Abstract
The effect of 6 years of cultivation and use of table-sugar (TS) on the biomass/terpene alkaloid productivities and rol gene expression were studied in a hairy root (HR) clone of Rauvolfia serpentina. The media cost could be reduced >94 % by replacing sucrose (SUC) with TS—an unexplored avenue for HR cultivation. The overall productivities increased over long-term cultivation with sugar proving superior to SUC for biomass (24.4 ± 2.11 g/l DW after 40 days to 17.31 % higher) and reserpine (0.094 ± 0.008 % DW after 60 days to 193.8 % more) production. The latter however revealed comparatively better yields concerning ajmaline (0.507 ± 0.048 % DW after 60 days to 61.98 % higher) and yohimbine (0.628 ± 0.062 % DW after 60 days to 38.32 % higher), respectively. PCR amplification of rol genes confirmed long-term expression stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The search for plant-based therapeutic agents has gained attention towards a century old medicinal plants, Rauvolfia serpentina Benth., also known as “snakeroot”, renowned for its root-specific terpene indole alkaloids (TIA) (Sudha et al. 2003). In addition to its traditional uses, Rauvolfia alkaloids have additional therapeutic applications to treat breast cancer, arrhythmia, human promyelocytic leukemia, cardiovascular diseases, hyperglycemia and hyperlipidemia, inhibitors of angiotensin-converting enzyme (ACE) and topoisomerase I & II that highlights their multifaceted uses (Dey and De 2011).
The endangered status of the plant and increasing demand for its bioactive materials have prompted biotechnological interventions with hairy root (HR) cultures having been introduced as the most useful production system (Madhusudanan et al. 2008). Although HR technology has gained preferential use over plant cell cultures owing to their multifarious advantages including prevalent genetic/biochemical stability (Chandra 2012), the question of inheritance of “HR” related transgenes and ensuing stability in growth and secondary metabolite production in prolonged cultures in tandem with the concern of cost-effectiveness, has raised serious awareness over the years (Guivarc’h et al. 1999).
There are contradictory findings with regard to the long-term stability in root morphology, biomass as well as secondary metabolite productivity (Lipp-Joao and Brown 1994; Maldonado-Mendoza et al. 1993; Baiza et al. 1999; Santos et al. 1999; Marconi et al. 2008). Former studies have indicated that any Ri T-DNA mediated alteration in metabolic performance may require an initial transitory phase, varying from months to several years, to reach a stabilized state (Geerlings et al. 1999; Peebles et al. 2009). This aspect bears profound implications on its future industrial applications and therefore needs to be optimized for each individual potential HR clone.
Likewise, the economical concerns of HR technology are still not credible as the production costs are still fairly high. Generally sucrose (SUC), the most abundantly utilized carbohydrate source for HR cultures, incurs the maximum cultivation cost and therefore its substitution with any low-cost alternative seemed most prudent, which has not been researched so far involving any HR system, to the best of our knowledge.
Therefore, the rationale of the present study was to delineate this responsibility through evaluating the effect of table-sugar (TS) as an operational low cost alternative of SUC on the long-term stability (>6 years) of a R. serpentina HR clone. Biomass and TIA production in tandem with the associated rol gene expression constituted the basis of our investigation.
Materials and methods
Induction and establishment of hairy roots
Several HR clones (30 in number) of R. serpentina were established >6 years back according to our earlier reported protocol (Madhusudanan et al. 2008). The established root clones were maintained through sub-culturing on every 20 days in liquid hormone-free half-strength MS (Murashige and Skoog, 1962) medium supplemented with either of the two different carbohydrates, viz. SUC or TS at 3 % (w/v) concentration and was incubated on a rotary shaker in the dark at 25 ± 1 °C under constant agitation (80 rpm) beyond 6 years.
Characterization for rol gene expression
DNA extraction from the HR culture as well as from the root of normal field grown plant (control) was done following the protocol described earlier (Madhusudanan et al. 2008). Consequently, the confirmation of the transformed nature of the HR clone as well as the stability of the transgene expression was done at the time of establishment and again after 6 years of maintenance through PCR amplification of the rol B (762 bp; forward: 5′-ATGGATCCCAAATTGCTATTCC-3′ and reverse: 5′-GTTTACTGCAGCAGGCTTCATG-3′) and rol C (539 bp; forward: 5′-ATGGCTGAAGACGACCTGTGT-3′ and reverse: 5′-CCGATTGCAAACTTGCACTC-3′) genes following a published procedure (Ayadi and Tremouillaux-Guiller 2003).
HPLC analysis
The dried HRs at different timepoints (10, 20, 30, 40, 50 and 60 days) were powdered and extracted with methanol/chloroform (1:1, v/v) for 3 times. The filtered extract was dried under vacuo and dissolved in methanol (1 mg/l). The chromatographic separation was achieved using a symmetry C18 column (5 μm, 450 × 4.6 mm2, Waters) at 25 °C, mobile phase consisted of solvent A 0.5 % acetic acid in water and solvent B acetonitrile in gradient elution mode with detector wavelength 254 nm and the injection volume was 20 μl. Gradient elution was started with 25 % solvent A at a flow rate of 0.4 ml/min; flow was increased to 0.8 ml/min at 19 min; at 22 min gradient was again modified to 70 % solvent A and flow rate of 0.8 ml/min, which was maintained as such up to 28 min there after initial condition was reverted back at 30 min.
Results and discussion
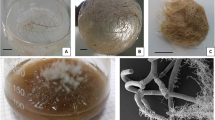
The established individual HR clones (30 in number) of R. serpentina demonstrated intense propensity towards dedifferentiation (Supplementary Fig. S1a), of which only 30 % root clones escaped, which multiplied in liquid hormone-free half-strength MS medium (Madhusudanan et al. 2008). Predisposition of HR cultures towards dedifferentiation on phytohormone-free basal media had been noted earlier in several other plant systems (Guivarc’h et al. 1999; Zdravkovic-Korac et al. 2003).
Most obviously, frequently reported observations have clearly indicated that dedifferentiation process has negatively influenced the biosynthetic potentials of HR cultures. Therefore, selection of “pure” HR clone of R. serpentina with callus-free contour has become a critical prerequisite for assured long-term consistency in growth and secondary metabolite production. Only three non-dedifferentiating clones exhibited the potential to synthesise the three major indole alkaloids (i.e. reserpine, yohimbine and ajmaline). Amongst these, only one clone (SR-9) grew through the successive culture passages over a period of 6 years (Supplementary Fig. S1b, c), while the rest survived <2 years with an equivalent production potentials (data not presented). Analogous consequences were also noted in case of carrot (Guivarc’h et al. 1999) and horse chestnut HR cultures (Zdravkovic-Korac et al. 2003). In a sense, such findings indicate the mandatory role of time interval for a HR clone to get stabilized in terms of its productivities, which should remain unaffected in course of sub-culturing passages. This has been eloquently emphasized involving Catharanthus roseus HR culture (Peebles et al. 2009).
Perceptible commercial attainments of the HR technology have engrossed our attention to the cost-effectiveness of the technique in tandem with the concern of “long-term” stability. While estimating the cost of HR cultivation, SUC has been recognised as the most cost-incurring ingredient. Therefore, replacement of SUC with sugar seemed to be the most tangible strategy that has not been deciphered hitherto for HR cultivation.
The growth kinetic analysis demonstrated a reasonably higher growth potential of the HR clone following long-term cultivation with either of the two carbohydrates (Fig. 1). Similar kind of unaltered growth performance over 1–6 years of HR maintenance has also been reported earlier with diverse plant systems, such as Cinchona officinalis ‘Ledgeriana’ (Geerlings et al. 1999), A. hippocastanum (Zdravkovic-Korac et al. 2003), coffea (Alpizar et al. 2008) and tomato (Lipp-Joao and Brown 1994).
Multiple studies have reported the stimulatory effect of different Ri T-DNA rol genes in escalating the secondary metabolism of HR clones, amongst which the rol B and C have won the maximum attention (Bulgakov 2008). Accordingly, unwavering secondary metabolite production in long-term HR cultures necessitates constancy in pRi T-DNA gene expression, which has been exemplified with contradictory reports (Hanisch ten cate et al. 1990; Guivarc’h et al. 1999). The PCR amplifications of rol B and C gene of the SR-9 HR clone yielded positive results confirming their expression stability consequent to 6 years of maintenance in either SUC or TS containing media (Supplementary Fig. S2). Such affirmative outcome also ascertained the required competence of TS in acting as a signal molecule to maintain the activation of the rol specific promoter. Consistency in expression of either pRi T-DNA or rol/gus genes during prolonged cultivation of HRs (Zdravkovic-Korac et al. 2003; Alpizar et al. 2008) corroborates our findings.
In terms of secondary metabolite productivity, the selected SR-9 HR clone characteristically produced three major terpene alkaloids (reserpine, ajmaline, yohimbine) since its establishment with either of the two employed carbon sources. Among these, the yohimbine content was highest, followed by that of ajmaline and reserpine in decreasing order. Their maximum accumulation coincided with 60 days of subculturing, as detected through HPLC prior to 6 years of upholding (data not presented).
Evaluation of the yield potentials at the same time points after 6 years of maintenance bears much relevance in context to its industrial applicability and therefore urged for detail articulation. Interestingly, the SR-9 clone maintained similar trend of production after 6 years of cultivation with either of the two carbon sources and yohimbine appeared to be the major alkaloid. Noticeable fluctuations in the yield of two other alkaloids (i.e. ajmaline and yohimbine) vis-a-vis growth phases could be noted, contingent upon the type of carbon source used (Fig. 2a). On the contrary, a continuous upsurge in the content of reserpine could be noticed corresponding to growth phases, which consequently assured production dexterity in the SR-9 clone (Fig. 2b).
Appreciably, the TS supplemented medium demonstrated 193.8 % more reserpine than that with SUC at 60 days of culture (Fig. 2b), which undoubtedly adds prefentiality to the use of the former considering its cost-effectiveness and commercial implications. Alternatively, the latter showed 38.32 and 61.98 % higher yields of yohimbine and ajmaline respectively than that with TS at the same time point (Fig. 2a).
In order to decipher the question of long-term consistency, comparative analyses were carried out with present performance of the SR-9 clone aligned with that of the 6 years prior potentials in terms of all the three terpene alkaloids at the optimum production phase (i.e. 60 days). Noticeably, productivity increased over the 6 years period and the highest upsurge was noted with ajmaline (296 % SUC and 184.6 % TS) followed by that of reserpine (128.6 % SUC and 14.63 % TS) and yohimbine (14.18 % SUC and 19.47 % TS) respectively (Table 1). Such escalation in secondary metabolite production can possibly be linked to the prolong in vitro culture mediated stress as has been demonstrated in case of Brugmansia candida HRs after 5 years of culture (Marconi et al. 2008). Biotechnological implications of stress as a regulating factor in enhancing mono TIA production has also been pragmatically evidenced by Matsuura et al. (2013).
Stability in sustaining the secondary metabolite productivity over 5 years of cultivation was reported earlier with Datura stramonium (Maldonado-Mendoza et al. 1993) and Hyoscyamus muticus (Sevon et al. 1998) HR clones. Our present findings will add credence to the long-term performance of HR cultures besides emphasizing the added benefit of the employed cost effective measures that triggered escalated growth and specific metabolite production potentials. In fact, the current study substantiates the idea that a reasonable period of time might realistically be needed before the biosynthetic potential of any HR clone becomes stabilized and insensitive to the in vitro culture stress, as well as to the negative feedback of the relatable metabolites. This will bear strong relevance on the threshold of impending industrial applications as has been elaborated earlier (Geerlings et al. 1999; Peebles et al. 2009).
Conclusion
It can be stated that the present findings report an economical approach that proficiently contributes to preserving the long-term benefits (>6 years) in terms of the rol gene expression and the overall biochemical and biomass production stabilities of the selected HR clone. The projected reduction of media cost (>94 %) through the replacement of SUC with table sugar will undoubtedly accelerate the pace of this know-how towards successful onset of forthcoming commercial consequence.
References
Alpizar E, Dechamp E, Lapeyre-Montes F, Guilhaumon C, Bertrand B, Jourdan C, Lashermes P, Etienne H (2008) Agrobacterium rhizogenes-transformed roots of coffee (Coffea arabica): conditions for long-term proliferation and morphological and molecular characterization. Ann Bot 101:929–940
Ayadi R, Tremouillaux-Guiller J (2003) Root formation from transgenic calli of Ginkgo biloba. Tree Physiol 23:713–718
Baiza AM, Quiroz-Moreno A, Ruiz JA, Loyala-vargas VM (1999) Genetic stability of hairy root cultures of Datura stramonium. Plant Cell Tissue Organ Cult 59:9–17
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Chandra S (2012) Natural plant genetic engineer Agrobacterium rhizogenes: role of T-DNA in plant secondary metabolism. Biotechnol Lett 34:407–415
Dey A, De JN (2011) Ethnobotanical aspects of Rauvolfia serpentina L. Benth ex Kurz. in India, Nepal and Bangladesh. J Med Plant Res 5:144–150
Geerlings A, Hallard D, Martinez CA, Lopes CI, Heijden RV, Verpoorte R (1999) Alkaloid production by a Cinchona officinalis ‘Ledgeriana’ hairy root culture containing constitutive expression constructs of tryptophan decarboxylase and strictosidine synthase cDNAs from Catharanthus roseus. Plant Cell Rep 18:191–196
Guivarc’h A, Boccara M, Prouteau M, Chriqui D (1999) Instability of phenotype and gene expression in long-term culture of carrot hairy root clones. Plant Cell Rep 19:43–50
Hanisch ten cate CH, Loonen AEHM, Ottaviani MP, Ennik L, Eldik GV, Stiekema WJ (1990) Frequent spontaneous deletions of Ri T-DNA in Agrobacterium rhizogenes transformed potato roots and regenerated plants. Plant Mol Biol 14:735–741
Lipp-Joao KH, Brown TA (1994) Long-term stability of root cultures of tomato transformed with Agrobacterium rhizogenes R1601. J Exp Bot 45:641–647
Madhusudanan KP, Banerjee S, Khanuja SPS, Chattopadhyay SK (2008) Analysis of hairy root culture of Rauvolfia serpentina using direct analysis in real time mass spectrometric technique. Biomed Chromatogr 22:596–600
Maldonado-Mendoza IE, Ayora-Talavera T, Loyola-Vargas VM (1993) Establishment of hairy root cultures of Datura stramonium. Characterization and stability of tropane alkaloid production during long periods of sub-culturing. Plant Cell Tissue Organ Cult 33:321–329
Marconi PL, Setten LM, Calcena EN, Alvarez MA, Pitta-Alvarez SI (2008) Changes in growth and tropane alkaloid production in long-term culture of hairy roots of Brugmansia candida. Electron J Integr Biosci 3:38–44
Matsuura HN, Rau MR, Fett-Neto AG (2013) Oxidative stress and production of bioactive monoterpene indole alkaloids: biotechnological implications. Biotechnol Lett 36(2):191–200. doi:10.1007/s10529-013-1348-6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Peebles CAM, Sander GW, Li M, Shanks JV, San KY (2009) Five year maintenance of the inducible expression of anthranilate synthase in Catharanthus roseus hairy roots. Biotechnol Bioeng 102:1521–1525
Santos PM, Figueiredo AC, Oliveira MM, Barroso JG, Pedro LG, Deans SG, Younus AKM, Scheffer JJC (1999) Morphological stability of Pimpinella anisum hairy root cultures and time-course study of their essential oils. Biotechnol Lett 21:859–864
Sevon N, Hiltunen R, Oksman-Caldentey KM (1998) Somaclonal variation in transformed roots and protoplast-derived hairy root clones of Hyoscyamus muticus. Planta Med 64:37–41
Sudha CG, Obul Reddy B, Ravishankar GA, Seeni S (2003) Production of ajmalicine and ajmaline in hairy root cultures of Rauvolfia micrantha Hook f., a rare and endemic medicinal plant. Biotechnol Lett 25:631–636
Zdravkovic-Korac S, Calic D, Druart PH, Radojevic L (2003) The horse chestnut lines harboring the rol genes. Biol Plant 47:487–491
Acknowledgments
The authors wish to express their sincere thanks to the Director, CSIR-CIMAP, for providing the facilities to carry out this research. Thanks are also due to Council of Scientific & Industrial Research (CSIR), New Delhi, India, for financial support to PP, RK and SS in the form of fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pandey, P., Kaur, R., Singh, S. et al. Long-term stability in biomass and production of terpene indole alkaloids by hairy root culture of Rauvolfia serpentina and cost approximation to endorse commercial realism. Biotechnol Lett 36, 1523–1528 (2014). https://doi.org/10.1007/s10529-014-1495-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-014-1495-4