Abstract
Objectives
To find an efficient and cheap system for NAD+ regeneration
Results
A NADH-ferricyanide dehydrogenase was obtained from an isolate of Escherichia coli. Optimal activity of the NADH dehydrogenase was at 45 °C and pH 7.5, with a K m value for NADH of 10 μM. By combining the NADH dehydrogenase, potassium ferricyanide and laccase, a bi-enzyme system for NAD+ regeneration was established. The system is attractive in that the O2 consumed by laccase is from air and the sole byproduct of the reaction is water. During the reaction process, 10 mM NAD+ was transformed from NADH in less than 2 h under the condition of 0.5 U NADH dehydrogenase, 0.5 U laccase, 0.1 mM potassium ferricyanide at pH 5.6, 30 °C
Conclusion
The bi-enzyme system employed the NADH-ferricyanide dehydrogenase and laccase as catalysts, and potassium ferricyanide as redox mediator, is a promising alternative for NAD+ regeneration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
NAD+ plays a major role in many biological oxidation–reduction reactions and its associated oxidoreductase has potential applications in synthetic chemistry (Chenault and Whitesides 1987). However, one fundamental obstacle to the large-scale use of NAD+-dependent oxidoreductases is the high cost of the cofactor when used as a stoichiometric reagent. Therefore, for the economic reasons, a favorable thermodynamic route for regeneration of NAD+ is desirable. Significant effort has been made to overcome the limitation of nicotinamide cofactors for possible large-scale industrial applications. Well-established enzymatic methods for NADH regeneration consist of formate dehydrogenase (Bommarius et al. 1998), alcohol dehydrogenase (Grunwald et al. 1986), and glucose dehydrogenase (Kaswurm et al. 2013) etc. There are, however, different strategies for regenerating NAD+, some of which involve enzymatic, chemical, photoelectrochemical, or electrochemical methods. Among these, enzymatic methods seem the most promising approach due to convenience and high efficiency; examples include glutamate dehydrogenase with 2-ketoglutarate (Lee and Whitesides 1985), alcohol dehydrogenase with acetaldehyde (Šalić et al. 2013), and lactate dehydrogenase with pyruvate (Chenault and Whitesides 1989). NADH oxidases are also used to catalyze NADH oxidation to NAD+ with O2 reduced to H2O2 (Geueke et al. 2003; Riebel et al. 2003). Moreover, two-enzyme systems are used to catalyze NAD+ regeneration (Aksu et al. 2009). However, many enzymatic methods used for the NAD+ regeneration needed the addition of co-substrate or catalase (Itoh et al. 1992).
In this contribution, we present a new alternative to regenerate NAD+ efficiently using NADH dehydrogenase obtained from E. coli, coupled with laccase from Agaricus bisporus. In the reaction system, potassium ferricyanide served as hydrogen acceptor from NADH (Kulikova 2005), and laccase was employed for the regeneration of electron acceptor potassium ferricyanide (Wang et al. 2013). Firstly, NAD+ was regenerated by NADH dehydrogenase and potassium ferricyanide was concomitantly reduced to potassium ferrocyanide. Secondly, potassium ferrocyanide was continuously oxidized to potassium ferricyanide by laccase, at the same time O2 was converted to H2O (Fig. 1). In the whole reaction process, there was no requirement for the addition of a sacrificial substrate or catalase (Jouanneau et al. 2006); only H2O was generated as by-product. Taking these advantages into account, the approach is attractive not only from the economic point of view, but also from ecological considerations.
Materials and methods
Chemicals
All chemicals used were the highest grade and were purchased from Sinopharm Chemical Reagent Corporation (Nanjing, China). Toyopearl DEAE-650M was obtained from Tosoh Corporation (Osaka, Japan).
Organism and growth
The bacterial strain used to produce NADH dehydrogenase was isolated from soil at Nanjing University of Science and Technology (Nanjing, China). It was identified by 16S rRNA sequencing as Escherichia coli with the designation of JZW506. It was grown in lysogeny broth (100 ml) in a 500 ml Erlenmeyer flask at 37 °C, 120 rpm for 18 h. The culture was centrifuged at 5000×g for 10 min and the cells were washed with distilled water three times.
Purification of NADH dehydrogenase
All procedures were performed under 4 °C or in an ice-water bath. Washed cells (from 1 l culture) were resuspended in 10 ml distilled water containing 200 μl mercaptoethanol (0.5 %, v/v), 1 ml Triton X-100 (1 %, v/v), and then were disrupted ultrasonically over 20 min. Ammonium sulphate fractionation was carried out collecting the pellet that formed between 40 and 70 % saturation. This was dissolved in 10 mM sodium phosphate buffer (pH 8). After being dialyzed against the same buffer, the crude preparation was applied to Toyopearl DEAE-650 M column (1.5 cm × 20 cm) which had been equilibrated with 10 mM sodium phosphate buffer (pH 8). The enzyme was eluted with a linear 0–1 M NaCl gradient in 10 mM sodium phosphate buffer (pH 8, 100 ml) at a flow rate of 1.0 ml/min and the active fractions were collected.
Biochemical properties of NADH dehydrogenase
The optimal pH of NADH dehydrogenase was analyzed at 30 °C in following buffers (final concentration, 50 mM): citrate/phosphate buffer (pH 5.6-8), and Tris/HCl buffer (pH 8–9). The optimal temperature of NADH dehydrogenase was analyzed at various temperatures from 25 to 70 °C in Tris/HCl buffer (pH 7.5, 50 mM). The pH stability of the NADH dehydrogenase was analyzed by incubating the enzyme at different pH buffers (pH 6–9, 50 mM) at 45 °C for 6 h. The thermal stability of the NADH dehydrogenase was analyzed by incubating the enzyme at different temperatures (30–50 °C) for 6 h. The Michaelis constant (K m) of NADH dehydrogenase toward NADH was determined at the condition of 0.2 mM potassium ferricyanide, Tris/HCl buffer (pH 7.5, 50 mM) and 45 °C using Lineweaver–Burk method. The K m value of NADH dehydrogenase toward potassium ferricyanide was determined at the condition of 0.1 mM NADH, citrate/phosphate buffer (pH 5.6, 50 mM) and 30 °C.
Enzyme activity assays
The NADH dehydrogenase activity was assayed at 30 °C by monitoring the decrease in NADH absorbance at 340 nm (ε340 = 6.22 mM−1 cm−1) for 1 min in citrate/phosphate buffer (pH 5.6, 50 mM) containing 1 mM potassium ferricyanide, 100 μM NADH, and a suitable amount of enzyme. One unit of enzyme activity was defined as the amount of enzyme which decreased 1 μmol NADH per min.
Laccase
Laccase was extracted from fruiting bodies of Agaricus bisporus. The experiments of laccase purification were performed as described by Wang et al. (2013).
Laccase activity was determined at 30 °C by following the increase in the absorbance of potassium ferricyanide at 420 nm (ε420 = 1.04 mM−1 cm−1) (Wang et al. 2013). The assay contained 1 mM potassium ferrocyanide, a suitable amount of enzyme, and citrate/phosphate buffer (pH 5.6, 50 mM). One unit of laccase activity was defined as the amount of enzyme oxidizing 1 μmol potassium ferrocyanide per min.
The bi-enzyme system for NAD+ regeneration
To investigate the influence of pH (3–8) on the bi-enzyme system for NAD+ regeneration, the reaction was carried out by monitoring the decrease of NADH absorbance at 340 nm in 50 mM citrate/phosphate buffer containing 300 μM potassium ferricyanide, 100 μM NADH, 0.05 U laccase, and 0.05 U NADH dehydrogenase. Absorbencies were converted into concentrations of NADH using the molar extinction coefficient (ε340 = 6.22 mM−1 cm−1). A control experiment was performed with equivalent volumes of distilled water instead of enzyme. To investigate the influence of temperature (30–50 °C) on the bi-enzyme system for NAD+ regeneration, the reaction was carried out in citrate/phosphate buffer (pH 5.6, 50 mM) containing 100 μM potassium ferricyanide, 1 mM NADH, 0.05 U laccase, and 0.05 U NADH dehydrogenase (Fig. 1). After 30 min, samples were taken and analyzed by HPLC.
To evaluate the efficiency of the chosen conditions, the reaction was performed on a rotary shaking incubator (140 rpm) at 30 °C in citrate/phosphate buffer (pH 5.6, 50 mM) containing 0.1 mM potassium ferricyanide, 10 mM NADH, 0.5 U laccase, and 0.5 U NADH dehydrogenase. A control experiment was performed with 10 mM NADH in citrate/phosphate buffer (pH 5.6, 50 mM). Samples were taken periodically and analyzed by HPLC.
HPLC analysis
The concentrations of NADH and NAD+ in reaction samples were monitored by HPLC using a C18 column (150 mm × 4.6 mm) with methanol/phosphate buffer (6.6 g Na2HPO4 l−1, 6.8 g KH2PO4 l−1) (6:94, v/v) was used as eluent at 0.6 ml/min at 25 °C. Detection was at 260 nm. A calibration curve was carried out with authentic samples of NADH and NAD+ from 0.01 to 10 mM.
Results and discussion
The properties of NADH dehydrogenase
The 16S rRNA sequence of the isolated strain of E. coli has been deposited in NCBI GenBank with accession number KT716302. The bacterium was grown in medium using 4-methylumbelliferyl-β-d-glucuronide (MUG) as substrate that emitted fluorescence when exposed to light at 365 nm (data not shown).
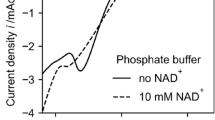
After two purification steps (ammonium sulfate fractionation followed by Toyopearl DEAE-650M column), the obtained NADH dehydrogenase was treated as a purified enzyme. Its activity was followed at 340 nm (Fig. 2). Without ferricyanide in the control experiment, no obvious absorption decrease was detected. Figure 2 also shows that low concentrations of NADH were decreased even though potassium ferricyanide was present.
The test of NADH dehydrogenase activity toward NADH. (Filled square) experiment: 1 mM potassium ferricyanide, 100 μM NADH, and a suitable amount of enzyme in citrate/phosphate buffer (pH 5.6, 50 mM) at 30 °C (filled diamond) control a: 100 μM NADH in citrate/phosphate buffer (pH 5.6, 50 mM) at 30 °C; (filled triangle) control b: 100 μM NADH, and a suitable amount of enzyme in citrate/phosphate buffer (pH 5.6, 50 mM) at 30 °C; (filled circle) control c: 1 mM potassium ferricyanide, 100 μM NADH in citrate/phosphate buffer (pH 5.6, 50 mM) at 30 °C
NADH dehydrogenase was optimally active at pH 7.5 and 45 °C (Fig. 3). More than 50 % of the activity was retained after 4 h at pH 7 and at 45 °C. It was not stable at pH 6 or 9, with only about 10 % activity maintaining after 4 h incubation at 45 °C. NADH dehydrogenase was stable under 40 °C, maintaining about 80 % of its activity following incubation for 6 h (Figs. 3, 4). NADH dehydrogenase had K m of 10 μΜ for NADH (Supplementary Fig. 1). NADH dehydrogenase had K m of 37 μΜ for potassium ferricyanide (Supplementary Fig. 2). Optimal activity of laccase was at 30 °C and pH 3 (Wang et al. 2013).
Regeneration of NAD+ by NADH dehydrogenase and laccase bi-enzyme system
There is a difference in optimal pH and temperature between NADH dehydrogenase (pH 7.5, 45 °C) and laccase (pH 3, 30 °C). More units of laccase were needed when NADH dehydrogenase was assayed at pH 7.5 due to the activity of laccase at pH 7.5 being very low, and vice versa. Therefore, we investigated the influence of pH and temperature on the catalysis of the bi-enzyme system, and optimal condition was at pH 5.6 and 30 °C (Fig. 5). We further investigated the stability of NADH dehydrogenase and laccase following incubation at 30 °C in citrate/phosphate buffer (pH 5.6, 50 mM). NADH dehydrogenase and laccase were stable under the optimal condition, maintaining about 50 % and 100 % of their activity after 8 h of incubation (Supplementary Fig. 3).
10 mM NADH was completely converted to NAD+ in less than 2 h under the chosen condition (Fig. 6). The result clarifies that NADH dehydrogenase can efficiently convert NADH to NAD+. Further investigations will focus on coupling the NAD+-regenerating system with NAD+-dependent oxidoreductases. During the process, we can calculate the turn number of NAD+ to evaluate the efficiency of the system. It should be mentioned here that potassium ferrocyanide is considered as harmful to the environment and to water organisms. Therefore, studies on replacing potassium ferricyanide by other mediators that are environment ally benign are underway.
Conclusion
We present a cheap, efficient system for NAD+ regeneration by coupling with NADH dehydrogenase from E.coli JZW506 and laccase from Agaricus bisporus. The research regarding NAD+ regeneration suggests that the system could be a potential approach for use in industrial biocatalytic application.
References
Aksu S, Arends IWCE, Hollmann F (2009) A new regeneration system for oxidized nicotinamide cofactors. Adv Synth Catal 351:1211–1216
Bommarius AS, Schwarm M, Drauz K (1998) Biocatalysis to amino acid-based chiral pharmaceuticals-examples and perspectives. J Mol Catal B Enzym 5:1–11
Chenault HK, Whitesides G (1987) Regeneration of nicotinamide cofactors for use in organic synthesis. Appl Biochem Biotechnol 14:147–197
Chenault HK, Whitesides GM (1989) Lactate dehydrogenase-catalyzed regeneration of NAD from NADH for use in enzyme-catalyzed synthesis. Bioorg Chem 17:400–409
Geueke B, Riebel B, Hummel W (2003) NADH oxidase from Lactobacillus brevis: a new catalyst for the regeneration of NAD. Enzym Microb Technol 32:205–211
Grunwald J, Wirz B, Scollar MP, Klibanov AM (1986) Asymmetric oxidoreductions catalyzed by alcohol dehydrogenase in organic solvents. J Am Chem Soc 108:6732–6734
Itoh S, Terasaka T, Matsumiya M, Komatsu M, Ohshiro Y (1992) Efficient NAD+-recycling system for ADH-catalysed oxidation in organic media. J Chem Soc Perkin Trans 1:3253–3254
Jouanneau Y, Meyer C, Jakoncic J, Stojanoff V, Gaillard J (2006) Characterization of a naphthalene dioxygenase endowed with an exceptionally broad substrate specificity toward polycyclic aromatic hydrocarbons. Biochemistry 45:12380–12391
Kaswurm V, Hecke WV, Kulbe KD, Ludwig R (2013) Guidelines for the application of NAD(P)H regenerating glucose dehydrogenase in synthetic processes. Adv Synth Catal 355:1709–1714
Kulikova VS (2005) NADH oxidase activity of gold nanoparticles in aqueous solution. Kinet Catal 46:373–375
Lee LG, Whitesides GM (1985) Enzyme-catalyzed organic synthesis: a comparison of strategies for in situ regeneration of NAD from NADH. J Am Chem Soc 107:6999–7008
Riebel BR, Gibbs PR, Wellborn WB, Bommarius AS (2003) Cofactor regeneration of both NAD+ from NADH and NADP+ from NADPH: NADH oxidase from Lactobacillus sanfranciscensis. Adv Synth Catal 345:707–712
Šalić A, Ivanković M, Ferk E, Zelić B (2013) ADH based NAD+ regeneration in a microreactor. J Chem Technol Biotechnol 88:1721–1729
Wang P, Yang J, Jiang L, Feng J, Yang C, Li D (2013) A bi-enzymatic system for efficient enantioselective bioconversion of racemic mandelic acid. J Mol Catal B Enzym 94:47–50
Weckbecker A, Groger H, Hummel W (2010) Regeneration of nicotinamide coenzymes: principles and applications for the synthesis of chiral compounds. Biosyst Eng I:195–242
Supplementary information
Supplementary Fig. 1—Lineweaver-Burk plot of the effect of NADH concentration on NADH dehydrogenase activity. Enzyme kinetic parameters were obtained by measuring the rate of A340 value decrease at various concentrations of NADH ranging from 12.5 μM to 400 μM at 45 °C in Tris/HCl buffer (pH 7.5, 50 mM).
Supplementary Fig. 2—Lineweaver-Burk plot of the effect of potassium ferricyanide concentration on NADH dehydrogenase activity. Enzyme kinetic parameters were obtained by measuring the rate of A340 value decrease at various concentrations of potassium ferricyanide ranging from 6.25 μM to 200 μM at 30 °C in citrate/phosphate buffer (pH 5.6, 50 mM).
Supplementary Fig. 3—Residual activity of NADH dehydrogenase and laccase following incubation at 30 °C in citrate/phosphate buffer (pH 5.6, 50 mM).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jizhong Wang and Chengli Yang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Yang, C., Chen, X. et al. A high effective NADH-ferricyanide dehydrogenase coupled with laccase for NAD+ regeneration. Biotechnol Lett 38, 1315–1320 (2016). https://doi.org/10.1007/s10529-016-2106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2106-3