Abstract
Objective
To investigate the effect of adipose tissue-derived mesenchymal stem cell (ASC) administered either systemically or locally in a murine model of bronchiolitis obliterans.
Results
When compared to controls, systemic treatment with 106 ASCs on D0 and a second dose on D7 significantly prevented tracheal obliteration 28 days after heterotopic tracheal transplantation (median of 94 vs. 16%; P < 0.01). A single dose tended towards less stenosis than controls, but did not reach statistical significance (28 vs. 94%; P = 0.054). On the contrary, repeated local injection was incapable of preventing tracheal obliteration when compared to a single injection or controls (37 vs. 71 vs. 87%). Two intravenous doses also tended to be better than two local injections (16 vs. 37%; P = 0.058), and were better than a single local dose (16 vs. 71%; P < 0.01).
Conclusion
A second dose of ASC, given systemically after 7 days, reduces luminal obliteration in a heterotopic tracheal transplantation model in mice, suggesting that ASC can be used to prevent obliterative bronchiolitis after lung transplantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung transplantation is the only possible treatment for many end-stage pulmonary diseases. Diminished long term survival when compared to other solid-organ transplants is explained by chronic lung allograft dysfunction, most commonly manifested as bronchiolitis obliterans syndrome (BOS), and as its pathological counterpart, obliterative bronchiolitis (OB) (Verleden et al. 2016). Alloimmune and non-alloimmune events are involved in the pathogenesis of the disease, which begins with small caliber airway epithelial lesion, followed by lymphocytic infiltration and subsequent fibrosis. The end result is airway obstruction (Jonigk et al. 2010).
Unfortunately, methods for treatment or prevention of BOS are limited. Because of that, several strategies have been evaluated, such as treatment with azithromycin (Vos et al. 2012), substitution of immunosuppressive drugs (Jiang and Nicolls 2014) and retransplantation (Warnecke and Haverich 2012), with poor results. Immunomodulation with stem cells (SC) may be a viable option. Nonetheless, the type and origin of cells and best route of administration are not yet clarified. Mesenchymal stem cells (MSC) have been tested for pulmonary diseases, such as chronic obstructive pulmonary disease, cystic fibrosis and asthma, with variable results (Conese et al. 2014). Adipose tissue-derived MSC seem particularly promising, partly due to their abundance in an easily obtainable tissue (Fraser et al. 2006), but their effects on OB scenarios have not been reported. These cells, adipose stem cells (ASC), are more attractive for their immunosuppressive capacity and their secretome than for their ability to differentiate in vivo (Ong and Sugii 2013). Besides that, ASC are considered immunoprivileged, i.e., are not attacked even by immunocompetent systems, as they do not express class II MHC or co-stimulatory surface molecules (Lindroos et al. 2011).
This study aims to evaluate the effect of ASC on airway obliteration, when administered either systemically or locally, in one or two doses, in a murine model of heterotopic tracheal transplantation (HTT).
Materials and methods
Animals
Donor Balb/C and recipient C57BL/6 male mice, 8–10 weeks old, 20–30 g, were provided by the Animal Experimentation Unit of Hospital de Clínicas de Porto Alegre (HCPA) and quarantined for a week. They were kept in standard six animals facilities, at 20–22 °C, 12 h light/dark shifts, fed with standard diet and water ad libitum. Animals were treated humanely in accordance with international regulations and with the Brazilian Federal Law 11.794/08 and Resolution 1000/2012 of the Federal Council of Veterinary Medicine, which rule animal research in Brazil. The study was approved by the Animal Research Ethics Commission at HCPA under the number 12-0066.
Study groups
We implanted two tracheas in each recipient, which were divided in six groups according to route of administration and number of ASC doses. One dose of ASC or phosphate-buffered saline (PBS) was administered on the same day of the tracheal implant (D0), immediately after the procedure. Groups of systemic (Syst) or local (Local) administration of ASC on D0 were named, respectively, Syst0 (n = 8 tracheas) and Local0 (n = 12). Those receiving an additional dose of ASC on D7 (7th day after transplantation) were named Syst0+7 (n = 12) and Local0+7 (n = 16). Control groups were named according to the route of administration on D0. All animals were killed on D28 (28th day after transplantation).
ASC culture
Epididymal fat was aseptically removed from male C57BL/6 mice and minced in a laminar flow chamber. Tissue was incubated at 37 °C for 30 min, after digestion with type 1 collagenase, 1 mg/ml (Sigma) in Dulbecco’s Modified Eagle Medium (DMEM) with glucose (1 g/l). The enzyme was subsequently inactivated by adding DMEM with 20% (v/v) fetal bovine serum (FBS). The resultant cellular suspension was centrifuged at 600×g for 10 min and the pellet resuspended in DMEM with 20% (v/v) FBS, with the addition of 100 U penicillin and 100 mg streptomycin (P/S) per ml solution. Cells were seeded in 6-well culture dishes and incubated in humidified atmosphere with 5% CO2, at 37 °C. After 72 h, non-adherent cells were washed. When reaching 80% confluence, adherent cells were removed with 0.25% trypsin/EDTA (Gibco) and kept in DMEM, 20% FBS and P/S solution. These cells were injected freshly between passages 3 and 6. Experimental protocols described by Gonçalves et al. (2012) were followed to characterize the cultures’ adipogenic and osteogenic differentiation capacities (see Supplementary Fig. 2). MSC were analyzed according to surface molecular markers including CD34, CD11bc, CD44, and CD90 (Becton–Dickinson, NJ, USA).
Heterotopic tracheal transplantation
Donor animals were anesthetized with ketamine (100 mg/kg, intraperitoneal) and xilazine (10 mg/kg, intraperitoneal). Tracheas were dissected through a cervicosternotomy and removed after being cut below the cricoid and at the main carina; immediately after that, donors were killed by cardiectomy. Tracheas were preserved in saline at 4 °C for 1 h, until implant. Recipients were anesthetized with 5% isoflurane, later adjusted to 1–2%, and the dorsum was prepared with alcoholic solution. In those of systemic treatment groups a single 1 cm vertical incision was made between the scapulae and the subcutaneous space was dissected bilaterally. One trachea was implanted on each side and the wound sutured with 6-0 mononylon (Ethicon, GA, USA). Animals from local injection groups received the implants through two 5 mm dorsal incisions, 2 cm apart, and subcutaneous dissection was minimal, only enough to place a trachea. This modification was necessary so that the tracheas would not move and ASC injections could be done around them with precision. Post-operative analgesia was done with tramadol, 10 mg/kg, IM. Recipients were killed by nuchal dislocation after isoflurane inhaled anesthesia.
ASC administration
ASC were systemically injected through the tail vein, always by the same veterinarian, with the mice in a small rodent restrainer. Cells (106) were resuspended in 120 µl PBS and 10–20 µl injected during approx. 3 min, through a 25 Ga needle. For the local treatment groups, animals were lightly sedated with isoflurane and 5 × 105 cells in 60 µl PBS were injected in bolus around each trachea, which were easily palpated at their implant site. Therefore, a total of 106 cells was administered to each animal in all treatment groups.
Morphometric analysis
Tracheas removed from the subcutaneous on D28 were fixed in 4% (v/v) formaldehyde for 24 h. They were then cut in half and embedded in paraffin with the cut side down, so that the middle portion of each trachea could be evaluated. Axial slices, 4 µm, were stained with hematoxylin and eosin (H&E) and photographed on optical microscope using the software QCapture Pro version 5.1.1.14 (QImaging, USA). Pictures were analyzed on software ImageJ version 1.45 s (NIH, USA). Internal boundaries of the cartilage (\( I \)) and tracheal lumen (\( L \)) were manually delimited and their areas calculated (Supplementary Fig. 1). Tracheal obliteration was obtained with the formula \( (I - L/L) \times 100 \).
Histological analysis
The same sections used for morphometric analysis were evaluated by a blinded pathologist. Each trachea was classified according to Boehler et al. (1997) criteria (Supplementary Table 1), with the aim of quantifying relevant OB histopathological aspects.
GFP-ASC distribution evaluation
Additionally, in order to better understand the biodistribution and persistence of ASC, eight animals were injected systemically and eight animals received local injections of green-fluorescent protein ASC (GFP-ASC). Methods for procurement, culture, expansion and injection of GFP-ASCs were identical to those described in the previous sections. Animals were killed at 1, 24, 48 and 72 h after ASC administration, and their lungs, orthotopic and graft tracheas, liver, spleen, thymus gland, heart, kidneys and axillar and inguinal lymph nodes were removed. Immunohistochemistry with anti-GFP antibodies was performed in search of the GFP-ASC.
Statistical analysis
Tracheal obliteration and histological score data were analyzed with non-parametrical Kruskal–Wallis test (KW) for comparisons among the 6 groups. Post hoc evaluation was done using pairwise Dunn’s test, with significance adjusted for multiple comparisons, as defined a priori. All tests ran on Predictive Analytics Software version 18 (SPSS Inc. 2009), using a significance level of α = 0.05.
Results
Characterization of MSC from adipose tissue
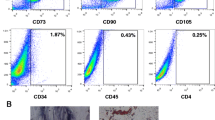
ASC with stable fibroblast-like phenotype were isolated by adherence separation. ASC were capable of differentiating into osteogenic and adipogenic cells when cultured under appropriate conditions (Supplementary Fig. 2). Flow cytometry with monoclonal antibodies showed ASC expressed CD90 and CD44 (more than 90% positivity), but lacked CD11b and CD34 (less than 10% positivity) (data not shown).
Prevention of bronchiolitis obliterans with ASC
Median tracheal obliteration for systemically treated groups Syst0, Syst0+7 and SystC were 28, 16 and 94%, respectively. KW test indicated at least one group was different from the others (P < 0.001). Post hoc analysis showed that group Syst0+7 had significantly less tracheal obliteration than control group (P < 0.001). Group Syst0 also had less obliteration than controls, but did not reach the critical statistical value (P = 0.054). There was no difference between treatment groups Syst0+7 and Syst0 (P > 0.05) (Figs. 1, 2).
Luminal obliteration (%) on all six groups. Data shown as median and quartiles. Obliteration on group Syst0+7 was significantly less than on SystC and Local0, and tended to be less than on Local0+7. Group Syst0 showed less obliteration than SystC, despite not reaching statistical level. *P < 0.01, 16 versus 94% when compared to Syst C; **P < 0.01, 16 versus 71% when compared to Local 0; # P = 0.058, 16 versus 37% when compared to Local0+7; ## P = 0.054, 28 versus 94% when compared to Syst C
Representative images of axial sections of tracheas from all groups after H&E staining. Upper row shows tracheal grafts from systemically treated animals and the bottom row shows locally treated grafts. Left, middle and right columns depict tracheas treated with two, one or no doses of ASC, respectively. The lumen (L) and obliterative tissue (*) are indicated. In these selected examples, Syst0+7 has an almost completely preserved lumen and Syst0 has little obliteration, while Local0+7 and Local0 present relevant obliteration and luminal area reduction; both controls are completely obliterated. Bar size 500 µm
In local administration groups, median obliteration for Local0, Local 0+7 and LocalC groups was 71, 37 and 87%. Despite group Local0+7 apparent lesser luminal reductions, the difference was not statistically significant (Figs. 1, 2).
Comparing systemic and local groups, it was shown that Syst0+7 group’s obliteration was less than Local0 (P < 0.001), and tended towards less stenosis than Local0+7 (P = 0.058). On the contrary, a single systemic injection did not have the same effect, when compared with either one or two local injections (P > 0.05).
Histological evaluation (Table 1) demonstrated significantly greater epithelial preservation on group Syst0+7 (P < 0.01), when compared to all others (median score of 3 vs. 4); there were no differences among the rest of them. On the criterion of alterations reflecting abnormal perfusion and ischemia, control groups and group Syst0+7 had a smaller score than other treatment groups (0 vs. 1; P < 0.01). Concerning lymphoplasmocytic infiltration, all groups that received at least one dose of ASC scored better than both controls (3 vs. 4; P < 0.01).
Distribution of ASCs
The parallel evaluation of the distribution of intravenous GFP-ASCs showed cells in the lung interstitium until day 3 (Fig. 3), but did not reveal any cells in the implanted tracheas or any other organs. Locally injected GFP-ASC did not migrate to other organs, including the adjacent graft, and remained at their injection site.
GFP immunostaining showing GFP-ASC homing at the lung at 1 h after systemic. 468 injection (a), and after 24 h (b), 48 h (c) and 72 h (d) (20×). GFP-ASC cells stain brown and are easily seen within the lung’s capillaries and interstitium (small arrows). GFP-ASC were not detected in the tracheas (data not shown) after intravenous injection. Despite close contact of the cluster of locally injected cells (large arrow) to the implants (e) (4×), migration to the tracheas did not occur after 24 h (f), 48 h (g) or 72 h (h) Bar size 500 µm
Discussion
This study compared the effect of ASC administered in one or two doses, by either systemic or local routes on tracheal stenosis after HTT. The peak of inflammation in the HTT model is approximately between days 7 and 10, and after day 14 the process may no longer be reversible (Brazelton et al. 1997). These data suggest that the second week is crucial in determining the graft’s fate. Considering a previous study using the same type of cell and the same model, which did not identify any effect with a single intravenous dose of ASC (Espinel et al. 2015), we decided to use a second dose after 7 days as an attempt to minimize the model’s inflammatory peak.
MSC activity on several pulmonary lesion scenarios is well documented (Brody et al. 2010). Bone marrow-derived MSC were more extensively tested than cells of other origins (Conese et al. 2014). We used ASC for their comparable characteristics (Lin et al. 2006) and the relative easiness of obtainment. In humans, visceral and subcutaneous adipose tissues have clearly different metabolic behaviors in diseases such as diabetes. This difference seems to reflect in better adipogenic in vitro differentiation seen in ASC from subcutaneous and mediastinal tissues (Russo et al. 2014), even though there are no morphologic or immunophenotypic distinctions between those cells and the ones of omental origin (Baglioni et al. 2009). We used epididymal fat because it is easily removed without contamination from other tissues, but possible peculiarities of ASC from various anatomical regions have not been evaluated in mice.
Among all groups, the one that had the tracheal lumen mostly preserved received two systemic doses of ASC, i.e., on the day of the implant and again after a week, indicating that the additional dose potentiates the effect of the first one. This observation may relate to how long ASC survive in the recipient, as there is evidence that MSC become apoptotic shortly after their intravenous infusion (Liu et al. 2012). Despite their short life, the global effect of ASC may persist through other mechanisms, such as activation of hematopoietic cells (Yang et al. 2012) or through phagocytosis of cellular debris by macrophages, altering the immune response (Lu et al. 2013). A second injection of ASC, precisely when the tracheas are the most inflamed, seems to create an additive effect. Even though other studies have tested MSC of embryonic (McIntyre et al. 2014), placental (Zhao et al. 2014) and umbilical cord origins (Cao et al. 2016) in the HTT model with positive results, none used them in more than one dose. Just like any immunosuppressive drug, regular doses may be necessary to keep rejection under control. Nonetheless, it is not known if adverse effects may add up after prolonged courses of treatment, since MSC are capable of inducing fibrosis under certain conditions by producing TGF-β, which is the greatest fibroblast activator known (Kirby and O’boyle 2011). Specifically, MSC found in lung grafts produce additional amounts of endothelin-1 in patients with BOS, and MSC are found in larger numbers in the bronco-alveolar lavage of sicker patients (Salama et al. 2011). Mechanisms that direct the actions of ASC to one path or the other are not completely understood.
Route of administration is important for the performance of ASC. We identified at least a trend towards a smaller effect when ASC were injected around the tracheas. Theoretically, promoting direct contact between cells and grafts should lead to a better control of inflammation. Our results contradict the thought that SC act paracrinally, as classically described. Supposedly, after initial pulmonary homing, cells would migrate to sites of inflammation (Hoogduijn et al. 2013), but several studies, including our own, have not been able to find MSC at those locations. Therefore, immunomodulation from the lung might be the main mechanism of MSC action. Possible explanation for a lesser effectiveness of ASC applied locally is the lack of an appropriate microenvironment, since the subcutaneous space is poorly vascularized and oxygenated, what may compromise ASC survival or limit their activity (Boehler et al. 1997). Analogously, in a model of ulcerative rectocolitis, intraperitoneal administration of ASC was also less effective in controlling inflammation than the intravenous route, in spite of the fact that the former directly contacted the cells and bowel (Gonçalves et al. 2014). Finally, dose–response curves for either local or systemic treatments with ASC are not known, and the answer to whether smaller or larger doses influence results is a matter for further research.
As expected, the group with the most preserved epithelium also had less stenosis. Therefore, preventing epithelial loss should be an essential strategy. That is confirmed by the less consistent association between lymphocytic infiltration and vascular changes with obliteration. Induction of chimeras, i.e., coating tracheas with cells from the recipient as well as from the donor, through tracheal instillation of epithelial cells from the host is another cellular therapy that might be successful (Zhao et al. 2013).
Finally, limitations must be considered. The HTT model, despite being technically simple and reproducible, uses a non-vascularized fragment of large airway without communication to the environment. That does not discredits the conclusions of this study, since the model is suitable for immunological intervention and has been used in more than a hundred studies in the past decade (Sato et al. 2009).
Conclusions
The immunosuppressive effect of ASC is greater when more than one dose is administered systemically, and multiple doses seem to have an additive effect, a finding not previously described in models of OB. Besides that, the intravenous route of administration is probably more effective than the local one, at least in the HTT model. This is particularly relevant in lung transplantation, since the local intratracheal delivery of ASC is feasible (Leblond et al. 2009). As the systemic infusion of MSC in humans is considerably safer than in animals, especially with autologous cells, their use as part of immunosuppressive regimens in humans may soon be tested, including lung/MSC co-transplantation and its use in ex vivo lung perfusion (Mohamed 2016). The incidence of neoplasms and opportunistic infections are the main concerns related to more intense immunosuppression, and clinical trials with long enough follow-up are necessary for clarification.
References
Baglioni S, Francalanci M, Squecco R, Lombardi A, Cantini G, Angeli R, Gelmini S, Guasti D, Benvenuti S, Annunziato F, Bani D, Liotta F, Francini F, Perigli G, Serio M, Luconi M (2009) Characterization of human adult stem-cell populations isolated from visceral and subcutaneous adipose tissue. FASEB J 23:3494–3505
Boehler A, Chamberlain D, Kesten S, Slutsky AS, Liu M, Keshavjee S (1997) Lymphocytic airway infiltration as a precursor to fibrous obliteration in a rat model of bronchiolitis obliterans. Transplantation 64:311–317
Brazelton TR, Adams BA, Cheung AC, Morris RE (1997) Progression of obliterative airway disease occurs despite the removal of immune reactivity by retransplantation. Transpl Proc 29:2613
Brody AR, Salazar KD, Lankford SM (2010) Mesenchymal stem cells modulate lung injury. Proc Am Thorac Soc 7:130–133
Cao XP, Han DM, Zhao L, Guo ZK, Xiao FJ, Zhang YK, Zhang XY, Wang LS, Wang HX, Wang H (2016) Hepatocyte growth factor enhances the inflammation-alleviating effect of umbilical cord-derived mesenchymal stromal cells in a bronchiolitis obliterans model. Cytotherapy 18:402–412
Conese M, Piro D, Carbone A, Castellani S, Di Gioia S (2014) Hematopoietic and mesenchymal stem cells for the treatment of chronic respiratory diseases: role of plasticity and heterogeneity. Sci World J 859817
Espinel JO, Uribe C, Meyer FS, Bringheti R, Kulczynski JU, Saueressig MG (2015) Cell therapy in the treatment of bronchiolitis obliterans in a murine model. Rev Col Bras Cir 42:181–188
Fraser JK, Wulur I, Alfonso Z, Hedrick MH (2006) Fat tissue: an underappreciated source of stem cells for biotechnology. Trend Biotechnol 24:150–154
Gonçalves FC, Paz AH, Lora PS, Passos EP, Cirne-Lima EO (2012) Dynamic culture improves MSC adhesion on freeze-dried bone as a scaffold for bone engineering. World J Stem Cells 4:9–16
Gonçalves FC, Schneider N, Pinto FO, Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP, Cirne-Lima EO, Meurer L, Paz AH (2014) Intravenous vs intraperitoneal mesenchymal stem cells administration: what is the best route for treating experimental colitis? World J Gastroenterol 20:18228–18239
Hoogduijn MJ, Roemeling-van Rhijn M, Engela AU, Korevaar SS, Mensah FK, Franquesa M, de Bruin RW, Betjes MG, Weimar W, Baan CC (2013) Mesenchymal stem cells induce an inflammatory response after intravenous infusion. Stem Cells Dev 22:2825–2835
Jiang X, Nicolls MR (2014) Working toward immune tolerance in lung transplantation. J Clin Investig 124(3):967–970
Jonigk D, Theophile K, Hussein K, Bock O, Lehmann U, Bockmeyer CL, Gottlieb J, Fischer S, Simon A, Welte T, Maegel L, Kreipe H, Laenger F (2010) Obliterative airway remodelling in transplanted and non-transplanted lungs. Virchows Arch 457:369–380
Kirby JA, O’boyle G (2011) Lung transplantation: the Yin and Yang of mesenchymal stem cells. Transplantation 92:129–130
Leblond AL, Naud P, Forest V, Gourden C, Sagan C, Romefort B, Mathieu E, Delorme B, Collin C, Pagès JC, Sensebé L, Pitard B, Lemarchand P (2009) Developing cell therapy techniques for respiratory disease: intratracheal delivery of genetically engineered stem cells in a murine model of airway injury. Hum Gene Ther 20:1329–1343
Lin Y, Liu L, Li Z, Qiao J, Wu L, Tang W, Zheng X, Chen X, Yan Z, Tian W (2006) Pluripotency potential of human adipose-derived stem cells marked with exogenous green fluorescent protein. Mol Cell Biochem 291:1–10
Lindroos B, Suuronen R, Miettinen S (2011) The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 7:269–291
Liu XB, Chen H, Chen HQ, Zhu MF, Hu XY, Wang YP, Jiang Z, Xu YC, Xiang MX, Wang JA (2012) Angiopoietin-1 preconditioning enhances survival and functional recovery of mesenchymal stem cell transplantation. J Zhejiang Univ Sci B 13:616–623
Lu W, Fu C, Song L, Yao Y, Zhang X, Chen Z, Li Y, Ma G, Shen C (2013) Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int J Cardiol 165:333–340
McIntyre BA, Alev C, Mechael R, Salci KR, Lee JB, Fiebig-Comyn A, Guezguez B, Wu Y, Sheng G, Bhatia M (2014) Expansive generation of functional airway epithelium from human embryonic stem cells. Stem Cells Transl Med 3:7–17
Mohamed MS (2016) Mesenchymal stem cells transplantation during ex vivo lung perfusion. J Heart Lung Transpl. doi:10.1016/j.healun.2016.10.010
Ong WK, Sugii S (2013) Adipose-derived stem cells: fatty potentials for therapy. Int J Biochem Cell Biol 45:1083–1086
Russo V, Yu C, Belliveau P, Hamilton A, Flynn LE (2014) Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl Med 3:206–217
Salama M, Andrukhova O, Jaksch P, Taghavi S, Kelpetko W, Dekan G, Aharinejad S (2011) Endothelin-1 governs proliferation and migration of bronchoalveolar lavage-derived lung mesenchymal stem cells in bronchiolitis obliterans syndrome. Transplantation 92:155–162
Sato M, Keshavjee S, Liu M (2009) Translational research: animal models of obliterative bronchiolitis after lung transplantation. Am J Transpl 9:1981–1987
Verleden SE, Sacreas A, Vos R, Vanaudenaerde BM, Verleden GM (2016) Advances in understanding bronchiolitis obliterans after lung transplantation. Chest 150:219–225
Vos R, Vanaudenaerde BM, Verleden SE, Ruttens D, Vaneylen A, Van Raemdonck DE, Dupont LJ, Verleden GM (2012) Anti-inflammatory and immunomodulatory properties of azithromycin involved in treatment and prevention of chronic lung allograft rejection. Transplantation 94:101–109
Warnecke G, Haverich A (2012) Lung re-transplantation: review. Curr Opin Organ Transpl 17:485–489
Yang X, Balakrishnan I, Torok-Storb B, Pillai MM (2012) Marrow stromal cell infusion rescues hematopoiesis in lethally irradiated mice despite rapid clearance after infusion. Adv Hematol 2012:142530
Zhao Y, Steidle JF, Upchurch GR, Kron IL, Lau CL (2013) Prevention of the second stage of epithelial loss is a potential novel treatment for bronchiolitis obliterans. J Thorac Cardiovasc Surg 145:940–947
Zhao Y, Gillen JR, Harris DA, Kron IL, Murphy MP, Lau CL (2014) Treatment with placenta-derived mesenchymal stem cells mitigates development of bronchiolitis obliterans in a murine model. J Thorac Cardiovasc Surg 147:1668–1677
Acknowledgments
This work was supported by Fundo de Incentivo à Pesquisa e Eventos (FIPE) of Hospital de Clínicas de Porto Alegre.
Supporting information
Supplementary Table 1—Histological assessment criteria according to Boehler et al. (1997).
Supplementary Fig. 1—An example of morphometric analysis.
Supplementary Fig. 2—Adipose stem cell characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lorenzi, W., Gonçalves, F.D., Schneider, N. et al. Repeated systemic administration of adipose tissue-derived mesenchymal stem cells prevents tracheal obliteration in a murine model of bronchiolitis obliterans . Biotechnol Lett 39, 1269–1277 (2017). https://doi.org/10.1007/s10529-017-2355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2355-9