Abstract
Objectives
To evaluate the biocatalytic characteristics of a new endo-β-1,4-d-mannan-degrading enzyme (ManP) from Paenibacillus sp. strain HY-8, a gut bacterium of the longicorn beetle Moechotypa diphysis.
Results
Purified ManP (32 kDa) with an N-terminal amino acid sequence of APSFAVGADFSYVPG displayed the greatest degree of biocatalytic activity toward locust bean gum (LBG) at 55 °C and pH 7.0. The enzyme degraded LBG, guar gum, ivory nut mannan, and mannooligosaccharides (M2–M5), but did not exhibit any hydrolytic activity against structurally unrelated substrates. The biocatalytic activity of ManP against LBG and guar gum was 695 and 450 U mg−1, respectively. Especially, enzymatic hydrolysis of mannobiose yielded a mixture of mannose (16.6 %) and mannobiose (83.4 %), although the degree of mannobiose degradation by ManP with was relatively limited.
Conclusion
The present results suggest that ManP is an endo-β-1,4-mannanase and is distinct from various other characterized endo-β-1,4-mannanases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In ecosystems, biological recycling of β-1,4-d-mannan polysaccharides, which are one of the main hemicellulosic components of plant biomass, is mediated by the cooperative action of diverse β-1,4-mannanase-producing bacterial and/or fungal species. These microorganisms generally produce various exo- and endo-type mannolytic enzymes with different biocatalytic functions for the complete degradation of β-1,4-d-mannans (Moreira and Filho 2008). Most β-1,4-mannanases are key endo-acting enzymes responsible for depolymerization of β-1,4-d-mannan polymers. They are affiliated with retaining glycoside hydrolase (GH) families 5, 26, 123, and 134 based on primary structural similarities (http://www.cazy.org/Glycoside-Hydrolases.html). β-1,4-Mannanases have drawn attention because they can be exploited as eco-friendly biocatalysts in various industrial processes, such as bleaching of softwood pulps, hydrolysis of coffee mannan, removal of mannan-containing stains in the detergent industry, production of poultry feed additives and prebiotic mannooligosaccharides, and degradation of galactomannan thickeners (van Zyl et al. 2010).
Similar to ruminants, including cattle and goats (Palackal et al. 2007), herbivorous insects contain diverse exo-symbiotic microorganisms in their digestive tracts that produce novel cellulolytic and/or hemicellulolytic enzymes with industrially valuable biocatalytic properties as well as unique molecular structures (Brune 2014). The longicorn beetle, Moechotypa diphysis, is a wood-feeding insect that has many fibrolytic bacteria in its gut to facilitate the intestinal digestion of plants (Park et al. 2007). Accordingly, we previously isolated a xylanolytic bacterium, Paenibacillus sp. HY-8 KCTC 10896BP, from the gut of M. diphysis and investigated the genetic and biocatalytic characteristics of its GH11 β-1,4-xylanase (Heo et al. 2006). Here, we describe the purification and biochemical properties of an extracellular endo-β-1,4-mannanase (ManP) produced by strain HY-8. ManP is the first mannan-degrading enzyme isolated and characterized from a gut bacterium of the longicorn beetle M. diphysis.
Materials and methods
Chemicals
A series of β-1,4-d-mannooligomers (M2–M5) and ivory nut mannan were purchased from Megazyme International Ireland Ltd. (Wicklow, Ireland). Carbohydrate polymers, such as chitosan, curdlan, and pectin, were obtained from USB Co. (Cleveland, USA). Poly(3-hydroxybutyrate) granules were microbially produced (Kim et al. 2011b). All other substrate compounds used in this study were provided by Sigma-Aldrich (St. Louis, USA).
Purification of β-1,4-mannanase
Extracellular β-1,4-mannanase (ManP) from Paenibacillus sp. HY-8 KCTC 10896BP (Heo et al. 2006) was produced by culturing the microorganism on a rotary shaker (200 rpm) for 48 h at 37 °C in two 2 l baffle flasks containing 500 ml LYM9 medium [5 g locust bean gum (LBG), 5 g yeast extract, 6.78 g Na2HPO4, 3 g KH2PO4, 0.5 g NaCl, 1 g NH4Cl, 1 ml of 1 M MgSO4, and 1 ml 0.1 M CaCl2 per liter of distilled water]. Following cultivation, the culture supernatant, which showed β-1,4-mannanase activity against LBG, was collected by centrifugation (5000×g) for 20 min at 4 °C. Purification of the enzyme was accomplished by (NH4)2SO4 precipitation (80 % saturation) and two consecutive chromatographies using HiPrep CM FF and HiLoad 26/60 Superdex 200 PG columns (GE Healthcare, Sweden) attached to a FPLC system (Amersham Pharmacia Biotech), according to the method described by Kim et al. (2011a) with minor modifications.
Protein analysis
To determine the relative molecular mass of purified ManP, SDS-PAGE of the denatured proteins was performed using a 12 % gel. The separated protein bands were visualized by staining the gel with Coomassie Brilliant Blue R-250. The N-terminal amino acid sequence of ManP was analyzed by automated Edman degradation of the peptides using a sequencer as described elsewhere (Kim et al. 2011a). The protein concentrations were measured using the Bradford reagent with bovine serum albumin as a standard.
Enzyme assays
β-1,4-Mannanase activity was assayed by determining the amount of reducing sugars released from LBG using 3,5-dinitrosalicylic acid reagent and mannose as a standard. The standard assay mixture (0.5 ml) was made up of 0.5 % LBG and appropriately diluted enzyme solution (0.05 ml) in 50 mM sodium phosphate buffer (pH 7). The biocatalytic reaction was routinely conducted at 55 °C for 15 min. One unit (U) of ManP activity for LBG and guar gums was defined as the amount of enzyme required to release 1 µmol reducing sugar per min under standard assay conditions.
Effects of pH, temperature, and chemicals on the β-1,4-mannanase activity
β-1,4-Mannanase activity of purified ManP was assessed from pH 3.5 to 10.5 at 50 °C for 15 min using the following buffer systems (50 mM): sodium citrate (pH 3.5–5.5), sodium phosphate (pH 5.5–7.5), Tris/HCl (pH 7.5–8.5), and glycine/NaOH (pH 8.5–11.0). β-1,4-Mannanase activity was also evaluated from 30 to 65 °C under the standard assay conditions. Thermal stability of ManP was assessed by determining its residual biocatalytic activity after holding at various temperatures for 15, 30, and 60 min, as previously described (Kim et al. 2011b). The stimulatory or inhibitory effects of various chemical compounds (1 or 5 mM) on the β-1,4-mannanase activity were ascertained after pre-incubation of ManP at 55 °C for 10 min in 50 mM sodium phosphate buffer (pH 7) with the compound of interest.
Analysis of the hydrolysis products
Biocatalytic hydrolysis of LBG (1.5 mg), ivory nut mannan (1.5 mg), and mannooligomers (M2–M5, each 1 mg) was performed using the purified ManP (5 μg) in 100 μl 50 mM sodium phosphate buffer (pH 7) for 12 h at 30 °C, during which time the enzyme retained more than 90 % of its original β-1,4-mannanase activity. After the reaction was completed, the degradation products were examined by LC–MS/MS, as previously described by Kim et al. (2011b).
Binding assay
Structurally different insoluble polymers, Avicel PH-101, chitin, chitosan, ivory nut mannan, curdlan, insoluble oat spelts xylan, lignin, and poly(3-hydroxybutyrate) granules, were employed to assess the substrate binding ability of ManP. To remove any water-soluble carbohydrates, the polymers were washed five times in 50 mM sodium phosphate buffer (pH 7) prior to the binding assay. The binding affinity of the enzyme to insoluble materials was then investigated by determining the residual β-1,4-mannanase activity and protein concentration in the supernatant collected from the reaction mixtures, as described elsewhere (Kim et al. 2011b).
Results and discussion
Purification and analysis of ManP
Similar to many cellulolytic and xylanolytic enzymes (Collins et al. 2005; Kim et al. 2016), most of microbial β-1,4-mannanases have been identified as acidic proteins (Moreira and Filho 2008). However, our preliminary experiments clearly showed that ManP did not bind to anion exchange resins such as DEAE-Sepharose, suggesting that the enzyme is a basic protein, analogous to an endo-β-1,4-mannanase from the blue mussel Mytilus edulis (Xu et al. 2002). Thus, purification of the enzyme was performed by (NH4)2SO4 precipitation (80 % saturation), cation exchange chromatography using CM-Sepharose resin, and gel permeation chromatography using Superdex 200 resin. After the three step purification procedure, ManP was purified 51-fold with an overall yield of 66 %.
SDS-PAGE analysis revealed that the relative molecular mass of ManP, which was isolated in an electrophoretically homogeneous state, was approx. 32 kDa (Fig. 1). The molecular size (32 kDa) of ManP on SDS-PAGE was similar to that (32.5 kDa) of a β-1,4-mannanase from Trichoderma harzianum T4 (Ferreira and Filho 2004), but smaller than those (>40 kDa) of other microbial multi-domain β-1,4-mannanases (Kim et al. 2011b; Zang et al. 2015). The N-terminal amino acid sequence of purified ManP was APSFAVGADFSYVPG, which did not share any obvious identity with other characterized β-1,4-mannanases available in the NCBI database.
SDS-PAGE of the purified ManP. Lane S standard marker proteins, lane 1 proteins after 80 % ammonium sulfate precipitation, lane 2 concentrated proteins after cation exchange chromatography on HiPrep™ CM FF 16/10, lane 3 concentrated proteins after gel permeation chromatography on HiLoad™ 26/60 Superdex™ 200 pg
Enzymatic characterization of ManP
ManP had activity against LBG at 55 °C in 50 mM sodium phosphate buffer (pH 7) (Fig. 2a, b). It maintained over 85 % of its original biocatalytic activity toward LBG after pre-incubation for 1 h from pH 4.5 to 9.5 (Fig. 2c). Under the optimal reaction conditions, the half-life of ManP was approx. 25 min (Fig. 2d), which is similar to the thermal properties of diverse mesophilic hemicellulases from invertebrate-symbiotic bacteria (Heo et al. 2006; Kim et al. 2011b).
Effects of pH (a) and temperature (b) on the biocatalytic activity of ManP and effects of pH (c) and temperature (d) on the stability of ManP. The optimal pH of the enzyme was evaluated using the following buffers (50 mM): sodium citrate (filled circle), sodium phosphate (open circle), Tris/HCl (filled square), and glycine/NaOH (open square). The pH stability of ManP was determined after pre-incubation of the enzyme for 1 h at a broad pH range of 3.5–11.0. The optimal temperature and thermal stability of ManP were assessed using 50 mM sodium phosphate buffer (pH 7.0), which was used to determine the pH stability of the enzyme
The stimulatory or inhibitory effect of divalent cations (each 1 mM) including Ni2+, Zn2+, and Sn2+ on the LBG-degrading activity of ManP was negligible (data not shown). However, the activity was increased by approx. 1.3-fold by adding 1 mM Mn2+ or Co2+, even though two thermostable β-1,4-mannanases from Bacillus subtilis WY34 (Jiang et al. 2006) and Bacillus sp. N16-5 (Ma et al. 2004) were significantly inactivated by 1 mM Mn2+. In addition, β-1,4-mannanase activity of ManP slightly increased when it was reacted with the substrate in the presence of Ca2+, Ba2+, Triton X-100, and sulfhydryl reagents such as sodium azide and N-ethylmaleimide. Conversely, the enzyme was completely inhibited by Trp-specific oxidizing reagents [Hg2+ (1 mM) and N-bromosuccinimide (5 mM)], similar to other functional homologues that contain Trp residues closely related to the enzyme-substrate interaction in their catalytic domains (Zolotnitsky et al. 2004). EDTA (5 mM) and Cu2+ (1 mM) inhibited the activity of ManP by approx. 28 %.
Substrate specificity
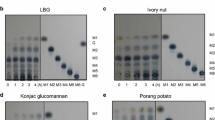
ManP readily decomposed two galactomannans (LBG and guar gum) under the optimized reaction conditions (55 °C, pH 7), although its ability to degrade galactomannans was less efficient than that of some other β-1,4-mannanases with a high specific activity (Jiang et al. 2006; Kim et al. 2011a, b). The specific activities of ManP toward LBG and guar gum were 695 and 450 U mg−1, respectively. However, like the β-1,4-mannanase produced by Cellulosimicrobium sp. HY-13 (Kim et al. 2011b), ManP did not show any detectable biocatalytic activity toward structurally unrelated polysaccharides such as carboxymethylcellulose, soluble starch, birchwood xylan, and pectin. The LBG-degrading activity of the enzyme assessed in this study was approx. 2- and 5.5-fold higher than that of an endo-β-1,4-mannanase from Trichoderma virens UKM1 (Chai et al. 2016) and that of a β-1,4-mannanase from Bacillus subtilis TJ-102 (Wang et al. 2013), respectively. In addition, the guar gum-degrading activity of ManP was approx. 1.7-fold higher than that of an endo-β-1,4-mannanase from T. virens UKM1 (Chai et al. 2016). Mannooligosaccharides of mannobiose (M2) to mannopentaose (M5) and β-1,4-d-mannan polysaccharides could be efficiently hydrolyzed by ManP, as determined by HPLC (Table 1). The biocatalytic depolymerization of LBG yielded a mixture of hydrolysis products of mannose (M1) to mannohexaose (M6), which mainly contained 54.1 % M5 and 31.8 % M6. In particular, the enzymatic degradation of M2 gave rise to the production of only 16.6 % M1 and the reaction proceeded very slowly. The formation of M1 as a hydrolysis product was also observed at below 4.5 %, when β-1,4-d-mannosidic materials were subjected to ManP-mediated biocatalytic degradation.
These results clearly showed that the substrate specificity of ManP capable of hydrolyzing M2 was significantly different from that of β-1,4-mannanases from Cellulosimicrobium sp. HY-13 (Kim et al. 2011a, b) Thielavia arenaria XZ7 (Lu et al. 2013), and B. subtilis WY34 (Jiang et al. 2006), which could not hydrolyze the same substrate to M1. In contrast to ManP, B. subtilis WY34 β-1,4-mannanase is inactive with mannotriose (M3) and mannotetraose (M4) as well as M2 (Jiang et al. 2006). Taken together, this evidence suggests that ManP is a new endo-β-1,4-mannanase that has unique hydrolysis patterns for β-1,4-d-mannosidic substrates, which are distinct from those of other microbial mannolytic enzymes. This endo-β-1,4-mannanase is expected to be useful as an animal feed additive because the viscosity caused by β-1,4-d-mannans during the ruminal decomposition of plant foods would be more efficiently reduced by ManP than other conventional endo-type β-1,4-mannanases. In particular, d-mannose and its mannooligosaccharides, which would be produced by ManP-mediated biocatalytic degradation of β-1,4-d-mannans, also inhibit Salmonella typhimurium and E. coli infections in the intestinal tracts of animals (Oyopo et al. 1989; Spring et al. 2000).
Binding ability of ManP to hydrophobic polymers
The binding ability of ManP in this study was assessed using diverse hydrophobic polymers with different microstructures: pentose- and hexose-based polysaccharides, PHB granules, and lignin. Table 2 shows that the enzyme strongly bound to Avicel and ivory nut mannan, but not to chitin, chitosan, curdlan, insoluble oat spelts xylan, or PHB granules. The binding capacities of ManP were comparable to those of other characterized functional homologues toward the same hydrophobic polymers. A β-1,4-mannanase from M. edulis (Xu et al. 2002) and a β-1,4-mannanase (Man26A) from Cellulomonas fimi (Stoll et al. 2000) did not bind to either cellulose or insoluble mannan. Although a β-1,4-mannanase (ManK) from Cellulosimicrobium sp. HY-13 bound strongly to Avicel, lignin, and PHB granules (Kim et al. 2011a). A β-1,4-mannanase (ManH) from Cellulosimicrobium sp. HY-13 showed strong binding affinities to chitosan and chitin as well as Avicel and ivory nut mannan (Kim et al. 2011b). The distinct binding affinities of ManP and the aforementioned β-1,4-mannanases to various hydrophobic polymers suggest that the molecular structure of the present enzyme is different from that of other β-1,4-mannanases.
Conclusion
The β-1,4-d-mannan-degrading enzyme (ManP) produced by Paenibacillus sp. strain HY-8 is the first endo-β-1,4-mannanase that has been isolated and biocatalytically characterized from mannolytic exo-symbionts resident in the gut of the longicorn beetle M. diphysis. The enzyme is distinguishable from other microbial β-1,4-mannanases by its N-terminal amino acid sequence, substrate specificities toward various mannose based-materials, and binding affinity to hydrophobic polymers. The results of this study demonstrate the ecological importance and contribution to the decomposition of plant biomass of the symbiotic gut bacteria of the longicorn beetle M. diphysis.
References
Brune A (2014) Symbiotic digestion of lignocellulose in termite guts. Nat Rev Microbiol 12:168–180
Chai SY, Bakar FDA, Mahadi NM, Murad AMA (2016) A thermostable endo-1,4-β-mannanase from trichoderma virens UKM1: cloning, recombinant expression and characterization. J Mol Catal B: Enzym 125:49–57
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Ferreira HM, Filho EXF (2004) Purification and characterization of a β-mannanase from Trichoderma harzianum strain T4. Carbohyd Polym 57:23–29
Heo S, Kwak J, Oh H-W et al (2006) Characterization of an extracellular xylanase in Paenibacillus sp. HY-8 isolated from an herbivorous longicorn beetle. J Microbiol Biotechnol 16:1753–1759
Jiang Z, Wei Y, Li D et al (2006) High-level production, purification and characterization of a thermostable & #x03B2;-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr Polym 66:88–96
Kim DY, Ham S-J, Lee HJ et al (2011a) A highly active endo-β-1,4-mannanase produced by Cellulosimicrobium sp. strain HY-13, a hemicellulolytic bacterium in the gut of Eisenia fetida. Enzyme Microb Technol 48:365–370
Kim DY, Ham S-J, Lee HJ et al (2011b) Cloning and characterization of a modular GH5 & #x03B2;-1,4-mannanase with high specific activity from the fibrolytic bacterium Cellulosimicrobium sp. strain HY-13. Bioresour Technol 102:9185–9192
Kim DY, Lee MJ, Cho H-Y et al (2016) Genetic and functional characterization of an extracellular modular GH6 endo-β-1,4-glucanase from an earthworm symbiont, Cellulosimicrobium funkei HY-13. Antonie Van Leeuwenhoek 109:1–12
Lu H, Zhang H, Shi P et al (2013) A family 5 & #x03B2;-mannanase from the thermophilic fungus Thielavia arenaria XZ7 with typical thermophilic enzyme features. Appl Microbiol Biotechnol 97:8121–8128
Ma Y, Xue Y, Dou Y et al (2004) Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 8:447–454
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Oyopo BA, Droleskey RE, Norman JO et al (1989) Inhibition by mannose of in vitro colonization of chicken small intestine by Salmonella typhimurium. Poult Sci 68:1351–1356
Palackal N, Lyon CS, Zaidi S et al (2007) A multifunctional hybrid glycosyl hydrolase discovered in an uncultured microbial consortium from ruminant gut. Appl Microbiol Biotechnol 74:113–124
Park D-S, Oh H-W, Jeong W-J et al (2007) A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J Microbiol 45:394–401
Spring P, Wenk C, Dawson KA, Newman KE (2000) The effects of dietary mannooligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of salmonella-challenged broiler chicks. Poult Sci 79:205–211
Stoll D, Boraston A, Stålbrand H et al (2000) Mannanase Man26A from Cellulomonas fimi has a mannan-binding module. FEMS Microbiol Lett 183:265–269
van Zyl WH, Rose HS, Trollope K, Görgens JF (2010) Fungal β-mannanases: mannan hydrolysis, heterologous production and biotechnological applications. Proc Biochem 45:1203–1213
Wang M, You S, Zhang S et al (2013) Purification, characterization, and production of & #x03B2;-mannanase from Bacillus subtilis TJ-102 and its application in gluco-mannooligosaccharides preparation. Eur Food Res Technol 237:399–408
Xu B, Hägglund P, Stålbrand H, Janson J-C (2002) Endo-β-1,4-mannanases from blue mussel, Mytilus edulis: purification, characterization, and mode of action. J Biotechnol 92:267–277
Zang H, Xie S, Wu H et al (2015) A novel thermostable GH5_7 & #x03B2;-mannanase from Bacillus pumilus GBSW19 and its application in manno-oligosaccharides (MOS) production. Enzym Microb Technol 78:1–9
Zolotnitsky G, Cogan U, Adir N et al (2004) Mapping glycoside hydrolase substrate subsites by isothermal titration calorimetry. Proc Natl Acad Sci USA 101:11275–11280
Acknowledgments
This work was supported by the Grants from the KRIBB Research Initiative Program (KGM2131622) and the Bio & Medical Technology Development Program (PRM0151611) of the Ministry of Science, ICT and Future Planning of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, D.Y., Chung, C.W., Cho, HY. et al. Biocatalytic characterization of an endo-β-1,4-mannanase produced by Paenibacillus sp. strain HY-8. Biotechnol Lett 39, 149–155 (2017). https://doi.org/10.1007/s10529-016-2228-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2228-7