Abstract
Light is an important signal for fungi. We analyzed the influence of blue light of various intensities and illumination times on growth, monascin (MS) and ankaflavin (AK) biosyntheses in Monascus strain M9. Blue light changed the color of colonies. The colonies grown in the dark were orange, but turned pale when exposed to continuous blue light. MS production increased by 12.5, 27, and 14.5 % under blue light of 100 lux for 15 min/day, 100 lux for 30 min/day, and 200 lux for 15 min/day, respectively, compared to growth in the dark. AK production increased by 14.4, 22, and 13 % under the same condition. MS and AK production decreased when exposed to blue light of 300 and 450 lux. The expression of pigment biosynthetic genes were analyzed by real-time quantitative PCR and correlated with phenotypic production of MS and AK.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light is a crucial environmental signal in nature for developmental and physiological processes in various organisms, including filamentous fungi. Light influences fungi in many aspects including mycelium development, conidia formation (Lee et al. 2006), and secondary metabolism (Miyake et al. 2005).
Blue light (455–470 nm) can stimulate spore formation, regulate circadian rhythms, induce carotenoid biosynthesis (Linden et al. 1997), inhibit mycotoxin production (Häggblom and Unestam 1979), and affect pigment accumulation in different fungi (Velmurugan et al. 2010; Babitha et al. 2008). Fungi can sense light because of various conserved photoreceptors. Light sensing has been studied in depth in Neurospora crassa, and both the perception and response to blue light have been characterized. These responses require the photoreceptors, WC-1 and WC-2 proteins (Ballario et al. 1996; Ballario and Macino 1997), to interact and form the white collar complex, which binds transiently to the promoters of light inducible genes upon light exposure to activate their transcription.
Monascus species are used in producing traditional oriental foods, such as red mold rice, and can produce various useful secondary metabolites, including pigments (natural coloring agents), γ-aminobutyric acid (GABA, a hypotensive agent), and monacolins (a group of anti-hypercholesterolemic agent). However, Monascus also produces the mycotoxin, citrinin (a nephrotoxic agent) (Jůzlová et al. 1996). Monascin (MS) and ankaflavin (AK) are two classical yellow pigments produced by Monascus. MS and AK possess anti-cancer (Akihisa et al. 2005), anti-inflammation (Cheng et al. 2011), anti-obesity (Jou et al. 2010), and anti-diabetes (Lee et al. 2011) properties, while also regulating cholesterol levels in the blood (Lee et al. 2010).
Light can influence the secondary metabolites of Monascus, including pigments, GABA, monacolins, and citrinin (Miyake et al. 2005; Velmurugan et al. 2010; Wang et al. 2012). However, little is known about the effect of the time and intensity of illumination on the secondary metabolites of Monascus. Many fungi react to the time and intensity of illumination in various ways. Rapid blue light can regulate photolyase gene expression and sporulation of Trichoderma harzianum (Berrocal-Tito et al. 1999). Blue light of varying intensities can also influence on the growth and ochratoxin A biosynthesis produced by five relevant fungi (Schmidt-Heydt et al. 2011). This study aims to investigate the effect of blue light intensity and illumination time on the growth as well as MS and AK production in Monascus strain M9. We have also determined the expression levels of the pigment biosynthetic gene cluster together with changes in pigment yield to elucidate the mechanism of blue light on Monascus.
Materials and methods
Strain and growth conditions
Monascus strain M9, was maintained on malt extract/agar (MEA) for 7 days at 30 °C. Spores were harvested with 3 ml sterile water and inoculated into 100 ml seed medium (60 g glucose, 20 g peptone, 10 g KH2PO4, 10 g NaNO3, and 5 g MgSO4 l−1; the initial pH of the medium was adjusted to 4.5 with lactic acid) in 250 ml flasks. The cultures were incubated at 30 °C for 30 h with shaking at 180 rpm. For pigment production, 3 ml spore suspension (adjusted spores to 106 ml−1) was inoculated into 50 ml rice medium (50 g rice powder, 1.5 g KH2PO4, 3 g NaNO3, and 1 g MgSO4·7H2O l−1) in a 12 mm culture dish. The dishes were incubated under static conditions at 30 °C for 7 days in the dark or exposed to blue light conditions.
Light exposure conditions
Five light chambers were constructed to enable incubation of cultures under different intensities of blue light. Each chamber was equipped with 9 W LEDs (460 nm) with the following conditions: chamber 1, light intensity 50 lux; chamber 2, 100 lux; chamber 3, 200 lux; chamber 4, 300 lux; and chamber 5, 450 lux. No heating effect by the LEDs could be detected. A dark chamber was used as control. Exposure times were 15, 30, 45, 60 min/day for each chamber.
Growth assessment
To observe the effects of blue light on mycelium development, 20 μl spore suspension was inoculated as a single point on MEA, exposed to blue light intensity of 450 lux or left in the dark for 10 days at 30 °C. Colony mycelium was observed directly and photographed on the 4th, 7th, and 10th days.
Extraction and HPLC analysis
Fresh mycelium was obtained from rice medium on the 7th day under blue light or dark condition, dried, and ground into powder. About 0.5 g powder was transferred into a 10 ml centrifuge tube. The preparations were extracted in triplicate with 3 ml of 75 % ethanol for 30 min on an ultrasonic bath and subsequently centrifuged at 2862×g for 10 min. The total supernate was merged and passed through RC 0.22 μm filters.
MS and AK were analyzed by HPLC. The testing system conditions were as follows. Eclipse XDB-C18 column (5 μm, 4.6 150 mm, Agilent, USA) was used at 25 °C, and isocratic elution was performed for 30 min using 0.05 % TFA in acetonitrile/water (62.5:37.5, v/v) as the mobile phase at 1 ml/min. MS and AK were detected by a DAD detector at 234 nm. MS and AK from sigma were used as standards.
Real-time quantitative PCR analysis
Total RNA was extracted from mycelia using the Plant RNA Kit (Omega, USA). First-strand cDNA was synthesized using the PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Japan), with the Oligo dT Primer 15. Gene expression was monitored by RT-qPCR and carried out using the SYBR Premix Ex Taq II (Takara, Japan). Primers for MpPKS5, mppR1, mppA, mppB, mppC, mppD, mppE, mppR2, MpFasA2, MpFasB2 (GenBank accession no. KC148521), and actin gene (GenBank accession no. AJ417880) were designed (Supplementary Table 1) by NCBI/Primer-BLAST to amplify a portion of the ten genes, and each production size was 100–250 bp to ensure the accuracy of RT-qPCR results. RT-qPCR was performed using the Stratagen Mx3000P (Agilent, USA) with the following cycling program: hold at 95 °C for 30 s, followed by a three-step PCR (42 cycles of denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s) and dissociation curve analysis (at 95 °C for 15 s, annealing at 60 °C for 30 s, then collecting the dissociation curve from 60 to 95 °C, finally at 95 °C for 15 s). Levels of 10 mRNA genes were normalized to actin gene, and relative changes in mRNA level treatment with sample in the dark as reference value were calculated from triplicate replicates of each using the 2−ΔΔCt method (Livak and Schmittgen 2001).
Results
Effect of blue light on M9 culture morphology
To gain an overview of the influence of blue light on the growth of Monascus, we inoculated M9 on MEA at 30 °C for 10 days under different light conditions (Fig. 1). No significant difference was observed on the colony size under dark or blue light at the same day, but significant changes were observed in colony color. The colonies in the dark (Fig. 1a) were an intense orange on the 4th, 7th, and 10th day but the mycelia exposed to continuous blue light (Fig. 1c) were pale. The center of the colony under dark conditions became deep red with increasing incubation time. It is interesting that the colony exposed to blue light 12 h/day (Fig. 1b) showed a series of two-tone concentric circles. The mycelia circles remained red in the dark but became white when exposed to blue light because of pigment production, which was accumulated in the dark and not under blue light. These observations indicate that M9 can sense and differentiate between blue light and the dark and respond with different patterns of development of mycelium and pigment production.
Culture morphology of M9 under different blue light condition. M9 was cultured on MEA under dark condition (a), blue light for 12 h/day (b), or kept under continuous blue light (c) for 10 days at 30 °C. The light intensity was 450 lux. From a–c left, middle, and right images are colony images photographed on the 4th, 7th, and 10th days, respectively
Effect of blue light intensity and illumination time on MS and AK production
To qualitatively analyze the pigment produced by M9, we performed HPLC–MS on the extracts obtained from mycelium grown in liquid rice medium. MS and AK, with molecular weights of 358 and 386, respectively, were identified as classical yellow pigments produced by M9 (Supplementary Fig. 1).
The effect of blue light intensity and illumination time on MS and AK production was analyzed by HPLC. MS and AK were determined in fermentation broth and cell extracts on the 7th day. MS and AK concentrations exhibited low levels in the broth culture; thus, measurements were performed with cell extracts.
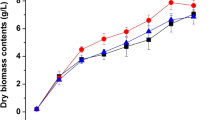
As shown in Fig. 2a, the intensity and time of illumination apparently influenced MS production. Under 300 and 450 lux conditions, MS production increasingly decreased compared with cells placed in the dark with illumination time of 15–60 min/day. The yields of MS under 200, 100, and 50 lux conditions initially increased and then decreased when exposure time was increased. MS production increased by 14.4, 21.8, and 12.7 % when exposed to blue light under 100 lux for 15 min/day, 100 lux for 30 min/day, and 200 lux for 15 min/day, respectively, compared with dark condition. Slight increases were also observed under 50 lux for 15, 30, and 45 min/day. The variation in the AK yield under different blue light conditions (Fig. 2b) was similar to MS. These results suggest that blue light may either induce or inhibit MS and AK production. The effects depend on light intensity and illumination time.
Effect of blue light intensity and illumination time on monascin (a) and ankaflavin (b) production. M9 was incubated in rice medium in the dark or under blue light of 50 lux (open square), 100 lux (filled circle), 200 lux (open up triangle), 300 lux (filled down triangle), and 450 lux (open diamond) illuminated for 15, 30, 45, and 60 min/day, respectively. Fresh mycelium was obtained from rice medium on the 7th day. The MS and AK were extracted from mycelium. The MS and AK contents were assessed by HPLC and given in absolute values dividing by the dry weight of mycelium. The data are represented as the mean ± SEM (n = 3)
Blue light is supposed to harm mycelia. MS and AK act as photo-protectants and accumulate in the cell to protect mycelia by absorbing blue light. Thus, MS and AK biosyntheses were induced when strains were exposed to low intensity at a proper illumination time. However, if the intensity of blue light becomes strong, pigment production is inhibited.
Effect of blue light on pigment biosynthetic gene expression
To understand the blue light effects on MS and AK production at the molecular level, we analyzed the expression level of pigment biosynthetic gene cluster by RT-qPCR. This gene cluster was a homolog of the pigment biosynthesis genes (GenBank accession no. KC148521) existing in the Monascus pilosus genome (Balakrishnan et al. 2013). According to the aforementioned pigment biosynthesis genes, ten targeted fragments were identified in M9 by colony screening with ten pairs of primers and named MpPKS5, mppR1, mppA, mppB, mppC, mppD, mppE, mppR2, MpFasA2, and MpFasB2.
As shown in Fig. 3, the order of expression levels of MpPKS5, mppR1, mppA, mppB, mppC, mppD, mppR2, MpFasA2, and MpFasB2 for the different illumination time each day was 30 min > 15 min > dark > 45 min > 60 min. Figures 2 and 3 show a similar change trend with phenotypic production of MS and AK under 100 lux condition, except for mppE. The expression level of genes increased parallel to the amount of pigment produced and decreased with reduced pigment. These results indicate that blue light stimulated or inhibited MS and AK production by upregulating or downregulating the expression levels of pigment biosynthetic gene cluster. Thus, the effects of blue light on pigment production in M9 were recognized as a biological control process.
Expression of pigment biosynthetic genes (MpPKS5, mppR1, mppA, mppB, mppC, mppD, mppE, mppR2, MpFasA2, and MpFasB2) under blue light or dark monitored by RT-qPCR. Gene expression test samples exhibited a one-to-one correspondence with those samples used for MS and AK tests under blue light of 100 lux condition. The transcriptional levels were normalized to those of the actin gene. The mRNA levels in the dark were used as the reference value. Data were expressed as the relative mRNA level for each gene and represented as the mean ± SEM (n = 3)
Discussion
Blue light influences mycelium development, MS and AK production, and pigment biosynthetic gene expression in Monascus strain M9. M9 exposed to continuous blue light is pale but is orange when grown in the dark. This suggests that blue light affects pigment accumulation in mycelium.
Miyake et al. (2005) reported that light of varying wavelengths had an influence on pigment production in M. pilosus strain IFO4520. Their observation suggests that red light enhanced pigment production but blue light inhibited pigment production. The same conclusion was also reached by Babitha et al. (2008), who reported that direct blue illumination totally suppressed pigment production in Monascus purpureus. By contrast, the present study found that blue light at a low intensity and proper illumination time (100 lux, 15 and 30 min/day; 200 lux, 15 min/day) enhanced the production of MS and AK but that with a high intensity (300 and 450 lux) and long exposure time (45 and 60 min) decreased pigment production. We speculate that blue light may inhibit or induce pigment biosynthesis in Monascus. The effect of blue light depends on light intensity, illumination time, and species. Light intensity and illumination time are important factors that affect the secondary metabolite biosynthesis of fungi. Schmidt-Heydt et al. (2011) analyzed the influence of blue light of varying intensities on the growth and ochratoxin A biosynthesis of five food relevant fungi. When the intensity of blue light was increased from 200 to 1700 lux, a drastic reduction in ochratoxin A biosynthesis occurred. The expression of photolyase gene (phr1) in T. harzianum was detected by light pulse. The expression level reached its maximum accumulation between 15 and 30 min and then started to decrease 60 min after the pulse (Berrocal-Tito et al. 1999).
This study is the first to correlate the expression levels of pigment biosynthetic gene with pigment production in Monascus. The expression of pigment biosynthetic gene transcripts increased when exposed to blue light under 100 lux for 15 and 30 min/day, and decreased when exposed for 45 and 60 min/day. These variations correlate quite well with pigment production. Gene mppE exhibited a different result in the amount of MS and AK produced. According to Balakrishnan et al. (2013), mppE belongs to the pigment biosynthetic gene cluster that exists in the M. pilosus genome as a putative dehydrogenase encoding gene. We suppose that MS or AK could be oxidized to other novel pigments by this dehydrogenase. Thus, the amount of MS and AK produced decreased with upregulating gene mppE expression.
Monascus can sense blue light because the majority of photoresponses in fungi are mediated by the photoreceptors that absorb blue light. We proposed that similar light regulatory protein, such as WC-1/WC-2, in N. crassa may exist in Monascus. Further studies are necessary to elucidate the molecular pathway of blue light in regulating pigment production.
References
Akihisa T, Tokuda H, Ukiya M, Kiyota A, Yasukawa K, Sakamoto N, Kimura Y, Suzuki T, Takayasu J, Nishino H (2005) Anti-tumor-initiating effects of monascin, an azaphilonoid pigment from the extract of Monascus pilosus fermented rice (red-mold rice). Chem Biodivers 2:1305–1309
Babitha S, Carvahlo JC, Soccol CR, Pandey A (2008) Effect of light on growth, pigment production and culture morphology of Monascus purpureus in solid-state fermentation. World J Microbiol Biotechnol 24:2671–2675
Balakrishnan B, Karki S, Chiu SH, Kim HJ, Suh JW, Nam B, Yoon YM, Chen CC, Kwon HJ (2013) Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl Microbiol Biotechnol 97:6337–6345
Ballario P, Macino G (1997) White collar proteins: passing the light signal in Neurospora crassa. Trends Microbiol 5:458–462
Ballario P, Vittorioso P, Magrelli A, Talora C, Cabibbo A, Macino G (1996) White collar—1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J 15:1650–1657
Berrocal-Tito G, Sametz-Baron L, Eichenberg K, Horwitz BA, Herrera-Estrella A (1999) Rapid blue light regulation of a Trichoderma harzianum photolyase gene. J Biol Chem 274:14288–14294
Cheng CF, Pan TM (2011) Protective effect of Monascus-fermented red mold rice against alcoholic liver disease by attenuating oxidative stress and inflammatory response. J Agric Food Chem 59:9950–9957
Häggblom P, Unestam T (1979) Blue light inhibits mycotoxin production and increases total lipids and pigmentation in Alternaria alternata. Appl Environ Microbiol 38:1074–1077
Jou PC, Ho BY, Hsu YW, Pan TM (2010) The effect of Monascus secondary polyketide metabolites, monascin and ankaflavin, on adipogenesis and lipolysis activity in 3T3-L1. J Agric Food Chem 58:12703–12709
Jůzlová P, Martínková L, Křen V (1996) Secondary metabolites of the fungus Monascus: a review. J Ind Microbiol 16:163–170
Lee K, Singh P, Chung WC, Ash J, Kim TS, Hang L, Park S (2006) Light regulation of asexual development in the rice blast fungus, Magnaporthe oryzae. Fungal Genet Biol 43:694–706
Lee CL, Kung YH, Wu CL, Hsu YW, Pan TM (2010) Monascin and ankaflavin act as novel hypolipidemic and high-density lipoprotein cholesterol-raising agents in red mold dioscorea. J Agric Food Chem 58:9013–9019
Lee BH, Hsu WH, Liao TH, Pan TM (2011) The Monascus metabolite monascin against TNF-α-induced insulin resistance via suppressing PPAR-γ phosphorylation in C2C12 myotubes. Food Chem Toxicol 49:2609–2617
Linden H, Ballario P, Macino G (1997) Blue light regulation in Neurospora crassa. Fungal Genet Biol 22:141–150
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Miyake T, Mori A, Kii T, Okuno T, Usui Y, Sato F, Sammoto H, Watanabe A, Kariyama M (2005) Light effects on cell development and secondary metabolism in Monascus. J Ind Microbiol Biotechnol 32:103–108
Schmidt-Heydt M, Rüfer C, Raupp F, Bruchmann A, Perrone G, Geisen R (2011) Influence of light on food relevant fungi with emphasis on ochratoxin producing species. Int J Food Microbiol 145:229–237
Velmurugan P, Lee YH, Venil CK, Lakshmanaperumalsamy P, Chae JC, Oh BT (2010) Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J Biosci Bioeng 109:346–350
Wang CL, Yang H, Chen MH, Wang YR, Li FJ, Luo C, Zhao SY, He D (2012) Real-time quantitative analysis of the influence of blue light on citrinin biosynthetic gene cluster expression in Monascus. Biotechnol Lett 34:1745–1748
Acknowledgments
We are grateful for the financial support provided by the National Natural Science Foundation of China (Grant No. 31330059).
Supporting information
Supplementary Table 1—Primers for RT-qPCR analyzing pigment biosynthetic genes.
Supplementary Figure 1—Mass spectra of monascin (a) and ankaflavin (b).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, C., Chen, D., Chen, M. et al. Stimulatory effects of blue light on the growth, monascin and ankaflavin production in Monascus . Biotechnol Lett 37, 1043–1048 (2015). https://doi.org/10.1007/s10529-014-1763-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1763-3