig. 1. Overall, 18 amino acid residues presented in the selected peptides (Table 1) with 4 amino acid residues (L, V, H, Y) had obviously higher observed frequency than their appearance in the original peptide library. The hydrophobic amino acid residue (V) and hydrophilic amino acid residue (Y) were about twofold more frequent. It appeared that hydrophobic and hydrophilic amino acid residues occurred alternately in the sequence as shown in Table 1, and all the peptides have charged residues (H, K, D, E). Such a structural layout may hint hydrophobic/hydrophilic interactions play a critical role in constituting the desired binding affinity, along with electrostatic interactions as an auxiliary factor, leading to a combination of the two forces for peptides and cell binding (Zita and Hermansson 1997).
Affinity binding capability of phage clones with affinity peptides
To determine the binding capacity and affinity strength, four identified phage clones (from Table 1) showed up more than once were chosen for ELISA assay. The selected clones were amplified and determined by phage titer assays prior to interact with targeted bacteria, and the phage peptide library was used as a control. As shown in Fig. 2, the phage displaying peptides AP2(VVSPDMNLLLTN) and AP3(GLHTSATNLYLH) exhibited a 3-times higher binding capacity against E. coli O157:H7 than that achieved with peptide library. As the best affinity binding peptide, AP3 was accordingly chosen for subsequent studies for construction of APs-modified gold electrode biosensor to E. coli O157:H7. It is noteworthy that AP3 identified in this work is very different structurally from other cellular affinity peptides reported previously.
Preparation and electrochemical characterization of AP-fabricated electrode
As shown in Scheme 1, the biosensor fabrication was realized by allowing the APs assemble on the surface of gold electrode through -SH residue of the peptide. The sequence GLHTSATNLYLHGGGC used in this work with three-glycine (G) residues act as a spacer and an external cysteine (C) that can act as an anchor onto gold electrode. CV and EIS were further conducted to demonstrate the attachment of the APs and bacteria onto the gold electrode surface. Figure 3A exhibits CVs of bare and APs modified gold electrodes tested in 5 mM potassium ferricyanide. The bare gold electrode incubated E. coli O157:H7 as a control. The symmetrical curves indicated that good reversibility of the reaction on the electrode surface. The CV curves of the bare gold electrode showed redox peak with current value as high as 43.54 μA. After immobilization of APs, the peak current intensity was decreased to 32.46 μA. It indicated that the peptide binding had impeded the electron transfer process required by the redox reactions on surface of the electrode (Liu et al. 2016). After incubation with E. coli O157:H7 cells (106 CFU/mL), the electron transfer rate was further restricted and the current peak value decreased to as low as 15.52 μA. On the other hand, bare gold electrode incubated with E. coli O157:H7 cells (106 CFU/mL) showed a peak current at 43.97 μA that is close to that of the bare electrode. Apparently, bare gold electrode could not capture cells, while affinity peptides assembled on the electrode can grab microbial cell to electrode surface.
Electrochemical properties of AP-fabricated electrodes. A Cyclic voltammotry (CV) of the gold electrode with different modifications. B Nyquist plots of electrode at different stages. (i) Bare gold; (ii) after incubating E. coli O157:H7; (iii) after immobilization of affinity peptides; (iv) APs modified electrode after binding E. coli O157:H7. The impedance spectra were recorded from 100 kHz to 0.1 Hz and AC amplitude with 5 mV
EIS is an effective method to evaluate surface modification of electrode, and toward that, Nyquist plot that shows the impedance spectra in the presence of the redox probe of [Fe(CN)6]3−/4− is adapted in the current study. Nyquist plot contains the solution phase resistance Rs, the charge transfer resistance Rct, and the Warburg impedance W. The semicircle diameter of the Nyquist curve accords reflects the charge transfer resistance, which is further related to modifications and interactions taking place on the surface. Figure 3B shows the impedance spectra of the bare gold electrode, the bare gold electrode incubating E. coli cells, and the electrode modified with APs and incubated with E. coli cells (106 CFU/mL). The bare gold electrode and the bare gold electrode incubated with E. coli cells displayed a very small semicircle, with Rct determined as 291.4 Ω and 207.0 Ω, respectively. That indicated good charge transfer on the electrode. After modification with APs, Rct value was boomed to 2048.0 Ω, reflecting much increased resistance against electron transfer on surface of the electrode. After binding of E. coli cells on the modified APs gold surface, since it is difficult for the redox species to penetrate bacteria layers, the Rct value was significant increased to as high as 15,710.0 Ω. All of the EIS tests demonstrated here suggest that the electrochemical biosensors fabricated with APs would allow a sensitive, selective and quantitative detection of the targeted bacterial pathogens.
Performance of AP-fabricated electrode for detection of E. coli O157:H7
We further evaluate the sensitivity of the AP-fabricated biosensors for the detection of E. coli O157:H7 by varying cell concentration. The Nyquist plots (Fig. 4A) of impedance spectra were recorded for increasing concentrations of E. coli O157:H7 ranged from 20 to 2 × 106 CFU/mL. As the cell concentrations increased, a significant impedance difference could be observed. At low frequencies (0.1–1 Hz), the different concentrations of bacterial cells had the effect of increasing the impedance in proportion to cell concentration. That is an indication that impedance changes are closely associated with charge transfer properties on the surface of electrode. This is the desired working situation under which the electrode transfer resistance of the redox probe can be correlated well to the number of captured bacterial cells on the electrode surface. For tests with higher frequencies, changes in cell concentration showed less influence on impedance, approaching to the status that leaving dielectric relaxation of small dipoles including water molecules in the buffer solution to becoming more dominant in affecting the apparent impedance (Mannoor et al. 2010).
A Electrochemical impedance spectra of APs biosensor for different E. coli O157:H7 concentrations from 2 × 10 CFU/mL to 2 × 106 CFU/mL: black filled square—2 × 10 CFU/mL, red filled circle—2 × 102 CFU/mL, green filled triangle—2 × 103 CFU/mL, blue filled inverted triangle—2 × 104 CFU/mL, light blue left filled right triangle—2 × 105 CFU/mL, violet right filled right triangle—2 × 106 CFU/mL. The impedance spectra were recorded from 100 kHz to 0.1 Hz and AC amplitude with 5 mV. B Relationship between RCT and log (E. coli O157:H7 concentrations from 2 × 102 to 2 × 106 CFU/mL) with its corresponding fitting
Quantitative analysis showed that changes in RCT(ΔRCT = RCT(APs−O157)−RCT(APs)) was about proportional to the logarithm of cell concentrations when varied in the range of 2 × 102 to 2 × 106 CFU/mL (see Fig. 4B). The low limit of detection (LOD) of the method appeared to 20 CFU/mL. This LOD is comparable to and maybe even slightly better than those reported for other methods that applied antimicrobial peptide and polyaniline modified impedimetric biosensor (Chowdhury et al. 2012) for E. coli O157:H7 detection.
Conclusions
In this study, we demonstrated the fabrication of a new biosensor based on EIS for the rapid detection of E. coli O157:H7 by assembly of E. coli O157:H7 affinity peptide identified through biopanning firstly. The specificity of sequence reflects affinity binding of peptide on E. coli O157:H7 is hydrophobic/hydrophilic interactions along with electrostatic interactions. It appeared the fabrication of electrode was efficient in terms of achieving cell recognition and quantification, offering at the same time good sensitivity and high specificity. The results demonstrate that the sensor could be operated easily and quickly (requires only ~ 30 min incubation), with a broad cell concentration range (20 to 2 × 106 CFU/mL). All of these make it attractive for in situ biosensing applications serving the rapidly growing demands in public health, environment, and food safety.
References
Bogomolova A, Komarova E, Reber K, Gerasimov T, Yavuz O, Bhatt S, Aldissi M (2009) Challenges of electrochemical impedance spectroscopy in protein biosensing. Anal Chem 81:3944–3949
Chowdhury AD, De A, Chaudhuri CR, Bandyopadhyay K, Sen P (2012) Label free polyaniline based impedimetric biosensor for detection of E. coli O157:H7 bacteria. Sens Actuators B 171–172:916–923
dos Santos MB, Agusil JP, Prieto-Simon B, Sporer C, Teixeira V, Samitier J (2013) Highly sensitive detection of pathogen Escherichia coli O157:H7 by electrochemical impedance spectroscopy. Biosens Bioelectron 45:174–180
Fang ZD, Laskey JG, Huang S, Bilyeu KD, Morris RO, Schmidt FJ, English JT (2006) Combinatorially selected defense peptides protect plant roots from pathogen infection. Proc Natl Acad Sci USA 103:18444–18449
Hwang HJ, Ryu MY, Park CY, Ahn J, Park HG, Choi C, Ha S-D, Park TJ, Park JP (2017) High sensitive and selective electrochemical biosensor: label-free detection of human norovirus using affinity peptide as molecular binder. Biosens Bioelectron 87:164–170
Katz BA (1997) Structural and mechanistic determinants of affinity and specificity and specificity of ligands discovered or engineered by phage display. Annu Rev Phys Chem 26:27–45
Kim YG, Lee CS, Chung WJ, Kim EM, Shin DS, Rhim JH, Lee YS, Kim BG, Chung J (2005) Screening of LPS-specific peptides from a phage display library using epoxy beads. Biochem Bioph Res Commun 329:312–317
Kulpakko J, Kopra K, Hänninen P (2015) Time-resolved fluorescence-based assay for rapid detection of Escherichia coli. Anal Biochem 470:1–6
Li Y, Afrasiabi R, Fathi F, Wang N, Xiang C, Love R, She Z, Kraatz HB (2014) Impedance based detection of pathogenic E. coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens Bioelectron 58:193–199
Liu X, Marrakchi M, Xu D, Dong H, Andreescu S (2016) Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens Bioelectron 80:9–16
Mannoor MS, Zhang S, Link AJ, McAlpine MC (2010) Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc Natl Acad Sci USA 107:19207–19212
Rao SS, Mohan KV, Gao Y, Atreya CD (2013) Identification and evaluation of a novel peptide binding to the cell surface of Staphylococcus aureus. Microbiol Res 168:106–112
Ruan C, Yang L, Li Y (2002) Immunobiosensor chips for detection of Escherichia coli O157:H7 using electrochemical impedance spectroscopy. Anal Chem 74:4814–4820
Vaisocherova-Lisalova H, Visova I, Ermini ML, Springer T, Song XC, Mrazek J, Lamacova J, Lynn NS Jr, Sedivak P, Homola J (2016) Low-fouling surface plasmon resonance biosensor for multi-step detection of foodborne bacterial pathogens in complex food samples. Biosens Bioelectron 80:84–90
Zita A, Hermansson M (1997) Determination of bacterial cell surface hydrophobicity of single cells in cultures and in wastewater in situ. FEMS Microbiol Lett 52:299–306
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos.31471659, 21636003 and 21303050) and the Natural Science Foundation of Shanghai (Grant No.19ZR1412400).
Supporting information
Supplementary Table 1—Enrichment of positive phage clones by subtraction biopanning
Supplementary Table 2—The peptides isolated from the 3rd and 4th round.
Supplementary Fig. 1—Bode plot for impedance measurement of an APs modified electrode, varying concentration of E. coli O157:H7 from 2 × 10 to 2 × 106 CFU/mL. (Filled rectangle) 2 × 10 CFU/mL. (Filled red circle) 2 × 102 CFU/mL, (Filled blue triangle) 2 × 103 CFU/mL. (Filled green inverted triangle) 2 × 104 CFU/mL. (Purple left sided triangle) 2 × 105 CFU/mL. (Filled light green right sided triangle) 2 × 106 CFU/mL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shi, F., Gan, L., Wang, Y. et al. Impedimetric biosensor fabricated with affinity peptides for sensitive detection of Escherichia coli O157:H7. Biotechnol Lett 42, 825–832 (2020). https://doi.org/10.1007/s10529-020-02817-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-020-02817-0