Abstract
Neural stem cells (NSCs) have great prospects in therapy for neurological disorders. However, the correlation between improved function and stem cell transplantation has not been fully elucidated. A non-invasive method for stem cell tracking is crucial for clinical studies. In the present study, NSCs were infected with lentiviral vectors, and the expression of transferrin receptor (TfR) in neural stem cells after lentivirus transfection (TfR-NSC) was confirmed by western blot analysis. TfR-NSCs were incubated with 1.8 nM ultra-small super-paramagnetic iron oxide nanoparticles (USPIOs) or transferrin (Tf)-conjugate of USPIO nanoparticles (Tf-USPIOs). Tf-USPIO enhanced the cellular iron content in TfR-NSCs 80 ± 18 % compared to USPIOs. These results demonstrated that TfR overexpressed in neural stem cells specifically internalized Tf-USPIOs. Furthermore, the results indicate that TfR reporter imaging may be a valuable way to evaluate the efficacy of neural stem cell treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neural stem cells (NSCs) are self-renewing, multipotent cells that can differentiate into neurons, astrocytes and oligodendrocytes (Alenzi and Bahkali 2011). They migrate and replace dying neurons (MacKlis et al. 2000) especially after treatment for traumatic brain injury (Ratajczak et al. 2011). However, the correlation between improved function and stem cell transplantation has not yet been fully elucidated and cell tracking studies are still at the preclinical stage. A method suitable for clinical research may be necessary before clinical application.
MRI is non-invasive and inherently three-dimensional; it provides high spatial resolution with no limitation for image depth. Currently, ultra-small super-paramagnetic iron oxide nanoparticles (USPIO) are the contrast agents most commonly used to label cells in vitro or in vivo for MRI. However, it does not account for cell viability, which results in dilution of the signal over time. This aspect of direct labeling limits its use for long-term monitoring. Unlike other methods of cell tracking, reporter genes can monitor stem cell biology in a longitudinal manner (Sun et al. 2009).
In the present study, we used human transferrin receptor (TfR), an endocytic receptor that internalizes transferrin (Tf), and have developed MRI for cell tracking by using TfR as a marker and Tf-USPIOs as MR imaging probes for this receptor. We determined whether NSCs transduced by lentivirus could stably express TfR without loss of function and whether Tf-USPIO could be specifically and efficiently internalized by TfR-NSCs and detected by MR imaging.
Materials and methods
Cell culture and virus production
NSCs were isolated and propagated by using the neurosphere method (Reynolds and Weiss 1992). They were cultured in 25-cm2 culture dishes at 500 spheres per 4 ml serum-free supplemented growth medium consisting of DMEM-F12 (HyClone, USA), Neurobasal (1:1, Gibco), B27 (1:50, Gibco), bFGF and EGF (0.02 mg/l each, Peprotech). All cell lines were cultured at 37 °C in a humidified atmosphere enriched in 5 % (v/v) CO2. The lentiviral vectors (pLenti6.3-TfR-IRES-eGFP and control pLenti6.3-MCS-IRES-eGFP) were produced in 293T cells as shown in Supplementary Fig. 1.
Transduction and selection of neural stem cells
NSCs were seeded at 8 × 104 cells per 100 mm dish 1 day before transduction. The medium was replaced with virus containing supernatant supplemented with 4 μg polybrene/ml (Sigma), and cells were incubated for 48 h. Infected stem cells were then replanted onto the standard neural stem cell culture medium. Three days later, small colonies emerged in the culture dish. Clones of NSCs were picked, expanded, and analyzed for enhanced green fluorescent protein (eGFP) expression using a MoFlo High Performance Cell Sorter (Beckman Coulter, USA). The eGFP-positive population was purified by fluorescence-activated cell sorting.
Western blotting
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (Beyotime, China). Protein samples (30 μg each) were loaded on an acrylamide/bisacrylamide SDS-PAGE gel, electrophoresed, and transferred to an Immobilon-P transfer membrane (PVDF; Bio-Rad, USA).The membranes were blocked in 5 % (w/v) non-fat dry milk/PBS for 1 h, incubated with anti-TfR antibody (1:1000; Epitomics, USA) and anti-β-actin antibody (1:1000; Beyotime, China), and subsequently incubated with secondary antibody (1:2000, Zhongshan Goldenbridge Biotech Co., Ltd., Beijing, China).
Preparation of Tf-USPIOs
USPIOs were obtained from Beijing Oneder Hightech Co. Ltd (Beijing, China), and Tf was obtained from Sigma-Aldrich. Tf-USPIOs were synthesized through 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide conjugation following the method described by Xie et al. (2011). The coupling reaction between USPIOs and Tf was investigated via the dynamic light scattering (DLS) method by monitoring the variation in the hydrodynamic size of the nanoparticles before and after the conjugation reaction. The amount of Tf in the resultant conjugate was quantified by the Bradford method.
Internalization of nanoparticles
TfR-NSCs were incubated with USPIOs and Tf-USPIOs (dissolved in medium) at different iron concentrations (0, 0.6, 1.2, and 1.8 nM) at 37 °C for 24 h. The cells were washed and collected. The intracellular iron content was determined using a fast sequential atomic absorption spectrometer as described by Xie et al. (2011).
Confocal microscopy
Cells were incubated with primary antibody (TfR, 1:100, Abcam) overnight at 4 °C, washed, and incubated with a secondary antibody conjugated to a fluorophore (1:1000, Biotium, USA) for 1 h. DAPI (Southern Biotech, USA) at 30 nM was added for 15 min at room temperature. Images were acquired using a confocal laser microscope.
Prussian blue staining of cells
Cells were incubated with 1.8 nM nanoparticles at 37 °C for 24 h, washed three times in distilled water, and fixed with paraformaldehyde (4 %, w/v). The cells were then treated with a Prussian blue staining solution containing 4 % (w/v) potassium ferrocyanide/6 % (w/v) HCl (1:1 vv) for 40 min. Subsequently, the cells were treated with Nuclear Fast Red for 5 min. The samples were then examined under a light microscope.
Transmission electron microscopy (TEM) imaging
The uptake and localization of the nanoparticles was assessed by TEM. Cells, grown on glass coverslips, were fixed with 2.5 % (w/v) glutaraldehyde in 0.1 M sodium cacodylate (pH 7.2) at 4 °C overnight. Slides were successively stained with 2 % (w/v) osmium tetroxide and 0.5 % (w/v) uranyl acetate and processed for ultrathin sectioning. Micrographs were taken with an electron microscope at 80 kV.
Cytotoxicity
The cytotoxicity of the nanoparticles was evaluated by using a cell counting kit (CCK-8; Dojindo Laboratories, Tokyo, Japan). TfR-NSCs were used for the cell viability studies. Medium containing USPIOs and Tf-USPIOs was added in a dilution series containing 0, 0.6, 1.2, 1.8, and 2.4 nM iron. The control well contained culture medium with no nanoparticles. The relative cell viability (%) was calculated by [A]test/[A]control × 100 according to the protocol of Xie et al. (2011).
Magnetic resonance imaging (MRI)
After incubation with various nanoparticles, cells were washed and then suspended in 300 μL 0.5 % agarose gel. MR images were acquired using a Bruker BioSpin 7.0 T scanner (Ettlingen, Germany). A total of 8 × 105 cells were embedded in gelatin for MR measurement according to a T2-weighted (T2w) sequence [FR-FSE (fast-recovery fast spin echo):TR (repetition time), 2500 ms; TE (echo time), 10, 20, 30, and 40 ms; FOV (field of view), 4 × 4 cm; matrix, 256 × 256; slice thickness, 2 mm].
Statistical analysis
Data were expressed as the mean ± standard deviation, and a paired t test was used to evaluate the statistical significance. A value less than 0.05 (P < 0.05) was considered statistically significant.
Results
Efficient lentivirus-mediated TfR gene transfer and selection
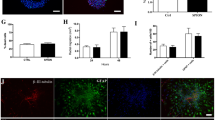
We used a lentiviral vector carrying a double-fusion reporter gene that stably expressed eGFP and TfR. Living cells were observed with a fluorescence microscope 48 h post-transfection. A strong green signal was visible in approx. 30–40 % of the cells, indicating that the transfection efficiency of the cells was 30–40 %. For stable transfection, the cells were screened for eGFP expression using a MoFlo High-Performance Cell Sorter. After three sorting rounds, more than 95 % of the population were eGFP-TfR(+) (Fig. 1).
A vector encoding a TfR-GFP fusion protein was transfected into NSCs. a Transfected cells visualized with fluorescent (left) and normal light (right) microscopes. Scale bars = 200 μm. b eGFP-positive cells were sorted and analyzed by FACS: 98.9 % of the cells were eGFP-positive after three rounds of sorting
Visualization of TfR expression in NSCs
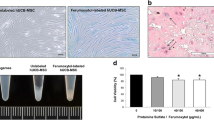
The degree of TfR expression in NSCs was visualized by western blot and confocal microscopy. TfR expression was observed in NSCs treated with lentivirus. Images of NSCs expressing TfR-eGFP complexes are shown in Fig. 2a. The protein expression induced by the transfection of NSCs was analyzed by western blot with TfR-specific antibodies. As shown in Fig. 2b, the TfR level in TfR-NSCs increased significantly.
Analysis of TfR-NSCs and Tf-USPIOs. a Confocal fluorescence microscopy of TfR-NSCs. Cells were double immunolabeled for eGFP (green) and TfR (red), and the nuclei were stained with DAPI (blue). b Western blot analysis in TfR-NSCs, eGFP-NSCs, and untransfected NSCs (UT). c Hydrodynamic size distribution profiles of the PEG-coated USPIO and the USPIO-Tf conjugate. Data are presented as the mean ± SD from three independent measurements
Characterization of Tf-USPIOs
The covalent coupling between USPIOs and Tf was investigated by the DLS method. The results (Fig. 2c) revealed that the initial hydrodynamic size of the USPIOs was 36 ± 0.7 nm, and the hydrodynamic size of the conjugates increased to 43.5 ± 0.08 nm. The size of Tf was about 4–5 nm as described by Pitek et al. (2012). The reasonable increase in the hydrodynamic size suggests that Tf coupled to the PEG-coated USPIOs via the EDC/sulfo-NHS mediated amidation reaction according to the protocol of Hu et al. (2006). The Bradford method was adopted to determine the quantity of Tf in an equal amount of the conjugates. In this way, approx. 10 Lf molecules were bound to each USPIO particle on average.
Analysis of nanoparticle uptake and cytotoxicity in TfR-NSCs
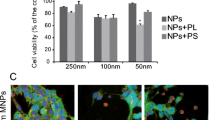
TfR-NSCs internalized Tf-USPIOs more efficiently than USPIOs at different nanoparticle concentrations (Fig. 3a): Tf-USPIOs enhanced the cellular iron content by 80 ± 18 % compared to bare USPIOs. After Prussian blue staining, TfR-NSCs incubated with Tf-USPIOs (Fig. 3b) contained large amounts of iron particles in the cytoplasm, whereas only a few particles were observed in the cytoplasm of cells cultured with USPIOs. The subcellular localization of the particles was observed by TEM after incubation with Tf-USPIO for 24 h. Large amounts of Tf-USPIOs were internalized and accumulated in the cytoplasm of TfR-NSCs (Fig. 3c). By contrast, very few USPIOs were seen in TfR-NSCs. Neither USPIOs nor Tf-USPIOs affected the metabolic activity in a concentration-dependent manner when added to the cells in the range of 0–2.4 nM (iron concentration; Fig. 3d).
Nanoparticles uptake and cytotoxicity assays. a Uptake curve of TfR-NSCs incubated with USPIOs or Tf-USPIOs. Data are presented as the mean ± SD from three independent measurements. **P < 0.01, ***P < 0.001. b TfR-NSCs incubated with 1.8 nM Tf-USPIOs (right) and USPIOs (left). Scale bars = 30 μm. c TEM images of Tf-USPIOs (right) and USPIOs (left). Scale bars = 0.5 μm. d CCK-8 cytotoxicity results for Tf-USPIOs or USPIOs. Data are presented as the mean ± SD from three independent measurements
Magnetic resonance imaging (MRI)
MR T2-weighted images of the nanoparticles cultured with TfR-NSCs are shown in Fig. 4. Cells treated with Tf-USPIOs showed a significant negative contrast enhancement, compared to cells incubated with bare USPIOs.
Discussion
In this study, we labeled NSCs using ligand-mediated receptor endocytosis. We showed that: 1. TfR, as an imaging marker transfected into NSCs, had no effect on cell activity; 2. TfR-NSCs specifically internalized Tf-USPIOs; 3. Tf-USPIOs were detectable by MR imaging.
TfR, the receptor for Tf, imports iron into cells by internalizing the Tf-iron complex through receptor-mediated endocytosis (Qian et al. 2002). To our knowledge, we are the first to transfect the TfR gene into NSCs. Using lentivirus, exogenous TfR was integrated into the genome of NSCs and continuous expression was obtained. After screening by flow cytometry, the expression of TfR was confirmed by western blot. Confocal microscopy showed that the TfR-eGFP fusion protein was expressed in NSCs. The average particle size of Tf-USPIOs was 43.5 ± 0.08 nm, even smaller than that of lactoferrin-conjugated super-paramagnetic iron oxide nanoparticles (Lf-SPIONs), which have been proven suitable for use as a negative MRI contrast agent. Lactoferrin (Lf) belongs to the Tf family. Lf-SPIONs were synthesized with Lf and SPIONs by the EDC method. Tf-USPIOs and Lf-SPIONs displayed similar magnetic properties. Hence, Tf-USPIOs are conducive to small cell endocytosis and imaging with MR. Figure 2c shows the curve of the conjugates is broader than that of USPIOs, but the variation of the conjugates is smaller than that of the USPIOs. There may be two reasons for the variation in material sciences: First, the conjugates do not contain the same number of Tfs; Second, the conjugates may contain unbound USPIO particles.
TfR expression and regulation can be visualized by NMR imaging using the magnetic TfR probe, and magnetic Tf conjugates can be used to amplify receptor expression (Moore et al. 1998). Tf-USPIOs and USPIOs were suitable for MR imaging (Wang et al. 2010). Tf-TfR plays a primary role in iron uptake across the blood brain barrier (BBB) (Moos et al. 2007). Scientists have developed a brain delivery probe by covalently conjugating lactoferrin to PEG-coated Fe3O4 nanoparticles to achieve receptor-mediated delivery of nanoparticles across the BBB (Qiao et al. 2012). In our work, Tf-USPIOs and USPIOs were added to the cell culture medium at different concentrations (0, 0.6, 1.2, and 1.8 nM) for 24 h. Prussian blue staining and TEM imaging showed that the uptake of Tf-USPIOs in TfR-NSCs was significantly higher than the uptake of USPIOs; this result was confirmed by measuring the cellular iron content (P < 0.01). A 7.0T MRI was used to analyze the MR signal intensity. A significant T2 signal decrease was observed with Tf-USPIO compared to USPIO. These findings demonstrate that Tf-USPIOs are a potential targeting MR contrast agent for TfR-NSCs. It was earlier reported that after blocking TfR with free Tf, the amount of iron content in the cytoplasm of transfected cells with TfR gene was decreased (Wang et al. 2010). The fate of Tf-USPIOs is one of problems in long-term tracking and they may leak out of cells due to exocytosis or cell death (Tachibanaa et al. 2010).
The toxicity of Tf-USPIOs is one of the most important concerns for their application. The CCK-8 assay showed that the cells grew normally even when exposed to a high concentration of Tf-USPIOs, and no noticeable cytotoxic effect was observed. Other studies have also shown nanoparticles have no significant cytotoxic effects on cells. Thus, iron oxide nanoparticles appear to be satisfactory in view of their proposed use as a single-dose diagnostic agent in human (Bourrinet et al. 2006). The differentiation potential of cells internalizing the Tf-USPIOs is also important. It has been reported that USPIOs labeling does not negatively affect cellular differentiation (Bakhru et al. 2012); however, additional future studies will be warranted to assess the genomic-level influence of Tf-USPIOs on stem cells before application.
Despite successfully demonstrating the feasibility of labeling TfR-NSCs with Tf-USPIOs and detecting Tf-USPIOs by magnetic resonance imaging, this study has several limitations. First, the experiment was carried out in vitro; we do not know if there are differences between in vitro and in vivo experiments. This will be addressed in our future research. Second, TfR is overexpressed in several malignancies, such as glioma, colon cancer, pancreatic cancer, and breast cancer (Recht et al. 1990; Prutki et al. 2006; Ryschich et al. 2004; Habashy et al. 2010). The risk of insertional mutagenesis may be increased (Baum et al. 2004). Finally, a lentiviral vector was used for gene transfer; this method has potential limitations and risks, including unfavorable immunological features.
In summary, we have successfully constructed a neural stem cell line expressing TfR that can specifically endocytose Tf-USPIOs. The cells could be useful in preclinical and clinical trials for cell tracking with MRI.
References
Alenzi F, Bahkali A (2011) Stem cells: biology and clinical potential. Afr J Biotechnol 10:19929–19940
Bakhru SH, Altiok E, Highley C, Delubac D, Suhan J, Hitchens TK, Ho C, Zappe S (2012) Enhanced cellular uptake and long-term retention of chitosan-modified iron-oxide nanoparticles for MRI-based cell tracking. Int J Nanomed 7:4613–4623
Baum C, von Kalle C, Staal FJ, Li Z, Fehse B, Schmidt M, Weerkamp F, Karlsson S, Wagemaker G, Williams DA (2004) Chance or necessity? Insertional mutagenesis in gene therapy and its consequences. Mol Ther 9:5–13
Bourrinet P, Bengele HH, Bonnemain B, Dencausse A, Idee JM, Jacobs PM, Lewis JM (2006) Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultra-small super-paramagnetic iron oxide magnetic resonance contrast agent. Invest Radiol 41:313–324
Habashy HO, Powe DG, Staka CM, Rakha EA, Ball G, Green AR, Aleskandarany M, Paish EC, Douglas Macmillan R, Nicholson RI, Ellis IO, Gee JM (2010) Transferrin receptor (cd71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat 119:283–293
Hu FQ, Wei L, Gao MY et al (2006) Preparation of biocompatible magnetite nanocrystals for in vivo magnetic resonance detection of cancer. Adv Mater 18:2553–2556
MacKlis JD, Magavi SS, Leavitt BR (2000) Induction of neurogenesis in the neocortex of adult mice. Nature 405:951–955
Moore A, Basilion JP, Chiocca EA, Weissleder R (1998) Measuring transferrin receptor gene expression by NMR imaging. Biochim Biophys Acta 1402:239–249
Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH (2007) Iron trafficking inside the brain. J Neurochem 103:1730–1740
Pitek AS, Mahon E, Monopoli MP et al (2012) Transferrin coated nanoparticles: study of the bionano interface in human plasma. PLoS ONE 7:e40685
Prutki M, Poljak-Blazi M, Jakopovic M, Tomas D, Stipancic I, Zarkovic N (2006) Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett 238:188–196
Qian ZM, Li H, Sun H, Ho K (2002) Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 54:561–587
Qiao R, Jia Q, Hüwel S, Xia R, Liu T, Gao F, Galla HJ, Gao M (2012) Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 6:3304–3310
Ratajczak J, Zuba-Surma E, Paczkowska K, Kucia M, Nowacki P, Ratajczak MZ (2011) Stem cells for neural regeneration-a potential application of very small embryonic-like stem cells. J Physiol Pharmacol 62:3–12
Recht L, Torres CO, Smith TW, Raso V, Griffin TW (1990) Transferrin receptor in normal and neoplastic brain tissue: implications for brain-tumor immunotherapy. J Neurosurg 72:941–945
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Ryschich E, Huszty G, Knaebel HP, Hartel M, Buchler MW, Schmidt J (2004) Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas. Eur J Cancer 40:1418–1422
Sun N, Lee A, Wu JC (2009) Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat Protoc 4:1192–1201
Tachibanaa Y, Enmib J, Maharaa A, Iidab H, Yamaoka T (2010) Design and characterization of a polymeric MRI contrast agent based on PVA for in vivo living-cell tracking. Contrast Media Mol Imaging 5:309–317
Wang K, Wang K, Shen B, Huang T, Sun X, Li W, Jin G, Li L, Bu L, Li R, Wang D, Chen X (2010) MR reporter gene imaging of endostatin expression and therapy. Mol Imaging Biol 12:520–529
Xie H, Zhu Y, Jiang W, Zhou Q, Yang H, Gu N, Xu H, Xu H, Yang X (2011) Lactoferrin-conjugated super-paramagnetic iron oxide nanoparticles as a specific MRI contrast agent for detection of brain glioma in vivo. Biomaterials 32:495–502
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30973225 and 81271316).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yongkun Guo and Yesen Zhang have contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Guo, Y., Wu, B. et al. Ligand-mediated endocytosis of nanoparticles in neural stem cells: implications for cellular magnetic resonance imaging. Biotechnol Lett 35, 1997–2004 (2013). https://doi.org/10.1007/s10529-013-1304-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1304-5