Abstract
Objectives
To genetically engineer Escherichia coli for the heterologous biosynthesis of triterpenoid, ambrein, the main bioactive component of ambergris, by constituting a novel squalene-derived ambrein biosynthetic pathway in E. coli.
Results
The ScERG9 gene encoding the squalene synthase (SS) was integrated into the E. coli genome to generate a squalene-producing strain that supplied the central precursor squalene for the formation of cyclic triterpenoids. The mutated squalene–hopene synthase (D377C SHC) and the tetraprenyl-β-curcumene cyclase (BmeTC) were co-expressed with SS to construct a novel ambrein biosynthetic pathway in E. coli. Ambrein was produced at 2.6 mg l−1.
Conclusions
An E. coli chassis for ambrein production was constructed by combining the squalene synthesis module with the downstream cyclization module.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The tricyclic triterpenoid, ambrein (C30H52O), is the major component of ambergris which is secreted from sperm whale intestines (Ohloff et al. 1977). Ambergris is one of the most valued animal perfumes and fragrances (Kovatcheva et al. 2004). Oxidative degradation of ambrein is responsible for the scent of ambergris (Tanimoto and Oritani 1997). Furthermore, ambergris is widely used in Eastern folklore medicine to treat common colds (Taha et al. 1998) and migraine, and is also an aphrodisiac (Shen et al. 2007). Ambrein possesses antinociceptive (Taha 1992), anti-inflammatory (Shen et al. 2007), and aphrodisiac properties (Taha et al. 1995). Ambergris is either obtained via whaling or is washed ashore (Taha 1989). However, natural sources of ambergris are increasingly limited due to the banning of commercial whaling by many countries. Thus, metabolic engineering of microorganisms has become a favorable alternative for ambrein production.
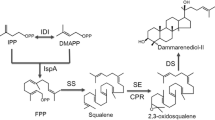
Although the endogenous ambrein pathway in sperm whales remains to be fully elucidated, Ueda et al. (2013) have developed a two-step enzymatic method for ambrein synthesis using the common triterpenoid precursor, squalene. The mutated squalene–hopene cyclase (D377C SHC) from Alicyclobacillus acidocaldarius catalyzes cyclization of squalene into the monocyclic 3-deoxyachilleol A (Sato and Hoshino 1999). The tetraprenyl-β-curcumene cyclase (BmeTC) from Bacillus megaterium is a multifunctional terpenoid cyclase that converts squalene into the bicyclic 8α-hydroxypolypoda-13,17,21-triene (Sato et al. 2011) and converts 3-deoxyachilleol A into the tricyclic ambrein (Ueda et al. 2013). However, heterologous production of ambrein in microbial systems has not been reported. Here, we selected Escherichia coli, as the host to realize the biosynthesis of ambrein (see Fig. 1).
The native methylerythritol-phosphate (MEP) pathway in E. coli can provide building blocks, including farnesyl pyrophosphate (FPP), for terpenoid production (Ajikumar et al. 2010). The first step in triterpenoid synthesis is condensation of two 15-carbon FPP molecules into 30-carbon intermediate squalene, which is catalyzed by squalene synthase (SS; Katabami et al. 2015). In the past decade, squalene production has been achieved by expressing SS from human (Katabami et al. 2015) or yeast (Li et al. 2016) in E. coli. Here, we co-expressed SS, D377C SHC, and BmeTC in engineered E. coli to achieve ambrein production and established a novel ambrein biosynthetic pathway.
Materials and methods
Strains and medium
Escherichia coli MEP43 was derived from strain ZF43 (Li et al. 2015) by deleting the β-carotene synthesis module. The MEP43 strain was used as the parent for establishing the ambrein-producing strain. E. coli DH5α was utilized to amplify the plasmids. All the strains and plasmids used or constructed in this study are summarized in Supplementary Table 1. E. coli strains were cultivated at 30 or 37 °C in lysogeny broth supplemented with 100 mg ampicillin l−1 and 34 mg chloramphenicol l−1 as required. E. coli strains were also cultivated at 30 °C in liquid terrific broth (TB) supplemented with 20 g glycerol l−1. Ampicillin (100 mg l−1) was added as required.
Genome editing and plasmid construction for gene expression in E. coli
The ScERG9 gene (Li et al. 2016) is the gene encoding the SS from Saccharomyces cerevisiae, the gene was PCR-amplified with the primers tERG9F and tERG9R using the pSQ1 plasmid (Li et al. 2016) as template. The ScERG9 operon, which is controlled by a strong promoter (J23119), was integrated into the E. coli genome at the lacZ locus through CRISPR-Cas9 genome editing (Li et al. 2015) to generate strain LK01. The 0.5 kb-homologous-arms, lacZ-up/lacZ-down, were amplified with the primers lacZuF, lacZuR, lacZdF, and lacZdR using the genomic DNA of E. coli MEP43 as template. The ScERG9 operon, lacZ-up, and lacZ-down were assembled to be the donor DNA via fusion PCR. The gRNA targeting the lacZ locus was expressed in pGRB-lacZ, which was constructed according to the methods of Li et al. (2015) using the primers lacZE1D and lacZE1U. The donor DNA and pGRB-lacZ were introduced into E. coli MEP43 to integrate ScERG9 into the genome, following the procedures described by Li et al. (2015).
The mutated squalene–hopene cyclase gene (D377C SHC) was synthesized with codon usage optimization. The tetraprenyl-β-curcumene cyclase gene (BmeTC) was amplified from the genomic DNA of B. megaterium (CGMCC 1.10466). The sequences for D377C SHC and BmeTC are shown in Supplementary Table 2. The D377C SHC gene fragment was amplified with the primers CASHC-SacIF/CASHC-BamHIR and cloned into p5C between the SacI and BamHI restriction sites to generate the pLs plasmid. The BmeTC gene fragment was amplified with the primers BmeTC-KpnIF/BmeTC-KpnIR and cloned into p5C at the KpnI restriction site to generate pLb. The D377C SHC-BmeTC co-expression cassette was constructed via fusion PCR, in which the individual D377C SHC and BmeTC fragments were amplified with the primers CASHC-EcoRIF/SHCBR and BmeTCSF/CBmeTC-KpnIR, respectively. Then, the D377C SHC-BmeTC cassette was cloned into p5C between the EcoRI and KpnI restriction sites to yield pLsb.
The oligonucleotide primers are listed in Supplementary Table 3.
Shake-flask cultures and metabolite extraction
Shake-flask cultures were performed in triplicate. E. coli recombinants were initially inoculated into 4 ml LB containing 100 mg ampicillin l−1 as required, and subsequently cultivated overnight at 30 °C and 220 rpm. The culture (0.3 ml) was then transferred into a 250 ml shake-flask containing 30 ml TB medium, and then incubated at 30 °C and 220 rpm. Upon reaching an OD600 of 0.6, gene expression was induced by adding 0.5 mM IPTG to the broth.
After 48 h of fermentation, metabolites were extracted using n-hexane following the method of Li et al. (2016). Samples were collected and then analyzed by GC–MS/HPLC.
Analytical methods
Putative squalene and ambrein products were identified using a GC–MS system which was the same as Li et al. (2016). Compound separation was achieved under the same operational conditions reported by Ueda et al. (2013). Squalene standard was purchased from Sigma-Aldrich. A gray ambergris sample (3.2 g; HuaZhuang Pha, China) was dissolved in n-hexane. The soluble fraction containing ambrein was analyzed by GC–MS and the total ion mass spectra were compared with the data from the NIST Database (Supplementary Fig. 1). The soluble fraction of ambergris in n-hexane was used directly as the ambrein standard for metabolite analysis.
Squalene produced by the recombinant strains was quantitatively analyzed by HPLC (Li et al. 2016), whereas ambrein was quantitatively analyzed by GC-FID. The ambrein yields were expressed as mean ± SD (bars) of three independent measurements (see Fig. 3). A GC system equipped with a Rxi-1HT capillary column (30 m × 0.32 mm × 0.25 μm) was used for analysis. Compound separation was achieved following the above mentioned heating process employed for GC–MS analysis. Epifriedelanol (C30H52O) was added to the samples as the internal standard to quantify ambrein yield (Supplementary Fig. 2). The relative response factor (RRF) was set to 0.99 (calculations are described in detail as a Supplementary method).
Results
Construction of ambrein biosynthetic pathway in E. coli
Following the presumptive ambrein synthetic pathway (Fig. 1), three reactions sequentially catalyzed by SS, D377C SHC, and BmeTC were incorporated in E. coli to achieve ambrein production. Firstly, the ScERG9 operon under the control of the constitutive J23119 promoter was assembled into the E. coli MEP43 genome to generate the transformed LK01 strain. Shake-flask fermentation of LK01 led to intracellular accumulation of squalene at 18.4 mg l−1. Afterwards, the recombinant plasmids pLs, pLb, and pLsb were introduced into LK01 strains to generate the strains LKs, LKb, and LKsb, respectively. The LKsb strain, which harbors the genes for the complete ambrein biosynthetic pathway, was cultured to evaluate ambrein production, LKs and LKb were taken as controls.
Identification of ambrein produced in E. coli
The strains LK01, LKs, LKb, and LKsb were cultivated according to the methods described above, and the corresponding intracellular metabolites were analyzed by GC–MS. Based on the relative retention times (Rt) and mass spectral comparisons with an external ambrein standard, the peak at Rt 19.1 min (Fig. 2a) corresponded to the desired ambrein product. Thus, strain LKsb produces ambrein in detectable levels. Ambrein production was not detected in strain LKs or LKb, but possible 3-deoxyachilleol A and 8α-hydroxypolypoda-13,17,21-triene were produced in LKs and LKb, respectively (Fig. 2a). Thus, heterologous biosynthesis of ambrein was successfully achieved via the co-expression of SS, D377C SHC, and BmeTC in E. coli.
Identification of ambrein produced by LKsb. a GC profiles of (A) squalene standard, metabolite extracts from the recombinant Escherichia coli strains (B) LK01, (C) LKs, harboring the pLKs plasmid, (D) LKb, harboring the pLKb plasmid, (E) LKsb, harboring the pLKsb plasmid and (F) ambrein standard, b mass spectrum of squalene in sample LK01, c mass spectrum of squalene standard, d mass spectrum of ambrein in sample LKsb, and e mass spectrum of ambrein standard. (1) squalene, (2) possible 3-deoxyachilleol A, (3) possible 8α-hydroxypolypoda-13,17,21-triene, and (4) ambrein (mass spectra of 2 and 3 not shown)
Effect of cultivation temperature on ambrein production
Effective control of cultivation temperature can help increase the ambrein yield in the recombinant strain LKsb. Temperatures ranging from 25 to 37 °C were tested to determine the optimum temperature for ambrein production. As shown in Fig. 3, 30 and 34 °C were optimal for ambrein biosynthesis in LKsb, corresponding to yields of ~ 2.5 mg l−1 which were 1.4- and 2.5-times higher than the yields obtained at 25 °C (1.8 mg l−1) and 37 °C (1 mg l−1), respectively.
Discussion
The triterpenoid ambrein, a crucial bioactive component of ambergris, is a valuable ingredient of perfumes and a potential medical feedstock. In this study, we constructed a novel ambrein biosynthetic pathway in genetically-engineered E. coli and provided an ambrein-producing system. To achieve this goal, an E. coli chassis (LK01) was constructed to supply precursor squalene, a key intermediate of the triterpenoid biosynthesis. The complete ambrein biosynthetic pathway was established by co-expression of D377C SHC and BmeTC with SS in the E. coli strain LKsb. Ambrein was produced at a yield of 2.55 mg l−1 in LKsb at the optimum temperature (Fig. 3). Thus, an alternative ambrein production platform was created in E. coli. To our knowledge, our study is the first to report ambrein production in recombinant microorganisms.
The established LKsb chassis can also be further engineered to improve ambrein yield by increasing the supply of precursors. For instance, introduction of the heterologous mevalonate pathway (Zhang et al. 2014) and modification of central metabolic modules (Jing et al. 2013) are two generally employed methods to enhance terpenoid productions in E. coli. The LKsb strain also generated an undesired by-product (peak 3 in Fig. 2a), which was likely caused by nonspecific catalysis of BmeTC (Fig. 1), so further engineering of BmeTC to improve its catalytic specificity might be useful for improving ambrein production.
To date, only a few triterpenoids have been developed for biosynthesis in engineered E. coli. The favorable formation of squalene and 2,3-oxidosqualene (Li et al. 2016) highlights the potential of E. coli as a host for triterpenoid production, and the present study also expanded the list of microbially derived triterpenoids. Our strategy used for constructing the ambrein synthetic pathway can also be employed to some other potential hosts such as S. cerevisiae. Thus, our findings provide new possibilities to achieve industrial-scale production of ambrein.
References
Ajikumar PK, Xiao WH, Tyo KE, Wang Y, Simeon F, Leonard E, Mucha O, Phon TH, Pfeifer B, Stephanopoulos G (2010) Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330:70–74
Jing Z, Li Q, Tao S, Zhu X, Xu H, Tang J, Zhang X, Ma Y (2013) Engineering central metabolic modules of Escherichia coli for improving β-carotene production. Metab Eng 17:42–50
Katabami A, Li L, Iwasaki M, Furubayashi M, Saito K, Umeno D (2015) Production of squalene by squalene synthases and their truncated mutants in Escherichia coli. J Biosci Bioeng 119:165–171
Kovatcheva A, Golbraikh A, Oloff S, Xiao YD, Zheng W, Wolschann P, Buchbauer G, Tropsha A (2004) Combinatorial QSAR of ambergris fragrance compounds. J Chem Inf Comput Sci 44:582–595
Li Y, Lin Z, Huang C, Zhang Y, Wang Z, Tang Y, Chen T, Zhao X (2015) Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab Eng 31:13–21
Li D, Zhang Q, Zhou Z, Zhao F, Lu W (2016) Heterologous biosynthesis of triterpenoid dammarenediol-II in engineered Escherichia coli. Biotechnol Lett 38:603–609
Ohloff G, Schulte-Elte KH, Müller BL (1977) Formation of ambergris odorants from ambrein under simulated natural conditions. Helv Chim Acta 60:2763–2766
Sato T, Hoshino T (1999) Functional analysis of the DXDDTA motif in squalene–hopene cyclase by site-directed mutagenesis experiments: initiation site of the polycyclization reaction and stabilization site of the carbocation intermediate of the initially cyclized A-ring. Biosci Biotechnol Biochem 63:2189–2198
Sato T, Hoshino H, Yoshida S, Nakajima M, Hoshino T (2011) Bifunctional triterpene/sesquarterpene cyclase: tetraprenyl-β-curcumene cyclase is also squalene cyclase in Bacillus megaterium. J Am Chem Soc 133:17540–17543
Shen YC, Cheng SY, Kuo YH, Hwang TL, Chiang MY, Khalil AT (2007) Chemical transformation and biological activities of ambrein, a major product of ambergris from Physeter macrocephalus (sperm whale). J Nat Prod 70:147–153
Taha SA (1989) Chemical investigation of the internal secretion of the sperm blue whale. Pak J Pharm Sci 2:105–110
Taha SA (1992) Studies on the mode of action of ambrein as a new antinociceptive compound. Jpn J Pharmacol 60:67–71
Taha SA, Islam MW, Ageel AM (1995) Effect of ambrein, a major constituent of ambergris, on masculine sexual behavior in rats. Arch Int Pharmacodyn Ther 329:283–294
Taha SA, Raza M, El-Khawad IE (1998) Effect of ambrein on smooth muscle responses to various agonists. J Ethnopharmacol 60:19–26
Tanimoto H, Oritani T (1997) Synthesis of (+)-ambrein. Tetrahedron 53:3527–3536
Ueda D, Hoshino T, Sato T (2013) Cyclization of squalene from both termini: identification of an onoceroid synthase and enzymatic synthesis of ambrein. J Am Chem Soc 135:18335–18338
Zhang H, Qiang L, Cao Y, Feng X, Zheng Y, Zou H, Hui L, Yang J, Mo X (2014) Microbial production of sabinene—a new terpene-based precursor of advanced biofuel. Microb Cell Factories 13:20
Acknowledgements
The present work was funded by the National Basic Research Program of China (“973” Program: 2012CB721105) and the Major Research Plan of Tianjin (16YFXTSF00460).
Supporting information
Supplementary method—effective prediction of relative response factor RRF for epifriedelanol/ambrein in GC-FID analysis via a carbon number-based approach.
Supplementary Table 1—strains and plasmids used.
Supplementary Table 2—DNA sequences of exogenous genes used for the construction of the ambrein biosynthetic pathway in Escherichia coli.
Supplementary Table 3—oligonucleotide primers used in this study.
Supplementary Fig. 1—identification of the authentic ambergris containing ambrein via GC–MS.
Supplementary Fig. 2—quantitative analysis of ambrein production via GC-FID.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ke, D., Caiyin, Q., Zhao, F. et al. Heterologous biosynthesis of triterpenoid ambrein in engineered Escherichia coli . Biotechnol Lett 40, 399–404 (2018). https://doi.org/10.1007/s10529-017-2483-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2483-2