Abstract
Objective
To develop a new expression system regulated by a ferric uptake regulator in which silicic acid is used as an inducer.
Results
Fur box (binding site for Fur) was substituted for the lac operator to regulate the expression of GFP with the lac promoter. Since the addition of supersaturated silicic acid invokes iron deficiency, supersaturated silicic acids were used as an inducer. GFP expression was dependent on silica concentration, and the expression level without silica was negligible. Basal expression level of this lac-Fur system was extremely low and, hence, lytic enzyme gene E from bacteriophage ϕX174 could be retained in this system. Furthermore, the expression of genes of interest was spontaneously initiated as the cell density increased and the costs of the inducer are considerably less than IPTG.
Conclusion
The combination of lac promoter and Ferric uptake repressor allowed the protein expression by supersaturated silicic acid as an inducer in an easy and cost-effective way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The heterologous protein expression system using E. coli is popular due to its ease of manipulation. Many promoter systems have been developed so far; however, most of them are constructed from a lac-derived regulatory element (Rosano and Ceccarelli 2014; Terpe 2006). This means that the basal level of expression is halted by a LacI (lac repressor), which binds to a lacO (lac operator) region, and induction can be achieved by adding the lactose analog, isopropyl-β-d-1-thiogalactopyranoside (IPTG). LacI is a relatively strong repressor; however, leaky expression occurs sometimes. This incomplete repression may lead to a problem when cloning genes and maybe toxic to the bacterial host. Thus, it is important to develop a tightly regulated expression system that permits cell growth until the moment of toxic gene induction (Anthony et al. 2004). Furthermore, the cost of production of recombinant protein in Lac-dependent system is excessive for industrial applications therefore, it is important to develop an efficient and economic expression system (Wu et al. 2010). The use of IPTG, especially, in commercial processes is hampered by its cost and toxicity. Thus, alternative promoters have been studied including by pH (Tolentino et al. 1992), anaerobiosis (Oxer et al. 1991), osmolarity (Herbst et al. 1994), and stationary growth (Shimada et al. 2004). To achieve a cost-effective expression system, alternative inducers that are less expensive and safe for the host can also be considered.
We found a silica-induced protein (Sip) in a thermophilic bacterium, Thermus thermophilus (Doi et al. 2009). Sip is the inducible protein when Thermus cells are cultivated with supersaturated silicic acid, and its amino acid sequence shows significant homology with periplasmic ferric-iron-binding ABC transporter. The mechanism by which Sip is induced by the addition of supersaturated silicic acids has been demonstrated (Fujino et al. 2016). Briefly, Sip, which chelates with Fe(III), is under control of Fur (Ferric uptake regulator) (Ratledge and Dover 2000). Due to the negatively-charged colloidal silica in a supersaturated solution, Fe(III) is absorbed on the colloidal silica and concentration of available iron is thereby decreased. To make up for iron deficiency, Fur dissociates from the promoter region of sip and then sip transcription is initiated.
In various bacteria, the transcription of genes involved in the iron acquisition system is negatively-controlled by Fur (Hantke 2001). Because the interactions of iron can lead to the generation of reactive oxygen species, such as peroxides and hydroxyl radicals, iron uptake needs to be tightly controlled. Indeed, the expression of Sip (homolog of ferric-iron-binding ABC transporter) when cultured in an iron-sufficient medium was negligible. Therefore, the protein expression system using supersaturated silicic acids via iron deficiency can be a tightly-regulated expression system. Furthermore, as the regulation system by Fur is common to various bacteria, it can be applied to the expression system in E. coli. Here we report a new protein expression system for E. coli in which supersaturated silicic acid is used as an inducer. This method is cost-effective and provides strictly controlled ways for recombinant protein expression.
Materials and methods
Bacterial strains, plasmids, and growth media
All experiments were performed using Escherichia coli JM109 (recA1, endA1, gyrA96, thi, hsdR17(r −K m +K ), e14− (mcrA −), supE44, relA1, Δ (lac-proAB)/F′[traD36, proAB +, lacI q, lacZΔM15]). E. coli were grown at 37 °C using lysogeny broth (LB) medium (10 g tryptone l−1, 5 g yeast extract l−1, and 10 g NaCl l−1) or LB medium containing sodium silicate at an appropriate concentration. The growth media were supplemented with 50 μg ampicillin ml−1 if needed.
Quantitative reverse transcriptase PCR
Cell cultures at the stationary phase were collected by centrifugation and re-suspended in freshly prepared LB medium or the media containing 600 mg silicic acids l−1. After further 1 h cultivation, the total RNA was extracted using the RNeasy mini kit (Qiagen). Representative genes under control of Fur, which is named as fecI (ferric citrate transport gene I), sodB (superoxide dismutase B), acnA (aconitase A), and fhuA (ferric hydroxamate uptake gene A), were chosen according to Hantke (2001). The relative expression levels of these genes compared to gyrB (DNA gyrase subunit B) were determined with quantitative reverse-transcriptase PCR using LightCycler 2.0 (Roche) with the One Step SYBR Primescript PLUS RT-PCR kit II (TaKaRa Bio).
DNA manipulation
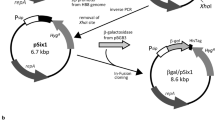
The plasmid pAcGFP1 (Clontech) has the pUC backbone, which provides a high-copy-number origin of replication, the lac promoter, green fluorescent protein gene from Aequorea coerulescens (AcGFP), and an ampicillin resistance gene. The procedures for plasmid construction are illustrated in Fig. 1. The SacI site was introduced by site-directed mutagenesis 10 bp upstream of the CAP binding site, and the resultant plasmid was named pAcGFP1-s. The putative promoter region of fhuA was amplified by PCR from E. coli genomic DNA and the fragment was inserted into pAcGFP1-s at SacI and NcoI sites (pPF/AcGFP1). To produce pPLFB/AcGFP1, the lac operator sequence was eliminated using the inverse PCR. The SacI site was introduced (pAcGFP1ΔlacO), and then, the Fur box consensus sequence (Baichoo and Helmann 2002; Escolar et al. 1998) was inserted at the site with oligo-nucleotide Fur cassette 1 and 2. Primers used and detailed sequences of the vectors constructed here are described in the Supporting information.
Scheme of the vector’s construction. pAcGFP1-s has a SacI site upstream of the CAP binding site, introduced by site-directed mutagenesis of the original plasmid, pAcGFP1. The PCR-amplified fhuA promoter region from E. coli and pAcGFP1-s were digested with SacI and NcoI and then ligated. The resultant plasmid, pPF/AcGFP1, was used for GFP reporter assay. The Lac operator sequence was removed from pAcGFP1 by the inverse PCR to generate the SacI site. Annealed oligonucleotides containing Fur box sequence were inserted at the site. pPLFB/AcGFP1 was subjected to GFP reporter assay. The GFP gene of pPLFB/AcGFP1 was substituted for gene E from ϕX174 at PstI and EcoRI site in the multi-cloning site (MCS) of the vector. Primers, oligonucleotides, and the PCR condition used are listed in Supplementary Table 1
GFP reporter assay
An overnight cell culture was transplanted to the fresh LB medium or LB medium containing silica at 1 % (w/v) and was cultured at 37 °C with shaking at 160 rpm. At intervals, aliquots of cultures were taken, centrifuged at 10,000×g for 3 min, washed and re-suspended in PBS (pH 7.2) to the same OD660 value (OD660 = 0.6). For each sample, 200 μl of cell suspension was added into a 96-well flat-bottom black plate. The relative fluorescence level was measured with a fluorescence plate reader. Excitation was 475 nm and emission was detected at 505 nm.
Toxic gene cloning and expression
The toxic gene for E. coli, named gene E from bacteriophage ϕX174 (Maratea et al. 1985), was amplified by PCR and cloned into pPLFB/AcGFP1 at PstI and EcoRI to replace AcGFP1 with gene E. The resultant plasmid (pPLFB/geneE) was transformed into E. coli JM109 and cultured in the LB medium or silica-containing LB medium. OD660 values were monitored over time to determine the cell growth.
Results and discussion
As described by Fujino et al. (2016), the addition of supersaturated silicic acids provokes the expression of Fur-regulated genes in thermophilic bacterium T. thermophilus. Since the regulation by Fur is widely found in bacteria, this phenomenon occurs even in E. coli. First, to screen the genes that are up-regulated by the addition of supersaturated silicic acid, the transcriptional level of Fur-regulated genes when cultured in supersaturated silicic acid-containing medium was determined by qRT-PCR. The transcriptional level of fecI, sodB, and acnA in silica-containing medium increased by 2–3 times that in normal media, whereas transcription of fhuA was enhanced by about 8 times. Threshold cycle (Ct) value in qRT-PCR were these; 24.4 ± 0.2 (fhuA, without silicate), 21.6 ± 0.7 (fhuA, with silicate), 20.1 ± 0.3 (gyrB, without silicate), and 20.1 ± 0.2 (gyrB, with silicate).
This result demonstrated that Fur-regulated genes were also transcribed by the addition of supersaturated silicic acid, and the promoter of fhuA seemed to be most effective in constructing a silica-inducible expression system. To evaluate the expression performance of the fhuA promoter, it was cloned into pAcGFP1-s at SacI and NcoI sites (pPF/AcGFP1) and the synthesis of GFP was measured. The transformant was cultivated in silica-containing LB medium at 0, 200, 400, and 600 mg l−1 for 24 h. Although the relative fluorescence level slightly increased by the addition of silica, each fluorescence value was almost the same as that of JM109 itself (data not shown), indicating that GFP was almost unexpressed. Although relative transcriptional level was enhanced by silica, the absolute strength of the pfhuA promoter might be weak. This weakness is also supported by qRT-PCR analysis. Although relative transcriptional level increased 8 times by the addition of silicic acids, Ct values of fhuA were higher than gyrB even in induced condition, indicating that fhuA transcripts were lower than gyrB.
To overcome the weakness of the fhuA promoter, the lac promoter was used for the protein expression. Since the nature of the induction by supersaturated silicic acids is the dissociation of Fur from the promoter region, a Fur binding site (Fur box) was introduced downstream of the lac promoter instead of the lac operator (pPLFB/AcGFP1). Figure 2 shows GFP expression under different concentrations of silicic acids. Even after 24 h little expression was observed below 200 mg l−1, while significant induction was observed at over 400 mg silicic acid l−1. At 800 mg l−1, the relative fluorescence (RF) value was almost the same as that at 600 mg l−1, indicating that 600 mg l−1 is an optimum value for silica induction. The RF value at 600 mg l−1 was 35 times compared with that with no inducer, which demonstrated that the leaky expression was quite low. When the original plasmid with the lac promoter and the lac operator (pAcGFP1) was transformed into JM109 and cultivated in the LB medium without IPTG, the RF value reached 22,295 ± 1400. This result also suggested that Fur provides tight regulation. Generally, high-copy-number plasmids such as those containing origins from pUC, ColE1, or pBR322 lead the leaky expression of the cloned gene. Therefore, low-copy-number plasmids such as pSC101 and pACYC are used to avoid undesired leaky expression (Dersch et al. 1994; Nakano et al. 1995). Although pPLFB/AcGFP1 is derived from pUC and the high-copy-number vector, the tightness of Fur regulation enabled less leaky expression in this system.
GFP expression induced by silicic acids. a GFP expression levels of JM109 with pPLFB/AcGFP1 under the different concentrations of silicic acids. 1 % inocula were cultivated for 24 h in the medium containing silicic acid listed below. Harvested cells were washed with PBS and re-suspended in PBS (OD660 = 0.6) to measure the relative fluorescence value. The values are expressed as mean ± standard deviation (SD) for three independent experiments. b Photograph of the non-induced (0 mg l−1) and induced (600 mg l−1) cells under the blue LED light
To evaluate the time-dependent response of pPLFB/AcGFP1, GFP yields along with growth in medium containing silica at 600 mg l−1 was measured (Fig. 3). After the OD600 value reached 0.6–1 and the RF value had continuously increased, GFP expression appeared to have been initiated. This may result from the availability of iron in the medium. In the initial medium containing silicic acid, iron is absorbed on colloidal silica and the amount of available iron might be low, although the amount of free iron might be sufficient for the cell growth. However, when cells reached the mid-growth phase, iron deficiency and the competition for iron occurred. Therefore, spontaneous induction was found at the mid-growth phase. Such induction is desirable in toxic gene expression to avoid the damage from gene products to the cells in the early growth stage. This phenomenon is like the ‘Overnight Express Autoinduction system (Novagen),’ which allows the induction of protein expression without monitoring cell density and without conventional induction with IPTG because medium components are metabolized differentially to promote growth to high density and automatically induce protein expression from lac promoters. In our system, competition for iron between silicates and cells might gradually occur and GFP expression is apparently recognized at the mid-growth phase. Of course, we tried the method of adding silicic acid after the cell density became high; however, the amount of GFP was less than that of the cultivation with silicic acids from the beginning. This may be due to LB medium containing sufficient iron and the cells grown in LB medium can thus take up sufficient iron and store it as bacterioferritin. If subsequent conditions become iron deficient, then E. coli can utilize the pre-formed bacterioferritin to maintain its growth (Abdul-Tehrani et al. 1999). Therefore, if silicic acid is added to the mid-growth phase culture, the cells that have already grown would be unaffected by the subsequent iron deficiency; bacterioferritin would then be passed on to daughter cells and it may take several generations for iron deficiency to be manifested. Thus, silicic acid should be added from the beginning of cultivation.
Based on the tight regulation observed with GFP reporter assays, this system could be applied for the cloning and expression of highly toxic gene products. To verify this potential, gene E form bacteriophage ϕX174 (Maratea et al. 1985) was cloned into pPLFB/AcGFP1 in place of GFP gene. Gene E induces cell lysis by the formation of a transmembrane tunnel structure in the cell envelope of E. coli (Witte et al. 1992). Its trace expression could cause the cell lysis and decrease the OD value. As shown in Fig. 4, the cells carrying pPLFB/geneE exhibited almost the same cell growth as the control cells, indicating that toxic genes could be stably retained and the expression was strictly regulated. On the other hand, when the cells carrying pPLFB/geneE were cultivated in the medium containing supersaturated silicic acids, the OD value decreased after 3 h cultivation. This result was consistent with those of GFP reporter assays, showing that the protein expression was initiated spontaneously after the cell density reached the mid-growth phase. This delay of expression might be an advantage for the expression of toxic genes.
Growth of E. coli harboring gene E from ϕX174. Closed dots show the OD660 value with pUC19 (without toxic gene) as a control. Closed and open squares indicate the OD660 values of pPLFB/geneE with zero silicic acids and 600 mg silicic acids l−1, respectively. The values are expressed as mean ± SD (n = 3)
Guan et al. (2013) reported the tightly regulated and Fur-dependent expression system with the combination of fhuA promoter from Vibrio cholerae and the consensus Fur binding sequence in E. coli. GFP yields, however, were lower and at the same level as the pBAD system (Guzman et al. 1995). The combination of the lac promoter and the Fur binding site described here could achieve both strict regulation and sufficient expression. As the depression of Fur responds to iron deficiency, 2,2′-dipyridyl (DP), which acts as an iron cheletor, can be applied. We also tried the GFP expression by adding 200 μM DP at the mid-growth phase of cell culture; however, the final yield after 24 h of cultivation was ~65 % compared to the induction of silicic acids. DP could work as a specific cheletor for iron but, basically, it can mask the divalent metal ions and toxicity for bacteria (Neilands 1982). The decrease in the expression level might be a result of the toxicity of DP and the addition of supersaturated silicic acid may be a mild way to invoke iron deficiency.
There are further advantages of using supersaturated silicic acid. The cost for a one liter culture at 600 mg silicic acid l−1 (Na2SiO3·9H2O as a source) is around $0.5, whereas that of IPTG is about $5 (at 0.3 mM. Furthermore, an affinity tag, which binds to the solid silica surface, called Si-tag, has been developed (Ikeda et al. 2010). Since supersaturated silicic acid contains colloidal silica, which are small particles retained in the solution when the fusion protein with Si-Tag is expressed, it will bind to the colloidal silica. This means that the induction and purification can be performed in one step.
In conclusion, the expression system described here exhibits notable advantages such as easy to handle, tight regulation, sufficient expression, cost-effective, and the potential of one-step expression and purification. This system will be a useful genetic tool for protein expression.
References
Abdul-Tehrani H, Hudson AJ, Chang YS, Timms AR, Hawkins C, Williams JM, Harrison PM, Guest JR, Andrews SC (1999) Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J Bacteriol 181:1415–1428
Anthony LC, Suzuki H, Filutowicz M (2004) Tightly regulated vectors for the cloning and expression of toxic genes. J Microbiol Methods 58:243–250
Baichoo N, Helmann JD (2002) Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J Bacteriol 184:5826–5832
Dersch P, Fsihi H, Bremer E (1994) Low-copy-number T7 vectors for selective gene expression and efficient protein overproduction in Escherichia coli. FEMS Microbiol Lett 123:19–26
Doi K, Fujino Y, Inagaki F, Kawatsu R, Tahara M, Ohshima T et al (2009) Stimulation of expression of a silica-induced protein (Sip) in Thermus thermophilus by supersaturated silicic acid. Appl Environ Microbiol 75:2406–2413
Escolar L, Perez-Martin J, de Lorenzo V (1998) Binding of the fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J Mol Biol 283:537–547
Fujino Y, Nagayoshi Y, Iwase M, Yokoyama T, Ohshima T, Doi K (2016) Silica-induced protein (Sip) in thermophilic bacterium Thermus thermophilus respond to low iron availability. Appl Environ Microbiol
Guan L, Liu Q, Li C, Zhang Y (2013) Development of a Fur-dependent and tightly regulated expression system in Escherichia coli for toxic protein synthesis. BMC Biotechnol 13:25
Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promoter. J Bacteriol 177:4121–4130
Hantke K (2001) Iron and metal regulation in bacteria. Curr Opin Microbiol 4:172–177
Herbst B, Kneip S, Bremer E (1994) pOSEX: vectors for osmotically controlled and finely tuned gene expression in Escherichia coli. Gene 151:137–142
Ikeda T, Ninomiya K, Hirota R, Kuroda A (2010) Single-step affinity purification of recombinant proteins using the silica-binding Si-tag as a fusion partner. Protein Expr Purif 71:91–95
Maratea D, Young K, Young R (1985) Deletion and fusion analysis of the phage phi X174 lysis gene E. Gene 40:39–46
Nakano Y, Yoshida Y, Yamashita Y, Koga T (1995) Construction of a series of pACYC-derived plasmid vectors. Gene 162:157–158
Neilands JB (1982) Microbial envelope proteins related to iron. Annu Rev Microbiol 36:285–309
Oxer MD, Bentley CM, Doyle JG, Peakman TC, Charles IG, Makoff AJ (1991) High level heterologous expression in E. coli using the anaerobically-activated nirB promoter. Nucleic Acid Res 19:2889–2892
Ratledge C, Dover LG (2000) Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54:881–941
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in microbial systems. Front Microbiol 5:341
Shimada T, Makinoshima H, Ogawa Y, Miki T, Maeda M, Ishihama A (2004) Classification and strength measurement of stationary-phase promoters by use of a newly developed promoter cloning vector. J Bacteriol 186:7112–7122
Terpe K (2006) Overview of bacterial expression systems for heterologous protein production: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 72:211–222
Tolentino GJ, Meng SY, Bennett GN, San KY (1992) A pH-regulated promoter for the expression of recombinant proteins in Escherichia coli. Biotechnol Lett 14:157–162
Witte A, Wanner G, Sulzner M, Lubitz W (1992) Dynamics of PhiX174 protein E-mediated lysis of Escherichia coli. Arch Microbiol 157:381–388
Wu H, Pei J, Jiang Y, Song X, Shao W (2010) pHsh vectors, a novel expression system of Escherichia coli for the large-scale production of recombinant enzymes. Biotechnol Lett 32:795–801
Acknowledgments
This research was supported in part by grants from the Asahi glass Foundation and JSPS KAKENHI Grant Numbers 23780085 and 26292182. The authors would like to thank Enago (www.enago.jp) for the English language review.
Supporting information
Supplementary Table 1—Primers used.
Supplementary Figure 1—Detailed sequences adjacent to promoter region that were constructed.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fujino, Y., Tanoue, R., Yokoyama, T. et al. A tightly regulated expression system for E. coli using supersaturated silicic acid. Biotechnol Lett 38, 1381–1387 (2016). https://doi.org/10.1007/s10529-016-2118-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2118-z