Abstract
Objective
To control the oscillatory behavior of the intracellular calcium ([Ca2+]i) concentration in endothelial cells via mechanical factors (i.e., various hydrostatic pressures) because [Ca2+]i in these cells is affected by blood pressure.
Results
Quantitative analyses based on real-time imaging showed that [Ca2+]i oscillation frequency and relative concentration increased significantly when 200 mm Hg pressure, mimicking hypertension, was applied for >10 min. Peak height and peak width decreased significantly at 200 mm Hg. These trends were more marked as the duration of the 200 mm Hg pressure was increased. However, no change was observed under normal blood pressure conditions 100 mm Hg.
Conclusion
We generated a simple in vitro model to study [Ca2+]i behavior in relation to various pathologies and diseases by eliminating possible complicating effects induced by chemical cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracellular Ca2+ is a universal second messenger involved in many signal transduction pathways (Bootman et al. 2012). It has attracted much attention in the study of physiological and/or pathological processes in cells. In most cases, elevated levels of [Ca2+]i are closely related to various pathologies, such as hypertension, inflammation, cardiac and neural diseases (Missiaen et al. 2000). Dynamic oscillatory changes in [Ca2+]i trigger biochemical signals that affect enzyme activities, gene expression, and other cellular functions (Song et al. 2012). This oscillatory function helps the cell avoid high levels of [Ca2+]i that are toxic over the long term. These findings suggest that [Ca2+]i needs to be studied in conjunction with its oscillatory changes.
Therefore, the concentration and/or dynamics of [Ca2+]i should be determined in advance for in vitro studies. Then, various pathological models can be conducted in relation to [Ca2+]i and we may further consider any treatments to have those pathological patterns to be normal or healthy status. Most studies have employed chemical cues to control [Ca2+]i behaviors and mimic pathological status for further drug screening (Hongo et al. 2015). However, chemical cues might evoke several signaling pathways or share the receptors with tested drugs which result in unclear effects. On the other hand, various studies of normal and pathological cells have adopted mechanical stimuli to allow for mechanical biomimetic conditions; cellular responses observed under biomimetic environmental conditions are expected to be more similar to those observed in vivo status, as in the human body (Estrada et al. 2011). Also, [Ca2+]i behavior of vascular endothelial and smooth muscle cells is closely related to blood pressure condition (Adamova et al. 2009). This implies that mechanical pressure may be a potential option for arbitrarily control of [Ca2+]i and mimic pathological status.
In this study, we employed hydrostatic pressure at different magnitudes and durations to investigate the potential of controlling [Ca2+]i concentration and/or oscillations in vitro to mimic pathological conditions without any chemical cues. The results are expected to contribute to additional studies on screening of potential drugs and explaining the basic mechanisms of normal or pathological signaling pathways by eliminating possible unclear effects of chemical cues currently adopted to control [Ca2+]i behavior.
Materials and methods
Acquisition, culture, and seeding of endothelial cells
Human umbilical vein endothelial cells were purchased from Lonza. A commercially-available endothelial growth medium (Lonza) was used for culture. The cells were kept at 37 °C under 5% CO2.
HP in a mini-chamber system
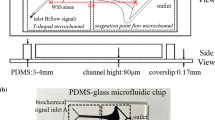
The custom-designed mini-chamber system used in the present study is shown in Fig. 1. It consists of a small chamber with a confocal dish, syringe pump, and pressure gauge (SMC Pneumatics). It was maintained at 37 °C by a water reservoir and gear pump.
After seeding the cells at passage #5 onto a confocal dish, we allowed 24 h for stabilization. Then, 100 or 200 mm Hg hydrostatic pressure (HP) was applied to simulate normal and hypertensive status, respectively. The duration of pressure engagement varied: 10 min for 100 mm Hg and 1, 5, 10, or 15 min for 200 mm Hg. Each application started 10 min after mounting the chamber onto a confocal microscope. For all groups, real time images were recorded during pressurization and another 10 min after de-pressurization. A group that was not exposed to pressure was included as a control. Therefore, the groups included were as follows: P0 (control), P100/10, P200/1, P200/5, P200/10, and P200/15, where the first and second numbers represent the pressure engaged and its duration, respectively.
Real-time recording of intracellular calcium behavior
Cells were loaded with 5 µM Fluo-4 AM calcium indicator (Molecular Probes) for 30 min. The solution was replaced with endothelial growth medium before pressurizing the cells. Real-time images were obtained during the experiments using a confocal laser microscope and recorded in a 12-bit format at 1 frame/s. [Ca2+]i intensity was detected using an argon laser at 488 nm. Five independent experiments were performed for each group.
Data analysis
Real-time changes in [Ca2+]i intensity in a single cell (Fig. 2a) were obtained using the LSM image browser (Carl Zeiss) based on a region of interest. Several oscillatory parameters which can explain signal characteristics were extracted and quantitatively analyzed. They were: frequency, area under the curve within a specific duration, peak height, peak-to-peak interval and width of each typical peak. These parameters have been widely chosen for [Ca2+]i behavior analyses (Song et al. 2012). MATLAB (R2015a, Mathworks, Inc.) was used in all processes.
Typical images observed along the time and procedure for oscillatory calcium intensity analysis. a Representative calcium images show relative changes in calcium intensity. b Original signal. Calcium intensity is expressed as a ratio of the baseline value. c Noise was removed by a low-frequency pass filter. d Baseline shift was determined using least squares methods; this shift was beneficial for peak finding. e Then, the peak and its width were determined. f Illustration of selected parameters
Raw oscillatory curves produced heterogeneous data with high noise (Fig. 2b), as reported previously (Christo et al. 2015). Therefore, noise was reduced using the moving-average low-pass filtering technique (Fig. 2c). The baseline for each curve was corrected using the asymmetric least squares method (He et al. 2014) (Fig. 2d). Peaks were detected with the peak-finding function in MATLAB and the selected parameters were extracted and/or calculated (Fig. 2e, f). The number of peaks within specific duration (before, during and after pressurization) was divided by the corresponding duration. Then frequency was obtained. However, the area under the curve during a specific time was calculated without baseline correction.
To avoid the long-term effect of fluorescence exposure on calcium intensity, all parameters were normalized to those in the control group. A one-way analysis of variance (ANOVA) was performed to identify any differences using SPSS ver. 19.0 K software (SPSS Inc.). P < 0.05 was considered statistically significant.
A total of 300–500 cells (>20% of all cells on a dish) were observed to examine oscillatory behavior in each group over five independent experiments. Assuming they were normal distribution we eliminated some data which were beyond 95% confidence interval.
Results
The typical signal of control group was shown in Fig. 3a. For 30 min consistent pattern was observed. This implies that [Ca2+]i oscillatory behavior was not affected by experiment duration, 30 min. No differences in any of the parameters were observed among the groups before the pressure was engaged, as expected (see the group “Before” in Fig. 3d–h). This finding suggests that the conditions, including temperature, were well controlled in all groups. However, [Ca2+]i oscillation pattern changed and became more complex during the 10 min pressurization (Fig. 3b, c). When lower pressure was engaged for 10 min (P100/10), the signal tended to return to the form before pressure was applied (Fig. 3b). However, the signal did not return to the form under higher pressure (P200/10; Fig. 3c). The accumulated 200 mm Hg HP made the pattern more complex, especially after pressurization. This trend was quantitatively analyzed and was shown in Fig. 3d–h. Frequency in P200/10 was significantly increased after pressurization (Fig. 3d). Moreover, the area under curve was also significantly increased (Fig. 3e). Meanwhile, peak height, peak width and peak-to-peak interval were significantly decreased in P200/10 after pressurization (Fig. 3f–h). All these findings suggest that higher pressure significantly affected and changed original [Ca2+]i oscillatory characteristics.
Typical signals showing relative intracellular calcium concentration, [Ca2+]i oscillatory behavior and their quantitative analyses under different magnitudes of hydrostatic pressure for 10 min. a–c Representative calcium oscillation pattern in each group. a P0 (control) group with no pressure. b Normal pressure for 10 min (P100/10). c High pressure for 10 min (P200/10). d–h Quantitative analyses of oscillatory parameters: d Oscillation frequency, e Area under the curve, f Peak height, g Peak width and h peak-to-peak interval. (*P < 0.05)
The 200 mm Hg treatment (i.e., high pressure group) changed the oscillatory patterns in the P0, P100/10, and P200/10 groups, and the changes remained after de-pressurization. Therefore, we further investigated the effects of 1-, 5-, 10-, and 15-min pressure duration of high pressure on oscillatory behavior. The typical patterns observed are presented in Fig. 4a–d, depending on the duration of higher pressure (200 mm Hg). Figure 4e–i present the results of quantitative analyses for various parameters selected. After 1 min of 200 mm Hg HP, the pattern tended to recover after the pressure was released (Fig. 4a). This can be confirmed in the graphs (Fig. 4e–i) that no significant difference of selected parameters between group P200/1 and control group was observed even after pressure was released. The typical signal of group P200/5 shows pattern irregular and heterogeneous (Fig. 4b). Frequency and area under the curve tended to increase when compared with those of control group. Meanwhile, peak height, peak width and peak-to-peak interval tended to decrease after pressurization. However, significant difference was not still found. When duration was 10 min pattern irregularity and heterogeneity were more observable, especially after pressurization. It can be supported by Fig. 4e–i that significant difference was begun to be observed in all parameters. When duration was extended to 15 min, remarkable irregularities were observed, even during engagement of HP (Fig. 4d). Again, significant differences in all parameters were confirmed (Fig. 4e–i).
Typical signals showing the effects of high pressure (200 mm Hg) duration. a–d Representative oscillation pattern in each group. a 200 mm Hg for 1 min (P200/1), b 200 mm Hg for 5 min (P200/5), c 200 mm Hg for 10 min (P200/10), (D) 200 mm Hg for 15 min (P200/15). e–i Quantitative analyses of oscillatory parameters: e oscillation frequency, f area under the curve, g peak height, h peak width, and i peak-to-peak interval. (*P < 0.05)
Discussion
Our results show that both pressure magnitude and duration affected EC calcium oscillatory behaviors. More interestingly, [Ca2+]i homeostasis was disrupted when higher pressure (P = 200 mm Hg) was applied for a certain periods (>10 min in this study) even after pressure was released. The group of P200/10 showed smaller peaks (lower peak height, narrow peak width) with higher frequency and higher calcium concentration relatively (increased area) than the group of P100/10. These trends were more observable when duration was increased up to 15 min under 200 mm Hg. Other previous studies (Gurkan et al. 2014; Hasel et al. 2005; Ohashi et al. 2007) observed the changes in endothelial cell morphology, related protein expressions and their function under various magnitudes and duration of pressurization. The changes in calcium oscillatory behaviors in this study were acquired within 1.0 h at most. Therefore, further study of morphological changes, related protein expression and cellular functions in relation to [Ca2+]i oscillatory behaviors is highly recommended.
We hypothesized that either higher pressure or longer time would cause cellular stress and abnormal calcium homeostasis and overload in the absence of chemical cues. In addition, the phenomenon became more observable when higher stress was applied. These findings can be interpreted similar to other reports demonstrating that [Ca2+]i overload under pathological conditions can cause cellular dysfunction or even death (Frosali et al. 2009; Vassalle and Lin 2004). Calcium overload has been used as an indicator in certain in vitro disease models for drug selection (Hongo et al. 2015; Katnik et al. 2006). The most notable advantage of this kind of simple experimental technique without any chemical cues is that a large amount of information can be obtained over a short time. Thus, the suggested method is suitable for use as a preliminary screening of drugs without using chemical cues which have been used to control [Ca2+]i concentration in vitro because those chemical cues may cause complex, unclear, or unexplainable outcomes. Based on our results, we suggest that our experimental model could be used as an in vitro pathological model for drug selection, which is related to dysfunction of [Ca2+]i in some types of cells, such as vascular cells under hypertension or neurons during edema.
Our study has some limitations that should be mentioned. We did not investigate the permeability of calcium ions during pressurization. However, it is well established that high pressure can alter the dynamics and structural characteristics of the cell membrane, in which permeability to molecules changes. Previous studies on the effects of high pressure on membrane structural or functional changes have been reported in various cell types. Nirmalanandhan et al. (2015) showed that elevated pressure (~300 mm Hg) caused the structure of the myeloid leukemia cell membrane to change to a solid gel-like state; consequently, reduced small molecule intake was detected. Bravim et al. (2010) demonstrated that high stress could damage or cause leaky membranes in Saccharomyces cerevisiae, which may also increase calcium intake from the extracellular environment. However, the HP magnitude used was more than 50 MPa, which is much higher than 200 mm Hg. Therefore, we set a hypothesis that the intake of calcium ion from outside during the pressurization is limited.
In addition, we did not investigate changes in gene or protein expression due to bio-mechanically invoked [Ca2+]i oscillation changes, as no specific pathological conditions or diseases were clarified beforehand. Therefore, further investigation is necessary focusing on a specific pathology or disease utilizing the suggested simple experimental techniques and related analyses.
References
Adamova Z, Ozkan S, Khalil RA (2009) Vascular and cellular calcium in normal and hypertensive pregnancy. Curr Clin Pharmacol 4:172–190
Bootman MD, Rietdorf K, Hardy H, Dautova Y, Corps E, Pierro C, Stapleton E, Kang E, Proudfoot D (2012) Calcium signalling and regulation of cell function. eLS. doi:10.1002/780470015902
Bravim F, de Freitas JM, Fernandes AAR, Fernandes P (2010) High hydrostatic pressure and the cell membrane. Ann NY Acad Sci 1189:127–132
Christo SN, Diener KR, Nordon RE, Brown MP, Griesser HJ, Vasilev K, Christo FC, Hayball JD (2015) Scrutinizing calcium flux oscillations in T lymphocytes to deduce the strength of stimulus. Sci Rep 5:7760
Estrada R, Giridharan GA, Nguyen MD, Roussel TJ, Shakeri M, Parichehreh V, Prabhu SD, Sethu P (2011) Endothelial cell culture model for replication of physiological profiles of pressure, flow, stretch, and shear stress in vitro. Anal Chem 83:3170–3177
Frosali S, Leonini A, Ettorre A, Di Maio G, Nuti S, Tavarini S, Simplicio PD, Di Stefano A (2009) Role of intracellular calcium and S-glutathionylation in cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta 1793:572–583
Gurkan S, Gur O, Yuksel V, Tastekin E, Huseyin S, Gur DO, Canbaz S (2014) The effect of distension pressure on endothelial injury and vasodilatation response in saphenous vein grafts: conversion of a bypass graft to a dead pipe. Kardiochirurgia i Torakochirurgia Polska 11:119–125
Hasel C, Dürr S, Bauer A, Heydrich R, Brüderlein S, Tambi T, Bhanot U, Möller P (2005) Pathologically elevated cyclic hydrostatic pressure induces CD95-mediated apoptotic cell death in vascular endothelial cells. Am J Physiol 289:C312–C322
He S, Zhang W, Liu L, Huang Y, He J, Xie W, Wu P, Du C (2014) Baseline correction for Raman spectra using an improved asymmetric least squares method. Anal Methods 6:4402–4407
Hongo Y, Takasu K, Ikegaya Y, Hasegawa M, Sakaguchi G, Ogawa K (2015) Heterogeneous effects of antiepileptic drugs in an in vitro epilepsy model-a functional multineuron calcium imaging study. Eur J Neurosci 42:1818–1829
Katnik C, Guerrero WR, Pennypacker KR, Herrera Y, Cuevasv J (2006) Sigma-1 receptor activation prevents intracellular calcium dysregulation in cortical neurons during in vitro ischemia. J Pharmacol Exp Therap 319:1355–1365
Missiaen L, Robberecht W, Van Den Bosch L, Callewaert G, Parys JB, Wuytack F, Raeymaekers L, Nilius B, Eggermont J, De Smedt H (2000) Abnormal intracellular Ca2+ homeostasis and disease. Cell Calcium 28:1–21
Nirmalanandhan VS, Hurren R, Cameron WD, Gronda M, Shamas-Din A, You L, Minden MD, Rocheleau JV, Schimmer AD (2015) Increased pressure alters plasma membrane dynamics and renders acute myeloid leukemia cells resistant to daunorubicin. Haematologica 100:e406–e408
Ohashi T, Sugaya Y, Sakamoto N, Sato M (2007) Hydrostatic pressure influences morphology and expression of VE-cadherin of vascular endothelial cells. J Biomech 40:2399–2405
Song S, Li J, Zhu L, Cai L, Xu Q, Ling C, Su Y, Hu Q (2012) Irregular Ca2+ oscillations regulate transcription via cumulative spike duration and spike amplitude. J Biol Chem 287:40246–40255
Vassalle M, Lin CI (2004) Calcium overload and cardiac function. J Biomed Sci 11:542–565
Acknowledgements
This work was supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (NRF-2014H1C1A1073148) and by the NRF Grant (NRF-2015M3A9B6073642).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y.R., Kang, Y.G., Shin, J.W. et al. Mechanical stimuli modulate intracellular calcium oscillations: a pathological model without chemical cues. Biotechnol Lett 39, 1121–1127 (2017). https://doi.org/10.1007/s10529-017-2354-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2354-x