Abstract
Objectives
To investigate the role of microRNA-126-5p (miR-126-5p) in acute lung injury induced by bronchial instillation of lipopolysaccharide (LPS), and to explore the potential target(s) of miR-126-5p in acute lung injury.
Results
In the mice with LPS-induced acute lung injury, the level of miR-126-5p in the pulmonary tissues was decreased by 41 % whilst pulmonary vascular endothelial growth factor-A (VEGFA) doubled in its mRNA content and increased threefold in its protein level. Similar results were observed in the alveolar type II (ATII) cells treated with LPS. By using luciferase reporter assay, we found that miR-126-5p inhibited VEGFA expression by targeting its 3′-untranslated region. In addition, overexpression of miR-126-5p attenuated LPS-induced reduction of epithelial sodium channel and aquaporin 1 in ATII cells
Conclusions
MiR-126-5p was down-regulated in LPS-induced acute lung injury in mice. Thus overexpression of miR-126-5p may alleviate acute lung injury by down-regulating VEGFA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute lung injury, a syndrome of acute hypoxemic respiratory failure, develops into acute respiratory distress syndrome (ARDS). Approximately 200,000 people annually suffer from acute lung injury/ARDS, with a mortality as high as 40 % (Matthay and Zemans 2011). Several novel therapeutic targets of acute lung injury/ARDS have emerged, including extracellular histones (Ward and Grailer 2014), matrix metalloproteinases (Aschner et al. 2014) and microRNA (miRNA) (Otsuki et al. 2015).
MiRNA is a class of non-coding, single-stranded RNA that precisely regulates gene expression by degrading the target mRNA or inhibiting its translation. It is involved in various physiological and pathological processes, especially in the pathological conditions of multiple diseases including lung injury. Blocking miR-21 ameliorates pulmonary edema caused by high volume tidal ventilation (Vaporidi et al. 2012). Overexpression of miR-454 alleviates lipopolysaccharide (LPS)-induced pulmonary inflammation and injury by modulating CXCL12 (Tao et al. 2016). In addition, miR-17 is involved in LPS-induced acute lung injury by regulating Forkhead box A1 (Xu et al. 2014).
MiR-126-5p was first identified in vascular endothelial cells with the primary function of modulating angiogenesis (Wang et al. 2008). It promotes proliferation of endothelial cells and inhibits atherosclerosis by suppressing delta-like 1 (Schober et al. 2014). Moreover, it restrains tumorigenesis and angiogenesis in gastric cancer by regulating vascular endothelial growth factor-A (VEGFA) (Chen et al. 2014). It also inhibits proliferation and oncogenicity of lung cancer cells via targeting VEGFA (Liu et al. 2009).
VEGFA is an isoform of the VEGF family. It promotes physiological and pathological angiogenesis and the growth of vascular endothelial cells (Yu et al. 2012). Up-regulation of VEGFA has been detected in multiple tumors wherein angiogenesis is essential for tumor growth (Patel et al. 2015; Zhang et al. 2015). As miR-126-5p was down-regulated in a rat model of ARDS induced by saline lavage and mechanical ventilation (Huang et al. 2014), this suggested a possible involvement of miR-126-5p and VEGFA in lung injury. Both miR-126-5p and VEGFA play a critical role in angiogenesis, yet their involvement and crosstalk in acute lung injury are unknown. Hence, in this study, we have explored the roles of and the crosstalk between miR-126-5p and VEGFA in acute lung injury.
Materials and methods
Ethical statement
Animal care and experimental procedures were conducted according to the methods approved by the Animal Care and Use Committee of China Medical University. All treatment procedures were in line with standards of the ethics committee.
Animal models
Healthy male C57BL/6 mice of 8 weeks old (Vital River) were randomly divided into four groups: sham, post-LPS 6, 12 and 24 h. After being anesthetized with 10 % (w/v) chloral hydrate (3.5 ml/kg), the mice were fixed on the operating platform. The neck skin was cut open, and the trachea was separated and exposed by blunt dissection. For induction of acute lung injury, LPS (Sigma) (from Escherichia coli 055:B5, and purified by phenol extraction) (7.6 μl/ml, 3 ml/kg) was rapidly injected into the trachea with a syringe. In the sham group, an equal volume of sterile saline was injected into the tracheal as the control. The mice were placed vertically and then inversed to allow even distribution of the LPS solution in the lungs. Thereafter, the wounds were sutured, and the mice were returned to the cage and allowed to wake up spontaneously.
Evans Blue (20 mg/kg) was injected into the tail vein of some mice 6, 12 or 24 h after LPS induction. 30 min later, the mice were anesthetized with 10 % (w/v) chloral hydrate (3.5 ml/kg), and arterial blood was collected by cardiac puncture. The blood samples were centrifuged at ~1000×g for 20 min, and the supernatant was collected and diluted 100 times. The intact left lung of Evans Blue-injected mouse was collected and lavaged with a same volume of sterile saline five times. The absorbance of the alveolar lavage fluid and the diluted serum was determined at 632 nm, and the relative alveolar permeability = A632 of alveolar lavage fluid/A632 of serum. For the Evans Blue-free mice, the left lungs was weighed and dried at 60 °C, and the relative pulmonary water content = (wet weight − dry weight)/wet weight.
Hematoxylin–Eosin (HE) staining
The lung tissues were fixed in 4 % (w/v) paraformaldehyde overnight, rinsed with water for 4 h, and dehydrated with graded concentrations of ethanol [70 % (v/v) for 2 h, 80 % (v/v) overnight, 90 % (v/v) for 2 h and 100 % (v/v) for 1 h twice], and hyalinized with xylene for 30 min. The tissues were paraffin-embedded, sliced into sections of 5 μm and dried on glass slides at 60 °C. Subsequently, the sections were dewaxed with xylene for 15 min twice and rehydrated with 100, 95, 85 and 75 % (v/v) ethanol. The sections were then stained with hematoxylin reagent for 5 min, soaked in 1 % (v/v) HCl/ethanol for 3 s, and stained with eosin for 3 min. Thereafter, the sections were dehydrated in 75, 85, 95 % (v/v) ethanol for 2 min each and 100 % (v/v) ethanol for 5 min twice, and hyalinized with xylene for 5 min twice. Finally after any residual liquid was removed, a half drop of neutral gum was added onto the section, which was then covered with a cover slip and dried at room temperature.
Real-time PCR
Total RNA was extracted from the lung tissues or cultured cells with total RNA rapid extraction kit according to the manufacturer’s protocol. Total RNA was reversely transcribed (RT) to cDNA by M-MLV reverse transcriptase (BioTeke) in the presence of oligo(dT) and random primers or the specific miRNA RT primers. All instruments in this section were treated with surface RNase Erasol, and all reagents were RNase-free.
To determine the levels of miR-126-5p and VEGFA mRNA, cDNA was used for real-time PCR analysis using 2 × Power Taq PCR MasterMix and SYBR Green, with U6 and β-actin as the respective control. PCR was as follows: 95 °C for 10 min, 40 cycles of 95 °C for 10 s, 60 °C for 10 s and 72 °C for 30 s, and finally 4 °C for 5 min. The data were calculated using the 2−ΔΔCt method. The sequences of all primers used in this study are shown in Supplementary Table 1.
Western blotting
Total proteins were extracted with tissue or cell lysis buffer, separated by SDS-PAGE and transferred onto a PVDF membrane. After blocking with 5 % (w/v) skimmed milk, the membrane was incubated with primary antibodies against VEGFA (1:400) (Boster), aquaporin 1 (AQP1) (1:500) (Boster) or epithelial sodium channel (ENaC) (1:500) (Bioss) at 4 °C overnight. After rinsing with TBST, the membrane was incubated with a HRP-labeled secondary antibody (1:5000) (Wanleibio) at 37 °C for 45 min, followed by signal exposure with ECL reagent. After removing the antibodies with stripping buffer, the PVDF membrane was incubated with relevant antibodies for detection of the internal control, β-actin.
Luciferase reporter assay
The luciferase reporter assay was performed to test whether miR-126-5p directly binds to the 3′-untranslated region (3′-UTR) of VEGFA mRNA. The luciferase reporter plasmid, pmirGLO-VEGFA 3′-UTR, was purchased from Wanleibio. Cells were seeded in a 12-well plate and cultured for 24 h. Thereafter, pmirGLO-VEGFA 3′-UTR or pmirGLO was co-transfected into cells with miR-126a-5p mimic/mutant/negative control (NC) with Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s protocol. Twenty four hour later, the cells were treated with dual-luciferase reporter assay system and detected for Firefly and Renilla signals. The targeting effect was then analyzed by calculating the Firefly/Renilla ratio.
Cell isolation and culture
The pulmonary tissues were isolated from healthy mice and washed with PBS to remove residual gas and blood. Trypsin 0.25 % (w/v) was transtracheally injected, and the tissues were digested at 37 °C for 15 min. The lobes were cut into small pieces, incubate with fetal bovine serum (FBS) and 0.25 % (w/v) DNase I for 10 min, and filtered through 100 and 60 μm filters. The filtrate was centrifuged at 114×g for 10 min at 4 °C, and the cell pellet was re-suspended with Dulbecco’s modified Eagle’s medium (DMEM). The suspension, on the top of a density gradient of 10 % (w/v) and 30 % (w/v) Percoll, was centrifuged at ~120×g for 20 min at 4 °C. The cells in layer of 10 % (w/v) Percoll and in the interface between 10 and 30 % (w/v) Percoll were collected, washed twice with DMEM, and cultured in DMEM supplemented with 10 % (v/v) FBS at 37 °C in 5 % (v/v) CO2. After adhering to the plate, the isolated alveolar type II (ATII) cells were ready for the subsequent experiments.
Statistical analysis
The data in this study were presented as mean ± standard deviation (SD) of three individual experiments in each group, and analyzed by one-way ANOVA test. P < 0.05 was considered statistically significant. (*P < 0.05, **P < 0.01, ***P < 0.001, ns no significance).
Results
The level of miR-126-5p was negatively correlated with VEGFA in LPS-induced acute lung injury
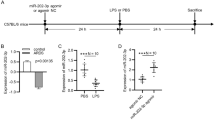
The mouse acute lung injury model was established by bronchial instillation of LPS. At post-induction 6, 12 and 24 h, the left lung was harvested and analyzed. HE staining showed that LPS induced inflammation and progressive lesions in the LPS-insulted lung tissues compared with the sham-operated control (Fig. 1a). We detected the alveolar permeability and pulmonary water content in each group. At 6, 12 and 24 h after LPS challenge, the alveolar permeability was increased 1.5-, 1.8- and 2.2-fold, respectively (Fig. 1b), and the water content was increased by 1.05-, 1.06- and 1.08-fold, respectively, as compared with the sham group (Fig. 1c). These results indicated that LPS-induced acute lung injury model was successfully established, and the LPS-induced pulmonary injury was the most prominent 24 h following bronchial instillation of LPS.
Induction of acute lung injury in mice by bronchial instillation of lipopolysaccharide (LPS). a Hematoxylin–Eosin (HE) staining of the lung tissues at various time points after LPS stimulation. Scale bars 100 μm. b Alveolar relative permeability at different time points afrer LPS insults. c Relative water content in the lung tissues at different time points after LPS insult. d The level of microRNA-126-5p (miR-126-5p) in the lung tissues at different post-LPS time points detected by real-time PCR. e, f The mRNA and protein levels of vascular endothelial growth factor-A (VEGFA) level in the lung tissues were detected by real-time PCR and western blot, respectively. (Compared to the sham group, *P < 0.05, **P < 0.01, ***P < 0.001, ns no significance)
To study the responses of miR-126-5p and VEGFA to LPS-induced acute lung injury, real-time PCR and western blotting were performed to detect the levels of miR-126-5p and VEGFA in the lung tissues at various time points after LPS insult. Twenty four hours after bronchial instillation of LPS, the level of miR-126-5p was decreased by 41 % compared to the sham-operated control, and the expression of VEGFA was increased 2.02-fold in the mRNA level and 2.88-fold in the protein level in acute lung injury (Fig. 1d–f).
The level of miR-126-5p is negatively correlated with VEGFA in LPS-treated ATII cells
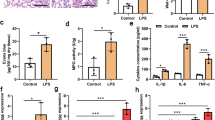
To verify the correlation between the levels of miR-126-5p and VEGFA in acute lung injury, ATII cells were isolated from mice the lungs of healthy mice and cultured in vitro. After being identified by microscopic observation (Fig. 2a), ATII cells were treated with LPS (1 μg/ml) for 2, 4, 6, 12 and 24 h. Subsequently, the expression levels of miR-126-5p and VEGFA were detected by real-time PCR and western blot analysis. Real-time PCR showed that LPS treatment of 2, 4, 6, 12 and 24 h decreased the level of miR-126-5p by 17, 30, 42, 51 and 54 %, respectively, and increased VEGFA mRNA level 1.2-, 1.5-, 1.9-, 2.3-, 2.3-fold, respectively, as compared to the control cells (Fig. 2b, c). Western blotting showed similar changes in VEGFA expression after stimulation (Fig. 2d).
The levels of miR-126-5p and VEGFA are negatively correlated in LPS-treated ATII cells. a Alveolar type II (ATII) cells were isolated from the lungs of healthy mice; scale bars 100 μm (left panel) and 20 μm (right panel). b The level of miR-126-5p in the ATII cells that were treated with LPS for different durations was detected by real-time PCR. c, d The mRNA and protein levels of VEGFA in the ATII cells treated with LPS for different durations were determined by real-time PCR and western blotting, respectively. (Compared to the untreated cells, *P < 0.05, **P < 0.01, ***P < 0.001, ns no significance)
MiR-126-5p down-regulated VEGFA expression by binding to its 3′-UTR
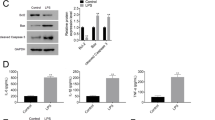
Several bioinformatic websites, such as TargetScan (http://www.targetscan.org/), PicTar (http://pictar.mdc-berlin.de/) and miRBase (http://www.mirbase.org/) were used to predict the targets of miR-126-5p. From this analysis, we found that VEGFA is a potential target of miR-126-5p because the seed sequence of miR-126-5p aligns perfectly with the 3′-UTR of VEGFA mRNA (Fig. 3a). For luciferase reporter assay, miR-126-5p mimic, miR-126-5p mutant and negative control (NC) oligos were synthesized, and a luciferase reporter plasmid containing VEGFA 3′-UTR was constructed. As shown in Fig. 3b, miR-126-5p mimic decreased luciferase activity by 53 % compared with NC, whereas miR-126-5p mutant did not affect luciferase activity. Next, we examined if overexpression of miR-126-5p affected the expression of VEGFA in ATII cells. Real-time PCR and western blot results showed that overexpression of miR-126-5p down-regulated the level of VEGFA mRNA by 39 % and VEGFA protein by 26 %, as compared to NC (Fig. 3c, d). The above results demonstrated that miR-126-5p inhibits VEGFA expression by directly targeting 3′-UTR of VEGFA mRNA.
MiR-126-5p down-regulated VEGFA expression by binding to its 3′-untranslated region (3′-UTR). a Alignment of miR-126-5p or miR-126-5p mutant with VEGFA 3′-UTR. b Luciferase reporter assay was performed to assess the effect of miR-126-5p mimic/mutant/negative control (NC) on gene expression. c, d The expression of VEGFA in miR-125-5p mimic- or NC-transfected cells and control cells (without any treatments) was detected by real-time PCR and western blotting. (**P < 0.01, ***P < 0.001, ns no significance)
MiR-126-5p enhanced alveolar fluid clearance
Since we observed a decline of pulmonary miR-126-5p and concomitant increase in alveolar permeability and pulmonary water content in LPS-induced acute lung injury, we next investigated if miR-126-5p was involved in alveolar fluid clearance. ENaC and AQP1 are critical proteins for the clearance of alveolar fluid. Western blots showed that 24 h LPS treatment decreased the level of ENaC and AQP1 by 54 and 48 % at 24 h, respectively. By contrast, overexpression of miR-126-5p markedly attenuated LPS-induced reduction of ENaC and AQP1 in ATII cells (Fig. 4a, b), suggesting that miR-126-5p may relieve the impairment of alveolar fluid clearance LPS-induced acute lung injury.
MiR-126-5p enhanced alveolar fluid clearance. a, b The expression of epithelial sodium channel (ENaC) protein and aquaporin (AQP1) in ATII cells with various treatments was detected different treatment detected by western blotting. ATII cells were treated with LPS for 24 h to model acute lung injury. (*P < 0.05, ***P < 0.001)
Discussion
In this study, we established a mouse model of acute lung injury by bronchial instillation of LPS. LPS is one of cytoderm components in Gram-negative bacteria and can evoke inflammatory response in mammals and cause persistent fever and even shock. Here, we introduced E. coli LPS into mouse lungs by bronchial instillation, in order to simulate pulmonary infection of the Gram-negative bacteria. HE staining, alveolar permeability and pulmonary water content assays showed significant inflammation, increased alveolar permeability and pulmonary edema in LPS-insulted lungs over time.
By determining the expression of miR-125-5p and VEGFA in the lung tissues and ATII cells, we found that miR-126-5p was decreased while VEGFA was increased both in the lung tissues and ATII cells after LPS treatment, suggesting a potential correlation of miR-126-5p with VEGFA in LPS-induced acute lung injury. Luciferase reporter assay demonstrated that miR-126-5p down-regulated VEGFA expression by directly binding to the 3′-UTR of VEGFA mRNA. As miR-126-5p was reduced in LPS-induced acute lung injury, we speculated that LPS-induced down-regulation of miR-126-5p may contribute to the overexpression of VEGFA and the progression of acute lung injury. In addition, our results also demonstrated that overexpression of miR-126-5p attenuated LPS-induced down-regulation of ENaC and AQP1 in ATII cells, suggesting that forced expression of miR-126-5p may ameliorate dysfunction of alveolar fluid clearance by maintaining the activity of AQP1 and ENaC.
AQP1 is a transmembrane protein that allows a single water molecule to pass through at a time, and it mainly exists in the capillary endothelium of alveolar, trachea and pleura. In addition to regulating fluid absorption and transport in the respiratory system, AQP1 also collaborates with ion channels and Na+-K+-ATPase to transport fluids in the thorax. ENaC is a Na+ channel protein that is expressed in pulmonary alveolar type I and type II (ATI, ATII) cells. ENaC actively transports Na+ from alveolar cavities into ATII cells, while Na+-K+-ATPase that is located on basement membrane transports Na+ to the pulmonary interstitium. Lack of any subunit of ENaC would lead to serious lung injuries and even death. For their importance to lung function, AQP1 and ENaC were examined in this study for the evaluation of alveolar fluid clearance. We found that the expression of AQP1 and ENaC declined in LPS-treated ATII cells, whereas the decline was attenuated when miR-126-5p was overexpressed. These results suggest that miR-126-5p plays a protective role against LPS-induced acute lung injury partially via maintaining the expression of ENaC and AQP1.
MiR-126-5p and VEGFA are known to play a critical role in angiogenesis. In this study, we demonstrated that miR-126-5p was down-regulated in LPS-induced acute lung injury in vivo and in LPS-treated mouse ATII cells in vitro. In addition, we showed that miR-126-5p inhibited the expression of VEGFA by directly targeting the 3′-UTR of VEGFA transcript. Furthermore, overexpression of miR-126-5p attenuated LPS-induced down-regulation of alveolar fluid clearance protein. These findings suggest a potential translational value of miR-126-5p to clinical acute lung injury/ARDS therapy.
References
Aschner Y, Zemans RL, Yamashita CM, Downey GP (2014) Matrix metalloproteinases and protein tyrosine kinases: potential novel targets in acute lung injury and ARDS. Chest 146:1081–1091
Chen H, Li L, Wang S, Lei Y, Ge Q, Lv N, Zhou X, Chen C (2014) Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget 5:11873–11885
Huang C, Xiao X, Chintagari NR, Breshears M, Wang Y, Liu L (2014) MicroRNA and mRNA expression profiling in rat acute respiratory distress syndrome. BMC Medical Genom 7:46
Liu B, Peng XC, Zheng XL, Wang J, Qin YW (2009) MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer 66:169–175
Matthay MA, Zemans RL (2011) The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6:147–163
Otsuki T, Ishikawa M, Hori Y, Goto G, Sakamoto A (2015) Volatile anesthetic sevoflurane ameliorates endotoxin-induced acute lung injury via microRNA modulation in rats. Biomed Rep 3:408–412
Patel KR, Vajaria BN, Begum R, Patel JB, Shah FD, Joshi GM, Patel PS (2015) VEGFA isoforms play a vital role in oral cancer progression. Tumour Biol 36:6321–6332
Schober A, Nazari-Jahantigh M, Wei Y, Bidzhekov K et al (2014) MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nature Med 20:368–376
Tao Z, Yuan Y, Liao Q (2016) Alleviation of lipopolysaccharides-induced acute lung injury by MiR-454. Cell Physiol Biochem 38:65–74
Vaporidi K, Vergadi E, Kaniaris E, Hatziapostolou M, Lagoudaki E, Georgopoulos D, Zapol WM, Bloch KD, Iliopoulos D (2012) Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 303:L199–L207
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN (2008) The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Develop Cell 15:261–271
Ward PA, Grailer JJ (2014) Acute lung injury and the role of histones. Transl Respir Med 2:1
Xu Z, Zhang C, Cheng L, Hu M, Tao H, Song L (2014) The microRNA miR-17 regulates lung FoxA1 expression during lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun 445:48–53
Yu M, Du F, Ise H, Zhao W, Zhang Y, Yu Y, Yao F, Yang J, Akaike T (2012) Preparation and characterization of a VEGF-Fc fusion protein matrix for enhancing HUVEC growth. Biotechnol Lett 34:1765–1771
Zhang SD, McCrudden CM, Kwok HF (2015) Prognostic significance of combining VEGFA, FLT1 and KDR mRNA expression in lung cancer. Oncol Lett 10:1893–1901
Supporting information
Supplementary Table 1—Sequences of reverse transcription and real-time PCR primers
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tang, R., Pei, L., Bai, T. et al. Down-regulation of microRNA-126-5p contributes to overexpression of VEGFA in lipopolysaccharide-induced acute lung injury. Biotechnol Lett 38, 1277–1284 (2016). https://doi.org/10.1007/s10529-016-2107-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2107-2