Abstract
Objective
To investigate the effects of ultrasmall superparamagnetic iron oxide (USPIO) labeling on the maturity or immune tolerance of immature dendritic cells (imDCs) as the success of immunotherapy with immature dendritic cells is highly dependent on immune tolerance.
Results
The feasibility of tracking implanted USPIO-labeled imDCs in vivo by magnetic resonance imaging (MRI) was explored. The effects of USPIO labeling on the immune tolerance of imDCs was examined. USPIO when higher than 200 μg/ml caused considerable damage to imDCs, induced imDC maturation, and impacted the immune tolerance of imDCs. USPIO labeling caused a dose-dependent increase in autophagosome formation in imDCs, and autophagy inhibitors prevented the maturation of imDCs while stimulating their immune tolerance.
Conclusions
We speculate that high concentrations of USPIO can be used to induce imDC maturation, and that this process is likely mediated through an autophagy-related pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendritic cells (DCs) play a critical role in the initiation of primary immune responses and can be classified as either tolerogenic immature dendritic cells (imDCs), that maintain immune tolerance, or immunogenic mature dendritic cells (mDCs), that are powerful stimulators of the immune response (Griffiths and O’Neill 2008). imDCs are highly efficient in antigen uptake and are capable of inducing T cell anergy or the generation of regulatory T cells (Tregs). Therefore, these cells can be used for specific cell therapies to induce immunological tolerance in transplantation and autoimmunity (Boks et al. 2012). imDCs exert potent immunosuppressive effects in the context of allograft rejection and allergy. However, the application of imDC vaccines in the clinical setting and the precise mechanisms underlying the immunosuppressive effects of these cells remain unclear.
Ultrasmall superparamagnetic iron oxide (USPIO), an iron oxide nanoparticle with diameters of approx. 30–50 nm, yields decreased signals on T2- and T2*-weighted images and has a relatively long half-life, making it an effective contrast agent for use in magnetic resonance imaging (MRI). Indeed, this agent is widely used for disease diagnosis and therapy. Characterizing the mechanism(s) of DC migration in vivo is crucial for both enhancing our understanding the immune response and treating diseases. While live cells can be labeled and tracked by MRI, direct cell labeling is generally associated with certain drawbacks, including cytotoxicity, suppressed proliferation, and impaired differentiation capacity (Bengel et al. 2005). The efficacy of MRI for visualizing SPIO-labeled monocytes and lymphocytes has been evaluated and has identified concentrations of these cells that are suitable for in vivo tracking; however, the influence of USPIO on cellular functions, as well as the optimal concentration of USPIO-labeled imDCs for in vivo tracking, remain unclear. In this study, we therefore investigated the viability and functionality of USPIO-labeled imDCs.
Autophagy is an intracellular process employed by various types of cell, including DCs, that is involved in packaging long-lived proteins and defective organelles into autophagosomes and delivering them to lysosomes for degradation (Cuervo et al. 2005). While excess autophagy has been suggested to induce the maturation of imDCs, the mechanism underlying this process remains poorly understood. Elevated autophagy is a widespread phenomenon in cells that have been treated with nanomaterials, indicating that USPIO, a type of nanoparticle, might activate this process. Additionally, induction of autophagy can facilitate antigen processing (Jagannath et al. 2009); however, limited data are available on the effects of autophagy on the other functions of imDCs.
In this study, we explored the minimal concentration of USPIO and the lowest number of imDCs required for detection by 1.5T MRI. Furthermore, we investigated whether MRI comprises a feasible method for monitoring transplanted imDCs. Lastly, we investigated whether USPIO promotes imDC autophagy, and whether autophagy might affect imDC maturation and the uptake/processing of antigens by comparing the levels of cytokine secretion and co-stimulatory molecule expression of labeled and unlabeled cells.
Materials and methods
Ethics statement
All experiments were conducted according to the Code of Ethics of the World Medical Association (Declaration of Helsinki), and all experimental protocols involving animals were approved by the Animal Care and Use Committee of Guangdong Province, China.
Nanomagnetic labeling materials
USPIO particles (diam: 30–50 nm) were provided by the Medical Imaging Center of Southern Medical University (Guangzhou, China). USPIO was incubated for 30 min at 50–2000 μg/ml with poly-l-lysine (PLL; Sigma) at 1.5 μg/ml in serum-free RPMI 1640 medium containing l-glutamine.
Cell preparation, cultivation, and labeling
Dendritic cells (DCs were derived from peripheral blood mononuclear cells (PBMCs) as previously described (Zhou and Chen 2012). PBMCs were isolated from the peripheral blood of New Zealand white rabbits via the auricular artery and cultured in complete medium (CM) in six-well polystyrene tissue culture plates for 4 h at 37 °C in an atmosphere containing 5% CO2. After incubation, non-adherent cells were discarded from the wells, and adherent cells (monocytes) were incubated in CM supplemented with granulocyte–macrophage colony-stimulating factor (GM-CSF; PeproTech, Rocky Hill, NJ, USA) at 50 ng/ml and 50 ng interleukin-4/ml (IL-4; PeproTech) for 6 days. Purity of DCs was assessed by expression of CD11c by flow cytometry. This was routinely >80%. For USPIO-labeling, imDCs (5 × 105 cells/well) were co-cultured with 1.5 ml of each USPIO-PLL mixture, respectively, for 12, 24, or 48 h.
Cell viability and proliferation
The Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) assay was used to assess the effects of USPIO-PLL on cell viability. The viability of cells was proportional to A450 value as determined using a microplate reader.
Levels of cell apoptosis and necrosis were measured using an Annexin V-FITC/PI Kit (BD Bioscience, Co.; San Jose, CA, USA), according to the manufacturer’s recommendation, and a FACSCanto flow cytometer (BD Biosciences).
Determination of intracellular iron content and MRI of USPIO-PLL-labeled imDCs
Iron concentrations within USPIO-labeled cells were quantified using a Ferrozin-based spectrophotometric assay.
For MRI analyses, different numbers of cells labeled with varying concentrations USPIO-PLL for 12 h were transferred into 1.5 ml Eppendorf tubes loaded with 1 ml 1% agarose. Samples were then subjected to MRI using a 1.5 T clinical whole-body MR system (GE Healthcare, Little Chalfont, UK) with a 12.7-cm receive-only knee coil (Zhang et al. 2015). Cells were imaged using the T2 mapping sequence [repetition time (TR) = 1 s; echo time (TE) = 16, 18, 20, 22 ms; band-width = 15.63; phase = 160; number of excitations (NEX) = 2] with a section thickness of 0.5 mm and a field of view (FOV) of 18 mm. Images were obtained with a matrix size of 256 × 160. The region of interest (ROI) for the signal intensity (SI) measurement was 19 mm2.
For MRI analysis of in vivo DC migration to the liver, imDCs were labeled with 200 μg USPIO/ml and injected into rabbits via the ear vein (106 cells/ml, 2 ml per rabbit); control animals were injected with an equal volume of physiological saline. After anesthesia and immobilization in the prostrate position, rabbits were scanned by MRI at 1, 3, and 7 days after cell transplantation. Imaging was performed with a knee coil on a 1.5 T MRI with 3/0.5 mm thick slices. MRI tracking was performed using T2-weighted sequences with fat suppression (T2WI-FS) with the following parameters: repetition time (TR) = 5500 ms; echo time (TE) = 106.6 ms; FOV = 14 mm; and matrix size, 256 × 256.
Analysis of the expression levels of surface antigens by flow cytometry and laser-scanning microscopy
Cells labeled with different concentrations of USPIO-PLL (0–500 μg/ml) for 12 h were harvested and stained with FITC-conjugated anti-CD86, APC-conjugated anti-CD14, and PE-conjugated anti-HLA-DR (BD Biosciences) for 30 min, and evaluated by flow cytometry and laser-scanning microscopy (LSM510/ConfoCor2; Carl Zeiss, Oberkochen, Germany).
ELISA and mixed leukocyte reaction (MLR)
The concentration of IL-10 in each sample was measured using ELISA. Concentrations were quantified by comparing the OD450 values of the samples to a standard curve.
MLR was assessed using a Cell Counting Kit-8 (Dojindo), according to the manufacturer’s instructions. Levels of lymphocyte proliferation were proportional to the OD450 value, which was determined using a microplate reader.
Endocytosis assay
Receptor-mediated endocytosis levels were assayed by evaluating uptake of FITC-dextran (40,000 MW; Sigma). Briefly, cells were labeled with different concentrations of USPIO-PLL (0–500 μg/ml) for 12 h, harvested, washed, and incubated with 100 μg FITC-dextran/ml diluted in PBS for 3 h at 37 °C. Cells were then washed once with PBS, resuspended in 200 ml PBS, and analyzed immediately by flow cytometry.
Detection of autophagy
Autophagic vacuoles were detected using LysoTracker Red DND-99 stain (Molecular Probes, Inc., Eugene, OR, USA) (Moon et al. 2009). Cells were placed in Prolong Gold antifade with DAPI to stain nuclei. imDCs were incubated with USPIO-PLL (0–500 μg/ml) for 12 h, washed three times with PBS, and then incubated with pre-warmed (37 °C) medium containing LysoTracker Red DND-99 for 1 h, washed once with PBS. Cells were then stained with DAPI. Cells were then examined under a laser-scanning microscope (LSM510/ConfoCor2); intense red dots within the cytoplasm were representative of autophagic vacuoles.
Statistical analysis
Each experiment was repeated at least three times. Data were presented as means ± standard deviations (SDs) and were analyzed by two-sample t-tests and one-way analysis of variance (ANOVA) with Bonferroni correction using Origin 7 software (OriginLab Corp., Northampton, MA, USA). p values < 0.05 were considered statistically significant (*p < 0.05, **p < 0.01, and ***p < 0.001, compared to controls).
Results
Cell differentiation
Compared with unstimulated cells, cells after stimulation exhibited markedly increased expression of CD11c, as determined by flow cytometry (Fig. 1).
Effects of USPIO-PLL on cell proliferation and viability
CCK8 assay analyses detected no significant differences between the viability of labeled cells and that of unlabeled cells after incubation with up to 2000 μg USPIO/ml for 12 h (Fig. 2a). However, statistical analysis of data after 24 and 48 h showed that cell viability decreased significantly as the concentration of USPIO increased to 1000 μg/ml. Consistent with these findings, while we detected no significant differences in the numbers of apoptotic or necrotic cells between labeled and unlabeled cells after 12 h by annexin V-FITC/PI staining (Fig. 2b), incubation with high concentrations of USPIO for 24 or 48 h resulted in increased apoptosis and necrosis (Fig. 2c, d). Together, these results indicate that USPIO affects the viability of imDCs in a concentration- and time-dependent manner.
Effects of ultrasmall superparamagnetic iron oxide (USPIO) labeling on cell proliferation and viability. a Immature dendritic cells (imDCs) were incubated with USPIO-PLL (poly-l-lysine) at different concentrations (0–2000 μg/ml) for 12, 24, or 48 h, and CCK-8 assays were used to examine cell proliferation and viability. (a–f) Control, 100, 200, 500, 1000, and 2000 μg USPIO/ml. b–d Survival rates of USPIO-labeled imDCs were determined by Annexin V-FITC/PI staining and flow cytometry at b 12 h, c 24 h, and d 48 h after labeling
USPIO-PLL labeling efficiency
In imDCs incubated with USPIO for 12 h, the intracellular iron content increased significantly with increasing concentrations of USPIO from 0 to 200 μg/ml and remained constant at USPIO concentrations from 200 to 500 μg/ml (Fig. 3). Notably, no significant differences in labeling efficiency were observed between cells incubated with 200 μg/ml for 12 h and those incubated with higher concentrations (500 and 1000 μg/ml) for 24 or 48 h, indicating that maximum labeling efficiency with low cytotoxicity could be achieved via incubation with USPIO at 200 μg/ml for 12 h.
In vitro and in vivo MR imaging of imDCs
To determine the detection threshold of USPIO-labeled imDCs on a 1.5 T MR scanner, we labeled imDCs with concentrations of USPIO ranging from 0 to 500 μg/ml for 12 h. As shown in Fig. 4a, the decrease in signal intensity was dependent on both the density of imDCs and the USPIO starting concentration. At 200 μg USPIO/ml, there was a distinguishable decrease in signal intensity (by approx. 31%) for 106 imDCs/ml (Fig. 4b), suggesting that injection of 1 × 106 imDCs/ml labeled with 200 μg USPIO/ml was optimal for distinguishing signal changes on the 1.5 T MRI scanner.
In vitro and vivo magnetic resonance imaging (MRI). a Different numbers of cells in each condition were embedded in 1 ml of 1% agarose and imaged on a 1.5 T MR system. Cells cultured in the absence of ultrasmall superparamagnetic iron oxide (USPIO) were used as controls. b Decrease in signal intensity caused by USPIO labeling of immature dendritic cells (imDCs), imaged with a 1.5 T MR-scanner using T2 mapping. c Rabbits were subjected to MRI analysis at different periods after bolus injection of USPIO-labeled imDCs; images of rabbits prior to bolus injection served as a control. d Quantification of the MRI signal intensity of three different slices within a region of interest within the rabbit liver (a–d control, 1 day after injection, 3 days after injection, 7 days after injection, respectively). Error bars represent standard deviations of the results obtained from three independent experiments
In vivo migration of USPIO-labeled imDCs was measured by MRI. Intravenous bolus injection of USPIO-labeled imDCs resulted in a strong signal loss in the liver tissue (Fig. 4c, d), and the livers of imDC-transplanted rabbits were darker than those of control rabbits. In addition, the minimal reduction in signal within the liver was observed at 1 day after injection, and this reduction increased continuously over the subsequent 7 days; these data indicate that labeled DCs can be used for long-term tracking and that transplanted DCs labeled with USPIO can be dynamically tracked in vivo by MRI.
Phenotypic analysis by flow cytometry and laser-scanning microscopy
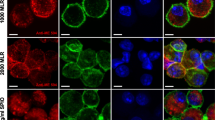
Compared with cells cultivated in USPIO-free medium, imDCs treated with 200 μg USPIO/ml exhibited reduced CD14 expression and markedly increased expression of CD86 and HLA-DR, as determined by flow cytometry (Fig. 5a). Moreover, these changes in expression occurred in a USPIO-dose-dependent manner. Similarly, microscopic analyses detected gradual decreases and increases in the fluorescence intensity of CD14, and of HLA-DR and CD86, respectively, in imDCs treated with 200 μg USPIO/ml (Fig. 5b). Together, these results indicate USPIO inhibits the expression of CD14 but promotes the expression of HLA-DR and CD86 expression, as compared to cells cultured in USPIO-free medium.
Detection of surface markers of immature dendritic cells (imDCs) by flow cytometry and laser scanning microscopy. a The expression of CD86, HLA-DR, and CD14 on imDC membranes was detected by flow cytometry analysis using marker-specific antibodies. b Cells were stained with fluorescein-labeled antibodies targeting CD86, HLA-DR, and CD14 and were observed under a laser-scanning confocal microscope. Bars 1 μm. imDCs were labeled with the indicated concentrations of ultrasmall superparamagnetic iron oxide (USPIO) for 24 h
Stimulation of lymphocyte proliferation and ELISA for detection of cytokine expression in imDCs
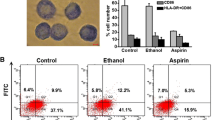
The capacity of rabbit imDCs to induce allogeneic T-cell proliferation from PBMCs was compared in an MLR (Fig. 6A). imDCs labeled with low concentrations of USPIO (0–200 μg/ml) did not exhibit obvious stimulation of proliferation when co-cultured with allogeneic PBMCs. Conversely, vigorous proliferation was observed in co-cultures of allogeneic PBMCs and imDCs labeled with high concentrations of USPIO (≥500 μg/ml). In addition, mixtures containing higher ratios of imDCs and T cells showed even greater proliferation rates upon PBMC stimulation. Moreover, as shown in Fig. 6b, incubation with low concentrations (≤200 μg/ml) of USPIO had almost no effect on IL-10 production in imDCs, while higher concentrations (500 μg/ml) of this compound dramatically decreased IL-10 concentrations.
Detection of immunity of immature dendritic cells (imDCs). a Mixed lymphocyte reaction containing imDCs and indicated concentrations ultrasmall superparamagnetic iron oxide (USPIO)-labeled imDCs with peripheral blood mononuclear cells (PBMCs) obtained from an allogeneic donor. b Analysis of IL-10 levels in 72 h mixed leukocyte reaction (MLR) supernatants (stimulator-responder ratio, 0.1) by enzyme-linked immunosorbent assay (ELISA). c Uptake of FITC-dextran by imDCs and indicated concentrations USPIO-labeled imDCs
Receptor-mediated endocytosis activity
Receptor-mediated endocytosis activity was assayed by measuring the uptake of FITC-dextran by flow cytometry (Fig. 6c). Exposure to USPIO substantially inhibited the phagocytosis of the antigen. Furthermore, increasing concentrations of USPIO were associated with greater inhibition of endocytosis. Thus, USPIO-treated DCs exhibit significantly lower endocytic capacity for FITC-dextran than untreated imDCs.
USPIO labeling induced autophagy in imDCs
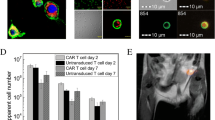
To measure the ability of USPIO to induce autophagy in imDCs, cells were treated with LysoTracker Red, which specifically labels acidic compartments, such as autophagic vacuoles. In addition, to detect the condition of imDCs, cells were treated with DAPI, that stains the nucleus of cells. As shown in Fig. 7, exposure of imDCs to USPIO for 12 h stimulated the production of acidic autophagic lysosomes in a concentration-dependent manner.
Autophagy influenced surface marker expression, T-cell stimulatory capacity, and endocytic capacity of imDCs in vitro
The expression levels of the cell surface co-stimulatory molecules CD86 and HLA-DR were obviously downregulated, while the expression level of CD14 was substantially upregulated, following addition of 3-methyladenine (3-MA, autophagy inhibitor) (Fig. 8a, b). In addition, 3-MA treatment resulted in increased production of IL-10 and decreased T-cell stimulatory capacity by imDCs (Fig. 8c, d). Furthermore, the endocytic capacity of imDCs treated with 3-MA was markedly higher than that of untreated cells (Fig. 8e). Finally, the number of autophagic vacuoles of imDCs treated with 3-MA was markedly less than untreated cells (Fig. 8f). Collectively, these results suggested that autophagy influences the surface marker expression, T-cell stimulatory capacity, IL-10 production, and endocytic capacity of imDCs in vitro. We therefore conclude that autophagy has influences the immunologic function of imDCs.
Autophagy affects the immunity of immature dendritic cells (imDCs). imDCs treated with the autophagy inhibitor 3-methyladenine (3-MA) exhibited deficiencies in immune activity. a, b Effects of 3-MA on surface marker expression in imDCs, as determined by a laser-scanning microscopy and b flow cytometry. c–e The effects of 3-MA on c the stimulation of T-cell proliferation, d the production of IL-10, and e the uptake of FITC-dextran, as determined by mixed leukocyte reaction (MLR), enzyme-linked immunosorbent assay (ELISA), and flow cytometry analyses, respectively. Scale bars 1 μm
Discussion
imDCs can act as potent immunosuppressors after allotransplantation by inducing immune tolerance. However, we currently lack an effective method for monitoring transplanted imDCs in vivo. MRI has been utilized for tracking cells in vitro and in vivo. In particular, the use of MRI for detecting cells labeled with USPIO particles, which provide strong changes in signals on T2 mapping and are composed of biodegradable, biocompatible iron (Corot et al. 2006), may represent a novel noninvasive method for tracking transplanted cells and tissues in the clinical setting.
In this study, imDCs incubated with 200 μg USPIO/ml for 12 h were readily detectable by MRI after injection in rabbits. This effect on MRI signal intensity in T2WI images was dependent on both the concentration of iron oxide per cell and the cell density. From these analyses, we concluded that incubation of 106 cells/ml with 200 μg USPIO/ml for 12 h yielded optimal detection of imDCs by whole-body MRI scanning. Notably, this signal was sustained for at least 7 days; however, whether the USPIO-loaded imDCs remained immature during this time period remains unclear.
imDCs are efficient in antigen phagocytosis and rarely express the required accessory signals for T-cell activation. Antigen presentation in the absence of co-stimulatory molecules can lead to clonal T-cell anergy (Lutz and Schuler 2002). Due to the Ag-dependent immunosuppressive ability of imDCs, these cells represent a promising candidate for clinical applications in cell transplantation therapy. Therefore, we next investigated whether USPIO influenced the immunosuppressive capacity of imDCs. We found that cells labeled with high concentrations of USPIO showed upregulation of HLA-DR (MHC class II) and CD86, as well as downregulation of CD14 and IL-10. Moreover, these cells exhibited reduced capacity for receptor-mediated endocytosis and were potent stimulators of allogeneic T-cell proliferation in mixed lymphocyte reactions. Therefore, we concluded that USPIO should be used at concentrations of 200 μg/ml or less for labeling of imDCs.
Nanomaterials, such as, USPIO cause different degrees of autophagy (Man et al. 2010). In this study, autophagy occurred following treatment of cells with USPIO, particularly at high concentrations, for 12 h. Moreover, autophagy influenced the surface marker expression, T-cell stimulatory capacity, IL-10 production, and endocytic capacity of imDCs in vitro. Therefore, we speculated that high concentrations of USPIO could induce imDC maturation, possibly through the autophagy pathway.
In conclusion, strong evidence supporting the use of USPIO-labeled imDCs for MRI-based monitoring of cell tracking in vivo is provided. It remains unclear whether USPIO-loaded imDCs are stimulated to a mature state in vivo after transplantation. If so, future studies will be needed to develop methods to ensure that these cells remain in an immature state after transplantation. Likewise, further studies are still required to confirm whether this potential new treatment modality may have applications in the clinical setting.
References
Bengel FM, Schachinger V, Dimmeler S (2005) Cell-based therapies and imaging in cardiology. Eur J Nucl Med Mol Imaging 32:S404–S416
Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, ten Brinke A (2012) IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction-a comparative study of human clinical-applicable DC. Clin Immunol 142:332–342
Corot C, Robert P, Idee JM, Port M (2006) Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev 58:1471–1504
Cuervo AM, Bergamini E, Brunk UT, Dröge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1:131–140
Griffiths KL, O’Neill HC (2008) Dendritic cells as immune regulators: the mouse model. J Cell Mol Med 12:1909–1914
Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL Jr, Eissa NT (2009) Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med 15:267–276
Lutz MB, Schuler G (2002) Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol 23:445–449
Man N, Chen Y, Zheng F, Zhou W, Wen LP (2010) Induction of genuine autophagy by cationic lipids in mammalian cells. Autophagy 6:449–454
Moon EK, Chung DI, Hong YC, Kong HH (2009) Autophagy protein 8 mediating autophagosome in encysting Acanthamoeba. Mol Biochem Parasitol 168:43–48
Zhang M, Zhou J, Wang J, Zhou Q, Fang J, Zhou C, Chen W (2015) Superparamagnetic iron oxide labeling limits the efficacy of rabbit immature dendritic cell vaccination by decreasing their antigen uptake ability in a lysosome-dependent manner. Biotechnol Lett 37:289–298
Zhou J, Chen WL (2012) Induction and identification of rabbit peripheral blood derived dendritic cells. Proc SPIE Int Soc Opt Eng 8214:138–139
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81471659 and 81630046). USPIO was kindly provided by Professor Xu Yikai (Department of Medical Imaging Center, Nan Fang Hospital, Southern Medical University, No. 1838 Guangzhou Avenue North, Guangzhou, Guangdong, 510515, China).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Zhang, S., Xu, W. et al. The function and magnetic resonance imaging of immature dendritic cells under ultrasmall superparamagnetic iron oxide (USPIO)-labeling. Biotechnol Lett 39, 1079–1089 (2017). https://doi.org/10.1007/s10529-017-2332-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2332-3