Abstract
Objective
To examine the effect of squalene on liver X receptors (LXRs) that regulate target genes associated with reverse cholesterol transport and thus control whole-body cholesterol homeostasis.
Results
To examine the effect of squalene on liver X receptors (LXRs) that regulate target genes associated with reverse cholesterol transport and thus control whole-body cholesterol homeostasis. Squalene significantly stimulated the transactivation of liver X receptor modulator LXRα and LXRβ. The mRNA expression of LXRs and their target genes, including ABCA1, ABCG1 and ApoE, was significantly induced in macrophages stimulated with squalene, resulting in removal of cholesterol from the cells. Notably, squalene did not induce higher hepatic triacylglycerol levels nor did it alter expression of sterol regulatory element-binding protein 1c (SREBP-1c) and FAS in hepatocyte cells, primarily because of its upregulation of Insig-2a, which delays nuclear translocation of SREBP-1c, a key hepatic lipogenic transcription factor.
Conclusion
Squalene has hypocholesterolemic effect through the activation of LXRα and β without inducing hepatic lipogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver X receptors (LXRs) are members of the nuclear receptor superfamily comprising several ligand-activated transcription factors. They play a critical role in the protection against atherosclerosis by upregulating the expression of ATP-binding cassette (ABC) proteins A1, G1, and apolipoprotein E (ApoE), increasing cholesterol efflux, stimulating reverse cholesterol transport (RCT) from peripheral tissues, and elevating high-density lipoprotein cholesterol (HDL-C) levels (Geyeregger et al. 2006). LXRs also AFFECT the systemic cholesterol levels by reducing intestinal cholesterol absorption and increasing biliary cholesterol excretion through the regulation of the transporters ABCG5 and ABCG8 (Repa et al. 2002). Treatment of atherosclerotic mice with synthetic LXR ligands, such as GW3965 and T0901317, inhibits the progression and promotes the regression of atherosclerotic plaques. Furthermore, the transplantation of macrophages lacking LXRα and β into a host predisposed to atherogenesis results in increased foam cell differentiation and arterial plaque formation, even after treatment with LXR agonists (Levin et al. 2005). Although synthetic LXR agonists have an obvious therapeutic interest, they may induce lipogenesis, leading to increasing plasma triacylglycerol concentrations and hepatic steatosis (Schultz et al. 2000). Thus, specific LXR ligands that do not induce fatty acid synthesis in the liver are of interest.
Squalene, a natural tripterpene, is often used in nutrition, health care, cosmetics and medicine. It has several beneficial properties as an anticancer agent, an antioxidant, a drug carrier, a detoxifier, as an aid to skin hydrating, and in providing emollient activities of adjuvants for vaccines (Spanova and Daum 2011). Squalene also has cardio-protective properties and hypocholesterolemic effects (Chan et al. 1996; Liu et al. 2009). Chan et al. (1996) reported that the treatment of elderly patients with hypercholesterolemia with a diet containing 850 mg squalene per day for 20 weeks significantly decreased the levels of total cholesterol by approx. 17%, the low-density lipoprotein cholesterol (LDL-C) by 22% and triacylglycerol by 5%. Although numerous studies have shown that squalene has anti-atherosclerotic and hypocholesterolemic effects, its direct molecular target remains unknown. In this study, the potential application of squalene as a novel LXR activator to reduce cholesterol levels was investigated.
Materials and methods
Isolation and purification of squalene from Schizochytrium mangrovei PQ6
Squalene was isolated and purified from dried biomass of S. mangrovei PQ6 as described by Hoang et al. (2014).
Cell culture and experiments
HepG2 and RAW 246.7 cells were obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in Dulbecco’s minimum essential medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin/streptomycin before treatment. All cells were grown in 5% CO2 at 37 οC. For the experiments, HepG2 cells were incubated with 600 μM palmitate to mimic hyperlipidemic conditions for 24 h. Then the lipid-loaded HepG2 and RAW 246.7 cells were treated with squalene (50 and 100 μM), synthetic LXRs agonist (T0901317; 1 μM) or 0.1% dimethyl sulfoxide (DMSO) as vehicle for a further 48 h. Each treatment was performed at least in triplicate.
Reporter gene assays
The transfection and reporter gene assays were performed with CHO-K1 cells (Hoang et al. 2012).
Cellular lipid measurements
Cellular and medium lipids were extracted (Hoang et al. 2012). The cellular and medium concentrations of cholesterol and triacylglycerol (TAG) were quantified enzymatically with an automated chemistry analyzer (Olympus Analyzers, Tokyo, Japan). Protein quantification was measured using the Bio-Rad protein assay kit for normalization.
Oil Red O staining
HepG2 cells were stained with Oil Red O as described by Hoang et al. (2012). Stained lipid images were taken with a digital camera. Lipid accumulation was quantified through 21-propanol extraction of Oil Red O from stained cells and read at 500 nm.
Determination of mRNA expression
Total RNA was extracted from RAW 246.7 and HepG2 cells using an RNAiso Plus reagent (Takara, Japan) as instructed by the manufacturer. Quantitative real-time PCR was performed in the LightCycler 2.0 system (Roche Diagnostics, Penzberg, Germany) using SYBR Green (Thermo). Expression levels were normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) by the normalized expression (CT) method according to the manufacturer’s guidelines. The primer sequences are shown in Supplementary Table 1.
Immunoblotting analysis
RAW 264.7 macrophages and HepG2 cells were lysed in ice-cold lysis buffer containing 10 mM Tris/HCl (pH 7.4), 0.1 M EDTA, 10 mM NaCl, 0.5% Triton X-100, and protease inhibitor cocktail (ThermoFisher). The lysate was clarified by centrifugation at ~10,000×g for 10 min at 4°C. Protein concentration was determined using a Coomassie protein assay reagent with bovine serum albumin as the standard. Protein samples (100 μg) were subjected to 8–12% SDS–PAGE and then transferred and immobilized on PVDF membranes. After blocking, the membranes were probed with primary antibody (anti-ABCA1, anti-SREBP 1, anti-Insig2; Santa Cruz Biotechnology) and then incubated with secondary antibody (anti-rabbit or anti-mouse immunoglobulin G; Thermo). Immunoreactive bands were stained with 3,3′-diaminobenzidine in 50 mM Tris/HCl (1 mg/ml with 0.1% H2O2, pH 7.2). The reaction was stopped by rinsing with water; the membrane was dried and scanned. Relative band intensities were determined using Gel-Pro Analyzer 4.0 software (Media Cybernetics). For each sample, target protein levels were normalized to a-tubulin (internal reference).
Statistics
Data are expressed as mean ± standard error of the mean (SEM). Differences were considered statistically significant at P < 0.05, assessed using Student’s t test.
Results and discussion
Squalene is an agonist ligand of LXRα and β
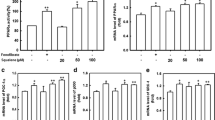
In the present study, squalene significantly increased the transactivation of both LXRα and LXRβ. Squalene increased the transactivation of LXRα by 60 and 70% at 50 and 100 μM, respectively; while T0901317 increased the transactivation of LXRα by 147% compared with the controls (P < 0.05) (Fig. 1a). Treatment with squalene 50 and 100 μM increased the transactivation of LXRβ by more than 30%, whereas T0901317 activated the LXRβ transactivation by 53% compared with the controls (P < 0.05) (Fig. 1b).
Squalene induced the transactivation of both LXR-α (a) and LXR-β (b). Cells were co-transfected with the reporter vector pGL4.35[luc2P/9XGAL4UAS/Hygro], pSV-β-galactosidase and either pFN26AhLXRα or pFN26AhLXRβ. Luciferase activity was assayed and normalized to that of β-galactosidase. *P < 0.05 versus controls (no treatment). Data are mean ± SEM
Squalene decreases cellular cholesterol and stimulates medium cholesterol by regulating the expression of LXRs and their responsive genes.
The activation of LXRs promotes cholesterol efflux, stimulates RCT in macrophages, and inhibits the accumulation of cholesterol in vitro and in vivo (Geyeregger et al. 2006). Therefore, we evaluated the cellular cholesterol concentration in RAW 264.7 macrophages stimulated with squalene. Squalene at 50 and 100 μM reduced the cellular cholesterol concentration by 10 and 14%, respectively, compared to the control cells (P < 0.05) (Fig. 2a). This was accompanied by an increased concentration of cholesterol in the culture medium treated with 50 and 100 μM squalene (P < 0.05; over 20% higher than the controls) (Fig. 2b). Similar but stronger trends were observed in cells stimulated with T0901317.
Squalene induced the efflux of cholesterol from RAW246.7 macrophage by upregulating the gene expression of LXRs and their target genes. a Cellular and b medium cholesterol concentrations in RAW246.7 macrophage. c–g mRNA expression of LXRα, LXRβ, ABCA1, ABCG1 and ApoE in RAW 246.7 cells, as measured by qPCR. h ABCA1 protein level in RAW 246.7 cells, as measured by immunoblotting. *P < 0.05, **P < 0.01, versus controls (no treatment). Data are mean ± SEM
LXRs play a critical role in cholesterol homeostasis by inducing the expression of several genes involved in cholesterol efflux as ABCA1 and ABCG1 transporters (Geyeregger et al. 2006). ABCA1 transfers both cholesterol and phospholipids from plasma membrane to lipid-free apolipoprotein A-I (apoA-I). This transporter is also crucial for the formation of nascent HDL particles in the liver. On the other hand, the function of ABCG1 is to transfer cholesterol to HDLs. ApoE is also an LXR target gene involved in cholesterol homeostasis, and mice lacking ApoE spontaneously develop atherosclerosis (Wouters et al. 2005). Taking into consideration our observation that squalene induced the cholesterol efflux, we further performed quantitative PCR (qPCR) and immunoblotting analysis of LXR target genes. We demonstrated that squalene increased the expression of LXRα and LXRβ mRNA, similar to T0901317 (Fig. 2c and d). Treatment with squalene (50 or 100 μM) increased the mRNA expression of LXRα in macrophages by 57–81% (P < 0.05) (Fig. 2c). The mRNA expression of LXRβ was upregulated in a dose-dependent manner, being increased by 61 and 93% in cells treated with 50 and 100 μM squalene, respectively (Fig. 2d). Squalene also significantly increased the mRNA expression of the LXR-responsive genes ABCA1, ABCG1 and ApoE in macrophages (P < 0.05). At 100 μM, squalene increased ABCA1, ABCG1 and ApoE mRNA expression by 46, 38 and 112% (P < 0.05), respectively, compared to the control group. In tandem with the upregulation of mRNA expression, the level of ABCA1 protein was also increased in macrophages stimulated with squalene (Fig. 2h). Similar but greater effects were observed in cells stimulated with T0901317; these findings suggest that squalene as an LXR ligand suppresses cholesterol accumulation by promoting an efflux pathway in macrophages, which could lead to the elevation of circulating levels of HDL-cholesterol and prevention of hypercholesterolemia and atherosclerosis.
Squalene reduces cellular TG concentrations by upregulation of Insig-2a in HepG2 cells
The role of LXR in the control of fatty acid metabolism has been implicated as a potential side effect of LXR therapy. The expression of fatty acid synthesis genes, including sterol regulatory element-binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) is blunted in mice carrying a targeted disruption in the LXRα gene. On the other hand, administration of synthetic LXR ligands in mice elevates plasma TAG levels, in part by inducing the hepatic lipogenic pathway (Schultz et al. 2000). In this study, stimulation of HepG2 cells with T0901317 increased cellular TAG levels, as previously reported, and also increased the expression of SREBP-1c and FAS (Fig. 3a–d). Notably, the cellular TAG concentrations decreased significantly with squalene; compared to the control, squalene at 100 μM significantly reduced the TAG level by 17% (Fig. 3a). Oil Red O staining showed similar results (Fig. 3b). Furthermore, squalene did not alter the mRNA and protein expression of SREBP-1c and expression of FAS (Fig. 3 c, d, f); this indicates that, unlike T0901317, squalene acts as a partial agonist that selectively activates LXRs without inducing hepatic lipogenesis. Thus, treatment with this compound may have practical implications for the prevention or treatment of dyslipidemia without undesirable side effects.
Squalene has modest effects on cellular triacylglycerol levels in HepG2 cells and the expression of hepatic lipogenesis genes. a Cellular triacylglycerol levels in HepG2 cells. b Oil Red O lipid staining of HepG2 incubated with squalene and T090131727. c–e mRNA expression of SREBP-1c, FAS and Insig-2a in HepG2 cells incubated with squalene and T0901317, as assessed by qPCR. f–g SREBP-1c and Insig-2a protein levels in HepG2 cells, as measured by immunoblotting. *P < 0.05, **P < 0.01, vs. controls (no treatment). Data are mean ± SEM
The mechanisms by which squalene does not affect expression of SREBP1c and FAS genes in hepatocytes are currently unclear. The endogenous LXR agonists cholesterol and oxysterols, inhibit the nuclear translocation of SREBP-1c by activating Insig-2a gene expression and, thus, did not stimulate FAS or SCD-1 gene expression in the mouse liver (Dang et al. 2009). Inhibition of SREBP processing by cholesterol and oxysterols is the major homeostatic mechanism that balances cellular cholesterol and fatty acid metabolism. When cholesterol is high, cholesterol binds to the sterol-sensing domain of SCAP and recruits insulin signaling protein (INSIG), thus retaining the INSIG—SCAP—SREBP complex in the ER, the expressions of SREBP-regulated gene were downregulated (Brown et al. 2002). In fact, Insig-2 is required for cholesterol and oxysterols to retain the SREBP—SCAP complex in the ER (Yabe et al. 2002). In our study, stimulation with squalene significantly increased Insig-2a mRNA and protein levels in HepG2 cells (Fig. 3e, g). At 100 μM, squalene increased mRNA and protein expression of Insig-2a by 44 and 37% (P < 0.05), respectively, compared to the controls. This suggests that squalene may delay nuclear translocation of SREBP-1c and retains the INSIG2-SCAP-SREBP-1c triple complex in ER and consequently does not alter the expression of SREBP-1c and its responsive hepatic lipogenic gene, FAS.
Albers et al. (2006) reported that the selective compared the effects of T0901317 to those of LN6500 in the induction of two LXR target genes, FAS and SCD-1 in the liver. T0901317 induced the expressions of FAS and SCD-1 while LN6500 showed no induction. In a co-activator recruitment assay, T0901317 recruited TRAP220 strongly, while LN6500 only recruited TRAP220 weakly, which may explain the lack of induction of FAS and SCD-1 expression by LN6500. Hence, it is possible that LXRs activation by squalene may recruit co-activators as well as LN6500 did. In addition, Svensson et al. (2003) reported that groups of trifluoro ethyl, phenyl and sulfonamide in T0901317 are essential for the interaction of T0901317 and LXRs, while structure of squalene only contains groups of methyl. Alternatively, it is also possible that squalene may not directly activate LXR proteins but indirectly by inducing endogenous ligand synthesis. Therefore, these possibilities should be examined in the future.
Conclusions
Squalene is a selective LXR modulator that regulates the expression of key genes in reverse cholesterol transport in macrophages without inducing lipogenesis in hepatocytes. Squalene can not only serve as a potent pharmaceutical agent for treatment of hypercholesterolemia and atherosclerosis, but also prevents the potential side effect of hepatic steatosis.
References
Albers M, Blume B, Schlueter T, Wright MB, Kober I, Kremoser C, Deuschle U, Koegl MA (2006) A novel principle for partial agonism of liver X receptor ligands. Competitive recruitment of activators and repressors. J Biol Chem 281:4920–4930
Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL (2002) Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol Cell 10:237–245
Chan P, Tomlinson B, Lee CB, Lee YS (1996) Effectiveness and safety of low-dose pravastatin and squalene, alone and in combination, in elderly patients with hypercholesterolemia. J Clin Pharmacol 36:422–427
Dang HX, Liu Y, Pang W, Li CH, Wang NP, Shyy JYJ, Zhu Y (2009) Suppression of 2,3-oxidosqualene cyclase by high fat diet contributes to liver X receptor-α-mediated improvement of hepatic lipid profile. J Chem Biol 284:6218–6226
Geyeregger R, Zeyda M, Stulnig TM (2006) Liver X receptors in cardiovascular and metabolic disease. Cell Mol Life Sci 63:524–539
Hoang MH, Jia Y, Jun HJ, Lee JH, Lee BY, Lee SJ (2012) Fucosterol is a selective liver X receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J Agric Food Chem 60:11567–11575
Hoang MH, Ha NC, Thom LT, Tam LT, Anh HT, Thu NT, Hong DD (2014) Extraction of squalene as value-added product from the residual biomass of Schizochytrium mangrovei PQ6 during biodiesel producing process. J Biosci Bioeng 118:632–639
Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG (2005) Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscl Thromb Vasc 25:135–142
Liu Y, Xu X, Bi D, Wang X, Zhang X, Dai H (2009) Influence of squalene feeding on plasma leptin, testosterone and blood pressure in rats. Indian J Med Res 129:150–153
Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ (2002) Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem 277:18793–18800
Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B (2000) Role of LXRs in control of lipogenesis. Genes Dev 14:2831–2838
Spanova M, Daum G (2011) Squalene – biochemistry, molecular biology, process biotechnology, and applications. Eur J Lipid Sci Technol 113:1299–1320
Svensson S, Ostberg T, Jacobsson M, Norstrom C, Stefansson K, Hallen D, Johansson IC, Zachrisson K, Ogg D, Jendeberg L (2003) Crystal structure of the heterodimeric complex of LXRα and RXRβ ligand-binding domains in a fully agonistic conformation. EMBO J 22:4625–4633
Wouters K, Shiri-Sverdlov R, van Gorp PJ, van Bilsen M, Hofker MH (2005) Understanding hyperlipidemia and atherosclerosis: lessons from genetically modified apoe and ldlr mice. Clin Chem Lab Med 43:470–479
Yabe D, Brown MS, Goldstein JL (2002) Insig-2, a second endoplasmic reticulum protein that binds SCAP and blocks export of sterol regulatory element-binding proteins. Proc Natl Acad Sci USA 99:12753–12758
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under Grant number 106-YS.06-2013.23. We would like to send our appreciation to all of supports from National Key Laboratory, Institute of Biotechnology, VAST. The LXRα, β and LXRE plasmids were a generous gift of Prof. Dr. Sung-Joon Lee, Korea University, Seoul, Korea.
Supporting information
Supplementary Table 1—Primers used for qRT-PCR.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hien, H.T.M., Ha, N.C., Thom, L.T. et al. Squalene promotes cholesterol homeostasis in macrophage and hepatocyte cells via activation of liver X receptor (LXR) α and β. Biotechnol Lett 39, 1101–1107 (2017). https://doi.org/10.1007/s10529-017-2345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2345-y