Abstract
A large set of mutants of CYP102A1 from Bacillus megaterium have human cytochrome P450-like activities and the ability to metabolize a number of marketed drugs and steroids. Here, we tested whether the CYP102A1 mutants could be used to produce hydroxylated human metabolites of 17β-estradiol (E2). A set of the mutants, which were generated by site-directed and random mutagenesis, was used to produce hydroxylated human metabolites of E2 in this study. Some of the tested mutants could regioselectively generate 2-OH E2 as a major metabolite but not other hydroxylated products. These results suggest that CYP102A1 mutants would be useful for the bioconversion of steroid hormones to hydroxylated products, which can be used for industrial applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Steroids play an important role as hormones in mammals, and a variety of steroids are widely used as anti-inflammatory, antiandrogenic, progestational, and anticancer agents, as well as in other applications (Holland 1999). Because it is difficult to conduct selective and efficient synthesis of hydroxylated derivatives of steroids by traditional chemical methods, transformation using enzymes is a useful alternative. A large number of bacterial strains that hydroxylate steroids have been isolated and characterized, and most positions of the steroid can be hydroxylated (Mahato and Gari 1997). CYP106A1 (Lee et al. 2014) and CYP106A2 (Virus and Bernhardt 2008) from Bacillus megaterium can catalyze the hydroxylation reaction of a set of steroids that generates novel hydroxylated derivatives. The hydroxylated steroids themselves can be drug leads, and they can also be used to make drug candidates after chemical modifications at the hydroxylated position.
17β-Estradiol (E2) is metabolized to multiple hydroxylated metabolites, including the catechol estrogens 2-OH E2, 4-OH E2, 16α-OH E2, and 4-hydroxyestrone, by human cytochromes P450 (P450) 1A1, 1A2, and 1B1 (Fig. 1). The carcinogenic effect of the E2 metabolites, such as 4-OH E2, 2-OH E2 and 16α-OH E2, are still controversial (Turan et al. 2004). The high cost of the hydroxylated E2 metabolites makes it difficult to study the effects of the metabolites on in vivo and in vitro systems.
P450 BM3 (CYP102A1) from B. megaterium is catalytically self-sufficient, containing both a heme domain responsible for substrate oxidation and a diflavin reductase domain responsible for electron transport. At present, a large set of CYP102A1 mutants are known to have human P450-like activities and the ability to metabolize a number of marketed drugs and steroids (Yun et al. 2007; Whitehouse et al. 2012; Caswell et al. 2013; Kang et al. 2014).
In this study, we tested whether the CYP102A1 mutants could be used to produce hydroxylated human metabolites of E2. We found that some of the tested mutants could generate 2-OH E2 regioselectively as a major metabolite but not other hydroxylated products.
Materials and methods
Chemicals
17β-Estradiol (E2), 2-hydroxyestradiol (2-OH E2), and 4-hydroxyestradiol (4-OH E2) were purchased from Sigma-Aldrich (St. Louis, MO). All of the other chemicals used were of the highest grade commercially available.
Construction of the CYP102A1 mutants by site-directed and random mutagenesis
The wild type CYP102A1 from B. megaterium ATCC 14,581 (Kang et al. 2011) and its 46 different mutants used in this study were prepared as described (Kim et al. 2008; Park et al. 2010; Kang et al. 2014). The mutants were selected based on their activity on a variety of human P450 substrates and drugs. Mutants #1–17 have mutations in the substrate channel and active site (Kim et al. 2008), and mutants #18–26 have mutations outside of the active site and substrate channel (Park et al. 2010). Chimera M16V2 and its 19 mutants (A1~H1), which were obtained by random mutagenesis of the heme domain, were created for a previous work (Kang et al. 2014). Each mutant bears amino acid substitution(s), relative to wild type CYP102A1, as summarized in Supplementary Tables 1 and 2.
Heterologous expression in Escherichia coli and purification of the wild type and mutated enzymes of CYP102A1
The wild type and 46 mutants of CYP102A1 were expressed in the E. coli strain DH5αF′-IQ and purified as previously described (Kim et al. 2008; Park et al. 2010; Kang et al. 2014). The CYP102A1 concentrations were determined from the CO-difference spectra using ε = 91 mM−1 cm−1. For all of the wild type and mutated enzymes, a typical culture yielded 200–700 nM P450.
Activity assay of 17β-estradiol (E2) hydroxylation catalyzed by the CYP102A1 mutants
The E2 hydroxylation activity of CYP102A1 was determined as previously described (Shimada et al. 1998), with slight modifications. Briefly, purified CYP102A1 (200 nM) was incubated with 200 µM substrate in 100 mM potassium phosphate buffer (pH 7.4) in 0.25 ml in the presence of a NADPH-generating system (final concentrations: 10 mM glucose 6-phosphate, 0.5 mM NADP+, and 1 IU/ml yeast glucose-6-phosphate dehydrogenase). Ascorbic acid (1 mM) was added to the reaction mixture to protect the E2 metabolites from oxidative degradation. After incubation for 20 min at 37 °C, the reactions were terminated with 0.5 ml ice-cold ethyl acetate and centrifuged at ~3,000×g for 10 min. The organic layers were combined, and the ethyl acetate was removed under N2. The reaction products were analyzed by HPLC using a Gemini C18 column (4.6 × 150 mm, 5 μm; Phenomenex, Torrance, CA) with a mobile phase of 33 % acetonitrile containing 0.5 % acetic acid with detection at 280 nm. The flow rate was 1 ml/min. The retention times for E2 and its metabolites were: 2-OH E2, 10.02 min; 4-OH E2, 16.5 min; and E2, 19.15 min.
LC/MS analysis
To identify the E2 metabolite produced by the CYP102A1 mutants, LC–MS analysis was carried out. The hydroxylation reaction of E2 by the CYP102A1 mutants was performed as described above. For the activity assays of human CYP1A2, a control experiment containing 50 pmol P450, 100 pmol rat NADPH-P450 reductase, and 50 μM L-α-dilauroyl-sn-glycero-3-phosphocholine was used. After extraction and centrifugation, the organic phases were evaporated under N2. Five microlitres of the reconstituted residue was injected onto the LC column. LC–MS analysis was performed on a Shimadzu LCMS-2010 EV system (Shimadzu, Kyoto, Japan), which included the LC–MS solution software. The separation was performed on a Shim-pack VP-ODS column (2 × 250 mm; Shimadzu) with a mobile phase of 50 % acetonitrile containing 0.5 % acetic acid at 0.15 ml/min. The column was maintained at 40 °C. To identify the metabolites, mass spectra were recorded by electrospray ionization in negative mode. The interface and detector voltages were 4.4 and 1.5 kV, respectively. The nebulization gas was at 1.5 l/min. The interface, curve desolvation line, and heat block temperatures were 250, 230, and 200 °C, respectively. The retention time and fragmentation patterns of the E2 metabolites and authentic compounds were compared to identify the chemical structure of the metabolites.
Stability of the CYP102A1 mutants during the reaction
To estimate the stability of the CYP102A1 mutants during the E2 oxidation reaction, we measured the CO-difference spectra of the reaction aliquots. After 100 nM of enzyme was incubated with 1 mM of substrate (E2) for 0.5, 1, 2, 3, and 4 h in the presence of the NADPH-generating system, the remaining concentration of P450 was measured.
Results and discussion
Hydroxylation of E2 by wild type CYP102A1 and its mutants
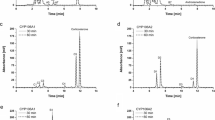
Initially, the catalytic activity of the wild type and a set of CYP102A1 mutants toward E2 was investigated at a fixed substrate concentration of 200 μM using HPLC (Fig. 2a). Although the wild type and some mutants did not show any detectable metabolites, 30 of 46 mutants tested here produced one metabolite hydroxylated at the 2-position (2-OH E2) with an apparent activity of >1 min−1 for E2 (Fig. 3). However, 4-OH E2 and 16α-OH E2, other human metabolites, were not detectable. The metabolite (2-OH E2) and substrate (E2) were identified by HPLC and LC–MS by comparison to authentic compounds. The retention time and fragmentation pattern of the major metabolite from E2 were matched to the 2-OH E2 standard (Fig. 2b). The turnover numbers from the 34 mutants for 2-OH E2 formation varied over a wide range. The wild type enzyme, as well as mutants #1, #2, #4 ~#7, #9, #11, B3, B10, E7, and G2, did not show activity for 2-OH E2 formation (<0.1 min−1). Nine mutants showed much higher catalytic activity (>10 min−1) for 2-OH E2 formation than those of human CYP1A1 (5.78 min−1) and CYP1A2 (7.68 min−1).
HPLC and LC–MS analyses of the hydroxylation of E2 catalyzed by human CYP1A2 and mutants of CYP102A1 from B. megaterium ATCC 14581. a The HPLC elution profile of E2 and its major metabolite produced by the CYP102A1 mutants and human CYP1A2. The peaks of the substrate (E2) and its metabolites (2-OH E2 and 4-OH E2) are indicated. Absorbance was monitored at 280 nm. b The LC–MS elution and TIC (total ion current) profiles of the metabolites generated by the CYP102A1 mutant and human CYP1A2. The mass spectra of the reaction samples showed two major peaks at 6.96 min (2-OH E2) and 10.5 min (E2). The mass spectra of the 2-OH E2 produced by the CYP102A1 mutant and CYP1A2 and E2 are also shown. The calculated mass for [M]− was 271 and 287 for E2 and 2-OH E2, respectively
Kinetic parameters and total turnover number (TTN) of E2 2-hydroxylation by the CYP102A1 mutants
Four mutants (#8, #13, #17, and G1) were selected to study the kinetics of the 2-hydroxylation of E2 (Table 1 and Supplementary Fig. 1). The k cat values of the mutants were in the range of 13–47 min−1, and the K m values were in the range of 34–125 µM. Although mutant #17 showed the highest k cat value, mutants #8 and G1 showed very high catalytic efficiency (k cat/K m) because of their low K m values.
The same mutants were used to measure the TTN (mol product/mol catalyst) for the 2-hydroxylation reaction of E2 (Supplementary Fig. 2). The overall TTN values were between 1,040 and 1,210 for the 2-OH E2 formation of mutants #8, #17, and G1 when the reaction mixture was incubated for 4 h in the presence of 1 mM substrate. Mutant #13 showed a TTN of only 122.
Stability of the CYP102A1 mutants as measured by CO-difference spectra
During the hydroxylation reaction of E2 by P450 in the presence of NADPH, the stability of the P450 enzymes was examined by measuring the CO-difference spectra of reaction aliquots at the indicated time. The stability of the mutants was quite different. Mutant #17, with the highest activity among the tested mutants, also showed the highest stability during the 4 h incubation. After 2 h incubation, <40 % of the intact P450 of mutants #8 and #13 remained under the tested condition (Supplementary Fig. 3). This result might be related to that of the TTN experiments (Supplementary Fig. 2).
According to the revised FDA guideline for the Safety Testing of Drug Metabolites (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm079266.pdf), any human drug metabolite “formed at >10 % of parent drug systemic exposure at steady state” should be subject to separate safety testing, which requires large quantities of the drug metabolites (MIST issue). Some drug metabolites of concern can be prepared by chemical methods, but others should be prepared by several enzyme sources, including purified human P450 enzymes. Some bacterial P450 s are competitive biocatalysts for the production of commercial drugs and steroids, due to their high activities and stabilities (Julsing et al. 2008; Whitehouse et al. 2012; Caswell et al. 2013; Kang et al. 2014). Therefore, CYP102A1 is now generally accepted to be a prototype monooxygenase for the development of versatile biocatalysts for use in drug discovery and synthesis (Urlacher and Girhard 2012; Kang et al. 2014).
In summary, using a set of CYP102A1 mutants and E2, a human P450 substrate, this work reveals that bacterial CYP102A1 enzymes catalyze the same reaction as human P450 s. The hydroxylation of E2 is catalyzed by a subset of the CYP102A1 mutants. One major hydroxylated product, 2-OH E2, was produced as a result of a hydroxylation reaction. Other hydroxylated products were not produced. 2-OH E2 formation was confirmed by HPLC and LC–MS by comparing the metabolite to the authentic 2-OH E2 compound. Thus, the CYP102A1 mutants efficiently produce 2-OH E2, an authentic human metabolite of E2.
References
Caswell JM, O’Neill M, Taylor SJ, Moody TS (2013) Engineering and application of P450 monooxygenases in pharmaceutical and metabolite synthesis. Curr Opin Chem Biol 17:271–275
Holl HL (1999) Recent advances in applied and mechanistic aspects of the enzymatic hydroxylation of steroids by whole-cell biocatalysts. Steroids 64:178–186
Kang JY, Kim SY, Kim D, Kim DH, Shin SM, Park SH, Kim KH, Jung HC, Pan JG, Joung YH, Chi YT, Chae HZ, Ahn T, Yun CH (2011) Characterization of diverse natural variants of CYP102A1 found within a species of Bacillus megaterium. AMB Express 1:1
Kang JY, Ryu SH, Park SH, Cha GS, Kim DH, Kim KH, Hong AW, Ahn T, Pan JG, Joung YH, Kang HS, Yun CH (2014) Chimeric cytochromes P450 engineered by domain swapping and random mutagenesis for producing human metabolites of drugs. Biotechnol Bioeng 111:1313–1322
Kille S, Zilly FE, Acevedo JP, Reetz MT (2011) Regio-and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution. Nat Chem 3:738–743
Kim DH, Kim KH, Kim DH, Liu KH, Jung HC, Pan JG, Yun CH (2008) Generation of human metabolites of 7-ethoxycoumarin by bacterial cytochrome P450 BM3. Drug Metab Dispos 36:2166–2170
Lee GY, Kim DH, Kim D, Ahn T, Yun CH (2014) Functional characterization of steroid hydroxylase CYP106A1 derived from Bacillus megaterium. Arch Pharm Res. doi:10.1007/s12272-014-0366-9
Mahato SB, Gari S (1997) Advances in microbial steroid biotransformation. Steroids 62:332–345
Park SH, Kim DH, Kim D, Kim DH, Jung HC, Pan JG, Ahn T, Kim D, Yun CH (2010) Engineering bacterial cytochrome P450 (P450) BM3 into a prototype with human P450 enzyme activity using indigo formation. Drug Metab Dispos 38:732–739
Rea V, Kolkman AJ, Vottero E, Stronks EJ, Ampt KA, Honing M, Vermeulen NP, Wijmenga SS, Commandeur JN (2012) Active site substitution A82 W improves the regioselectivity of steroid hydroxylation by cytochrome P450 BM3 mutants as rationalized by spin relaxation nuclear magnetic resonance studies. Biochemistry 51:750–760
Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP, Gillam EM (1998) Recombinant human cytochrome P450 1B1 expression in Escherichia coli. Arch Biochem Biophys 357:111–120
Turan VK, Sanchez RI, Li JJ, Li SA, Reuhl KR, Thomas PE, Conney AH, Gallo MA, Kauffman FC, Mesia-Vela S (2004) The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17beta-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16alpha-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol 183:91–99
Urlacher VB, Girhard M (2012) Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol 30:26–36
Virus C, Bernhardt R (2008) Molecular evolution of a steroid hydroxylating cytochrome P450 using a versatile steroid detection system for screening. Lipids 43:1133–1141
Whitehouse CJ, Bell SG, Wong LL (2012) P450BM3 (CYP102A1): connecting the dots. Chem Soc Rev 41:1218–1260
Yun CH, Kim KH, Kim DH, Jung HC, Pan JG (2007) The bacterial P450 BM3: a prototype for a biocatalyst with human P450 activities. Trends Biotechnol 25:289–298
Zehentgruber D, Hannemann F, Bleif S, Bernhardt R, Lütz S (2010) Towards preparative scale steroid hydroxylation with cytochrome P450 monooxygenase CYP106A2. Chem BioChem 11:713–721
Acknowledgments
This research was supported by the Next-Generation BioGreen 21 program (SSAC, grant#: PJ00948303), Rural Development Administration, Republic of Korea, and the the Bio-industry Technology Development Program (111,052-04-3-SB010), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Conflict of interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cha, G.S., Ryu, S.H., Ahn, T. et al. Regioselective hydroxylation of 17β-estradiol by mutants of CYP102A1 from Bacillus megaterium . Biotechnol Lett 36, 2501–2506 (2014). https://doi.org/10.1007/s10529-014-1628-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1628-9