Abstract
Transglutaminase-mediated cross-linking has been employed to optimize the mechanical properties and stability of tissue scaffolds. We have characterized tissue transglutaminase (TG2)-mediated cross-linking as a useful tool to deliver biologically-active TGF to mesenchymal stem cells (MSCs) and direct their differentiation towards a chondrogenic lineage. TGF-β3 is irreversibly cross-linked by TG2 to collagen type II-coated poly(l-lactic acid) nanofibrous scaffolds and activates Smad phosphorylation and Smad-dependent expression of a luciferase reporter. Human bone marrow-derived MSCs cultured on these scaffolds deposit cartilaginous matrix after 14 days of culture at 50 % efficiency compared to chondrogenesis in the presence of soluble TGF-β3. These findings are significant because they suggest a novel approach for the programming of MSCs in a spatially controlled manner by immobilizing biologically active TGF-β3 via cross-linking to a collagen-coated polymeric scaffold.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transglutaminases (TGases) function by catalyzing the formation of covalent N-ϵ-(γ-glutamyl) lysine amide bonds between individual protein strands to form a permanent network of polypeptides. In mammals, the family of TGases is composed of eight catalytically-active enzymes expressed in various patterns in virtually all organs and tissues (Nurminskaya and Belkin 2012; Lorand and Graham 2003).The ubiquitous presence of cross-links in the body as mediated by TGase suggests that biomaterials cross-linked by TGases would have improved biocompatibility as well as enhanced stability (Collighan and Griffin 2009). Multiple studies have shown that the cross-linking of biomaterials by tissue transglutaminase (also known as transglutaminase 2 or TG2) enhances cell attachment, spreading, and differentiation at the biomaterial interface. Several mechanisms have been suggested to underlie these effects including: alterations in the elasticity of cross-linked matrices (Chau et al. 2005); crosslinking of cell surface-associated fibronectin that may affect its binding to integrins (Akimov et al. 2000; Chau et al. 2005) and thus alter matrix–cell interactions and downstream signaling; and regulation of intracellular signaling by TG2 auto-cross-linking to protein-coated scaffolds (Shanmugasundaram et al. 2012a) due to the ability of TG2 to interact with various cell surface receptors (Nurminskaya and Belkin 2012).
In articular cartilage, TG2 contributes to the resistance of tissue to high stress and to control cell differentiation (Demignot et al. 1995; Rosenthal et al. 1997; Summey et al. 2002; Johnson et al. 2001). In addition, TG2-mediated cross-linking of a number of cartilage components including fibronectin, collagen II, osteonectin, and osteopontin (Jones and Messersmith 2007) is believed to improve matrix stability. In cartilage tissue engineering, TG2 has been explored for several applications. TG2 cross-linking of collagen matrices promoted chondrogenic differentiation in human bone marrow-derived mesenchymal stem cells (hBMSC) (Shanmugasundaram et al. 2012a) and helped to sustain the phenotype of intervertebral disc fibrochondrocytes incorporated into a mixture of elastin-like peptides (McHale et al. 2005). TG2 has been also used as biological glue in the repair of articular cartilage, improving the adhesive strength between two pieces of cartilage by as much as 40 %—superior to a commercial tissue sealant (Jurgensen et al. 1997). In addition, TG2 cross-linking of biologically active molecules has been used as an effective approach to functionalize biomaterials. For example, fibrin matrix modified by cross-linking the active fragment of human parathyroid hormone (PTH(1-34)) supported improved bone formation in vivo (Arrighi et al. 2009).
Cartilage bioengineering with stem cells (including hBMSCs) commonly depends on the presence of TGF growth factors to induce chondrogenic differentiation and promote deposition of cartilaginous matrix (reviewed by Freyria and Mallein-Gerin 2012). The main drawback of TGFs is their cost when used continuously as a culture medium supplement for weeks to stimulate chondrogenesis in vitro. We proposed that TGF-β can be cross-linked to a matrix, providing high local morphogen concentrations and sustained presence to direct cell differentiation. Here, we employed TG2 to functionalize a collagen matrix with cross-linked TGF-β3 protein and tested the ability of the modified scaffold to stimulate chondrogenic differentiation of hBMSCs. We show that TGF-β3 cross-linked to collagen-coated poly(l-lactic acid) (PLLA) scaffolds by TG2 retains biological activity and induces chondrogenesis in hBMSCs. This proof-of-principle study identifies a new application for TG2 in tissue engineering to fabricate pre-cast functionalized scaffolds that can drive local differentiation of MSCs in vivo.
Materials and methods
PLLA scaffold preparation
Biodegradable nanofibrous scaffolds were prepared by electrospinning of PLLA (Sigma-Aldrich) in a 10–12 % (w/v) hexafluoroisopropanol (HFIP) (Sigma-Aldrich, MO) as described in our previous study (Shanmugasundaram et al. 2012a). In brief, electrospinning was performed at room temperature and at 3 ml/h at 20 kV. The average nanofibrous scaffolds were approx. 150 μm in thickness. Prior to use, PLLA scaffolds were cut into disks of 6 mm diameter for 96-well plates and 11 mm diameter for 24-well plates. Scaffolds were washed in 70 % (v/v) ethanol, then in PBS, and coated overnight with 0.1 mg/cm2 collagen type I or collagen type II (Chondrex, Inc., WA) according to the published protocol (Shanmugasundaram et al. 2012a). To cross-link TGF-β3 to collagen-coated PLLA nanofibers, 0.01 U/ml purified liver TG2 enzyme (Sigma-Aldrich, MO) was incubated with scaffolds and 10 ng/ml TGF-β3 (ProSpec, NJ) for 8 h. Cross-linked scaffolds were rinsed in PBS to remove non-bound TGF-β3 and TG2 before use for cell cultures.
hBMSC expansion and culture
Three independent samples of hBMSCs were purchased from Lonza Walkersville Inc., MD. Cells were expanded in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10 % (v/v) fetal bovine serum and 1 % antibiotic/antimycotic (Invitrogen, CA) at 37 °C, 5 % CO2. Cells were used at passages 2 and 3.
Cell attachment assay and cell spreading analysis
hBMSCs attached on collagen-coated PLLA scaffolds were detected using the calcein AM cell viability kit according to the manufacturer’s instructions (Trevigen, MD) at 2 and 18 h after seeding. For quantitative assessment, cells were counted with a hemacytometer under a fluorescence microscope. At least four fields per scaffold were analyzed and at least three scaffolds per condition were used. To analyze cell spreading, FITC-conjugated anti-actin antibody (AC-40, 1:250, Abcam) was used on fixed cells permeabilized with 0.01 % Triton X-100. Nuclei were counterstained with diamidino-2-phenylindole.
hBMSC differentiation
For hBMSC chondrogenesis, cells were seeded at 1.75 × 105/cm2 (2.5 × 104 cells per well in a 96-well format), and maintained in chondrogenic medium [DMEM–high glucose supplemented with 10−7 M dexamethasone, 0.1 mM ascorbic acid, 1 % ITS premix (BD Biosciences, NJ), 1 mM sodium pyruvate, 0.35 mM l-proline, 4 mM l-glutamine and 1 % penicillin/streptomycin. Purified TGF-β3 (ProSpec, NJ) was either added to the chondrogenic medium at 10 ng/ml or cross-linked to the scaffolds by TG2 as described above. Medium was changed twice a week. Quantitative analysis of the deposited sulfated glycosaminoglycan (sGAG) was performed with Blyscan kit following a 3 h papain extraction at 65 °C. Cell number was estimated with Crystal Violet stain.
Luciferase assay
TGFβ-dependent luciferase reporter (encoding luciferase under a Smad-dependent promoter) and control “promoterless” A10 VSMC lines were established by stable transduction with Cignal Reporter lentiviruses (Invitrogen) (Beazley et al. 2012). hBMSCs were infected with lentivirus encoding Smad-dependent luciferase (Invitrogen) and plated on PLLA scaffolds for 48 h. Cell lysates were prepared using Promega reagents (cell lysis buffer) and frozen at −20 °C. Luciferase activity was measured in lysates using Luciferase Assay System (Promega) and normalized to total LDH activity in the lysate, determined using the LDH cytotoxicity kit (BioVision, CA).
Results and discussion
Collagen coating of PLLA scaffolds promotes cell adhesion and chondrogenesis
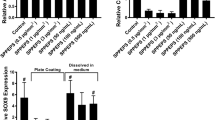
To optimize the experimental model, we examined adhesion and chondrogenic differentiation of hBMSCs seeded on PLLA scaffolds coated with either collagen type I or collagen type II. Both collagens enhanced cell adhesion and spreading on nanofibrous scaffolds within 2 h (Fig. 1a) but collagen type II was slightly more efficient after a longer incubation (up to 18 h) (Fig. 1b). Similarly, TGF-β3 induced chondrogenesis was more efficient in hBMSCs cultured on the collagen type II-coated PLLA scaffolds for 14 days, as estimated by the deposition of Alcian Blue-positive sGAG matrix per cell (Fig. 1c). Therefore, this type of scaffold was chosen for further experiments.
Optimization of the experimental model of chondrogenesis in BMSC on nanofibrous PLLA scaffolds by collagen coating. a Visualization of cell morphology by immunostaining for actin cytoskeleton in hBMSCs cultured on non-coated PLLA scaffolds (Ctrl) or scaffolds coated with collagen type I (Coll I) or type II (Coll II). b, c Quantitation of cell attachment and spreading of hBMSCs on PLLA scaffolds either non-coated (Ctrl) or coated with collagen type I (C-I) or collagen type II (C-II). b Cell attachment is shown as fold increased in the number of attached cells compared to control PLLA scaffolds. Collagen coating enhances attachment (3 independent experiments, **p < 0.01). c Deposition of cartilaginous glycosaminoglycan (GAG) rich matrix by hBMSCs is enhanced on the collagen type II-coated PLLA scaffolds compared to collagen type I-coating as compared to GAG deposition on control untreated PLLA scaffolds
TG2 cross-linked TGF-β3 retains its biological activity
TG2-mediated cross-linking of proteins can alter their activities (Cooper et al. 1997). Therefore, we sought to examine whether TGF-β3 retains its biological activity after cross-linking to collagen type II-coated PLLA nanofibers. The hBMSCs infected with Smad-dependent luciferase reporter virus (Smad-hBMSCs) were divided into 4 groups: (1) cells grown on collagen II-coated PLLA (coll II-PLLA) scaffolds, (2) cells grown on coll II-PLLA scaffolds that were pre-incubated with TG2 alone, (3) cells grown of coll II-PLLA scaffolds pre-incubated with TGF-β3 alone, and (4) cells grown on coated coll II-PLLA scaffolds pre-incubated with TG2 and TGF-β3. Smad-dependent luciferase expression was analyzed in all groups 48 h after seeding on the scaffolds. In groups 1–3, no significant induction of luciferase was observed (Fig. 2a, black bars). In contrast, in Smad-hBMSCs grown on coll II-PLLA scaffolds with TGF-β3 cross-linked by TG2, an approx. fourfold (p < 0.05) induction of Smad-dependent luciferase expression was detected (Fig. 2a, dark gray bar). Control Smad-hBMSCs grown on coll II-PLLA scaffolds in the continuous presence of soluble 10 ng/ml TGF-β3 expressed only 50 % more luciferase (Fig. 2a, light gray bar) suggesting a specific effect of cross-linked TGF-β3.
TGF-β3 cross-linked to collagen-coated PLLA nanofibers retains its biological activity: a expression of the Smad-dependent luciferase reporter in hBMSCs cultured for 48 h on scaffolds pre-treated for 8 h with TG2 (0.01 U/ml) alone, TGF-β3 (10 ng/ml) alone, or both in combination to allow for TGF-β3 cross-linking to the scaffolds. Cell exposed to soluble TGF-β3 (10 ng/ml) were used as a positive control. Two independent experiments with three repeats in each were performed (*p < 0.05). b Levels of phospho-Smad2/3 were analyzed in equal numbers of hBMSCs seeded on the PLLA scaffolds in the absence of TGF-β3, or in the presence of TG2 cross-linked TGF-β3 or soluble TGF-β3 (10 ng/ml). The bar graph presents quantitative analysis of a similar Western blot study for levels of phosphorylated Smad2/3 proteins in hBMSCs cultured for 48 h on PLLA scaffolds cross-linked with TG2 (0.01U/ml) and the indicated amounts of TGF-β3. Cells exposed to soluble TGF-β3 at the same concentrations served as positive controls
Activation of Smad-dependent signaling in hBMSCs grown on the TGF-β3-modified scaffolds was further confirmed by the analysis of Smad phosphorylation (Shi and Massague 2003). In this study, we compared the dose-dependent effects of TGF-β3, either cross-linked to the scaffold or supplemented to the medium, on Smad phosphorylation. Soluble TGF-β3 efficiently induced Smad phosphorylation at 10 ng/ml and this effect was only slightly enhanced at higher doses of TGF-β3 (Fig. 2b). In contrast, Smad phosphorylation in hBMSCs seeded on coll II-PLLA scaffolds pre-incubated with the TG2/TGF-β3 mixture increased in a dose-dependent manner (Fig. 2b), suggesting that either only a fraction of TGF-β3 molecules were attached to the collagen-coated scaffold or that partial reduction in the activity of TGF-β3 results from its cross-linking. In summary, these experiments established that biologically active TGF-β3 can be cross-linked by TG2 to collagen-coated PLLA scaffolds and suggested that the TGF-collagen scaffolds may stimulate chondrogenic differentiation in hBMSCs.
Irreversible cross-linking of TGF-β3 to collagen II scaffolds by TG2
In the next experiment, we addressed whether TG2-mediated cross-linking of TGF-β3 to coll II-PLLA scaffolds is reversible and whether the growth factor is released from the scaffold with time. Coll II-PLLA scaffolds were first incubated with TG2 and 5, 10 or 20 ng TGF-β3/ml and then incubated in serum-free medium for 1, 3 or 7 days. Medium conditioned by these scaffolds was tested on TGFβ-dependent luciferase reporter cells for its ability to induce luciferase expression indicative of the presence of soluble TGF-β3. Cells exposed to medium containing 10 ng soluble TGF-β3/ml were used as a positive control in this analysis to estimate the possible levels of luciferase induction. As shown in Fig. 2c, none of the media conditioned by TG2/TGF-β3-modified scaffolds induced luciferase expression indicating that TGF-β3 is irreversibly cross-linked to scaffolds, and is not released into the medium, allowing for the precise local delivery of this morphogen on modified scaffolds in vivo.
TGF-β3 requirement is limited to early stages of in vitro chondrogenesis
It is intuitive to assume that cells seeded on the TGF-β3-modified scaffolds would be exposed to the active growth factor for a limited time since chondrogenic differentiation associates with induction of proteases that may remove scaffold proteins by proteolysis. Therefore, we examined whether a short-term exposure to TGF-β3 is sufficient to stimulate chondrogenic differentiation in hBMSCs cultured on the coll II-PLLA scaffolds. In these studies, cells were exposed to chondrogenic medium supplemented with TGF-β3 for 3 or 7 days only and then the medium was replaced with 10 % FBS without TGF-β3. In control cultures, cells were continuously exposed to the TGF-β3-containing chondrogenic medium for 14 days (Fig. 3a). Even a 3-day treatment with TGF-β3 was sufficient to induce a 28 % (±4 %, p < 0.02) increase in cartilaginous GAG deposition, while a 7-days exposure induced an additional 43 % (±15 %, p < 0.01) increase in GAG deposition as compared to untreated control cultures. Interestingly, the presence of TGF-β3 in the culture medium for an additional week, from day 7 to day 14, had only a slight and statistically insignificant effect on GAG production as compared to the 7 day treatment (p > 0.05), suggesting that TGF-β3 acts to commit hBMSCs to chondrogenic lineage and supports early stages of differentiation but is less involved in maturation of the committed chondrocytes. This result assures the feasibility of inducing chondrogenesis in hBMSCs by cross-linked TGF-β3 even if this activity diminishes with time.
Regulation of chondrogenic differentiation in hBMSCs by TGF-β3: a deposition of GAG-rich matrix by hBMSCs is stimulated by soluble TGF-β3 (10 ng/ml) supplemented to cells for only 3 or 7 days following cell seeding. Cells exposed to soluble TGF-β3 for 14 days served as positive control. Three independent experiments with three repeats were performed. (*p < 0.05, **p < 0.01). b GAG deposition was analyzed in hBMSCs cultured on collagen type II-coated PLLA nanofiber scaffolds for 14 days. Prior to cell seeding, scaffolds were incubated overnight with TG2 (0.01 U/ml), TGF-β3 (10 ng/ml) or a mixture of the two to allow TGF-β3 cross-linking to the scaffolds. Cell-seeded scaffolds exposed to fresh aliquots of soluble TGF-β3 for 14 days served as a positive control. Experiments were repeated in triplicate. *p < 0.05
TGF-β3 cross-linked to scaffold by TG2 promotes chondrogenic differentiation in hBMSCs
Lastly, we analyzed GAG deposition by hBMSCs induced to undergo chondrogenic differentiation on coll II-PLLA scaffolds in the presence of either cross-linked or soluble TGF-β3. A significant 25 % increase in GAG deposition was induced by cross-linked TGF-β3 (p < 0.05) and this constituted about one-third of the GAG production induced by the common protocol with continuous presence of soluble TGF-β3 (Fig. 3b). Therefore, our study demonstrates for the first time that TGF-β3 irreversibly cross-linked by TG2 to coll II-PLLA scaffolds retains its biological activity and is capable of inducing chondrogenic differentiation of hBMSCs. These findings are significant because they provide a novel approach for local programming of MSCs for chondrogenic differentiation. Recent identification of endogenous mesenchymal cells and progenitors in the articular cartilage (Alsalameh et al. 2004; Williams et al. 2010) combined with the described new approach for local stimulation of their differentiation by immobilized TGFβ may define a new strategy for the induction of chondrogenic differentiation in vivo in a desired location such as damaged articular cartilage, thus avoiding common in vitro manipulations with cells pre-implantation. In addition, immobilization of biologically active TGFβ by TG2-mediated cross-linking may be useful in efficient design of dual MSC-derived tissue implants, such as osteochondral constructs.
Previously, we have shown that TG2 crosslinks collagen type XI absorbed on PLLA scaffolds and auto-crosslinks itself. These modifications stimulate cell adhesion in hBMSCs and promote the deposition of cartilaginous matrix (Shanmugasundaram et al. 2012b). Further, TG2 attenuates later stage chondrogenic differentiation in hBMSCs (Shanmugasundaram et al. 2012b) and mesenchymal limb bud progenitors (Nurminsky et al. 2011) delaying their transition into the pre-hypertrophic stage. In contrast, continuous exposure of committed articular chondrocytes to TG2 has been shown to stimulate abnormal maturation towards hypertrophy in these cells (Johnson et al. 2003). Stage-specific expression of various cell surface receptors during chondrogenic differentiation may underlie this twofold effect of TG2. As discussed above, it is intuitive to assume that proteases secreted by differentiating hBMSCs will rapidly remove from the modified scaffold both TGF-β3 and TG2 (assuming its auto-crosslinking to collagen type II), and therefore the putative effect of TG2 will be limited to enhancement of the initial stages of TGF-β3-induced chondrogenesis including cell adhesion.
A new finding of this study is that TG2 cross-links active TGF-β3 to collagen-coated scaffolds in an amount sufficient to support chondrogenic differentiation in hBMSCs. Our results demonstrating that soluble TGF-β3 was more efficient than the immobilized form in inducing GAG production suggest that cross-linking of the TGF-β3 molecules to collagen-coated scaffolds may be limited by the restricted number of glutamine sites for TG2-mediated crosslinking in collagen type II similar to the previously described collagens type III, V, XI and XVI (Bowness et al. 1989; Kleman et al. 1995; Akagi et al. 2002). Mixing different collagens with each other or with non-collagenous matrix proteins, such as fibronectin, may allow for a more efficient incorporation of TGF-β3 to generate a more potent pro-chondrogenic scaffold. Studies are underway to optimize the amount of cross-linked TGF-β3 to achieve maximal efficiency of chondrogenesis in hBMSCs.
Conclusions
To date, TG2 has been utilized in tissue bioengineering mostly to increase the mechanical stability of various bioengineered scaffolds (Orban et al. 2004; Chau et al. 2005; Garcia et al. 2009; Spurlin et al. 2009; Ciardelli et al. 2010). Here, we demonstrate that TG2-mediated cross-linking is a useful tool to deliver in a spatially controlled manner biologically active TGFβ capable of directing the differentiation of hBMSCs towards a chondrogenic lineage. In contrast to the PTH peptide that required functionalization with a transglutaminase substrate sequence (Arrighi et al. 2009), TGF-β3 by itself is a substrate for TG2 and can be directly cross-linked to collagen type II matrix. Therefore, the TGF-β3-enriched collagen-coated PLLA scaffolds described here may be directly implanted into damaged cartilage to instruct neocartilage formation by mesenchymal cells. Further, it is expected that TG2-mediated cross-linking of other growth factors to similar scaffolds may find applications in various fields of tissue engineering.
References
Akagi A, Tajima S, Ishibashi A, Matsubara Y, Takehana M, Kobayashi S, Yamaguchi N (2002) Type XVI collagen is expressed in factor XIIIa + monocyte-derived dermal dendrocytes and constitutes a potential substrate for factor XIIIa. J Investig Dermatol 118:267–274
Akimov SS, Krylov D, Fleischman LF, Belkin AM (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148:825–838
Alsalameh S, Amin R, Gemba T, Lotz M (2004) Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum 50:1522–1532
Arrighi I, Mark S, Alvisi M, von Rechenberg RB, Hubbell JA, Schense JC (2009) Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials 30:1763–1771
Beazley KE, Eghtesad S, Nurminskaya MV (2012) Quercetin attenuates warfarin-induced vascular calcification in vitro independently from Matrix Gla protein. J Biol Chem 288(4):2632–2640
Bowness JM, Tarr AH, Wiebe RI (1989) Transglutaminase-catalysed cross-linking: a potential mechanism for the interaction of fibrinogen, low density lipoprotein and arterial type III procollagen. Thromb Res 54:357–367
Chau DY, Collighan RJ, Verderio EA, Addy VL, Griffin M (2005) The cellular response to transglutaminase-cross-linked collagen. Biomaterials 26:6518–6529
Ciardelli G, Gentile P, Chiono V, Mattioli-Belmonte M, Vozzi G, Barbani N, Giusti P (2010) Enzymatically crosslinked porous composite matrices for bone tissue regeneration. J Biomed Mater Res A 92:137–151
Collighan RJ, Griffin M (2009) Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 36:659–670
Cooper AJ, Sheu KR, Burke JR, Onodera O, Strittmatter WJ, Roses AD, Blass JP (1997) Transglutaminase-catalyzed inactivation of glyceraldehyde 3-phosphate dehydrogenase and alpha-ketoglutarate dehydrogenase complex by polyglutamine domains of pathological length. Proc Natl Acad Sci USA 94:12604–12609
Demignot S, Borge L, Adolphe M (1995) Transglutaminase activity in rabbit articular chondrocytes in culture. Biochim Biophys Acta 1266:163–170
Freyria AM, Mallein-Gerin F (2012) Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury 43:259–265
Garcia Y, Hemantkumar N, Collighan R, Griffin M, Rodriguez-Cabello JC, Pandit A (2009) In vitro characterization of a collagen scaffold enzymatically cross-linked with a tailored elastin-like polymer. Tissue Eng Part A 15:887–899
Johnson K, Hashimoto S, Lotz M, Pritzker K, Terkeltaub R (2001) Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am J Pathol 159:149–163
Johnson KA, van Etten D, Nanda N, Graham RM, Terkeltaub RA (2003) Distinct transglutaminase 2-independent and transglutaminase 2-dependent pathways mediate articular chondrocyte hypertrophy. J Biol Chem 278:18824–18832
Jones ME, Messersmith PB (2007) Facile coupling of synthetic peptides and peptide-polymer conjugates to cartilage via transglutaminase enzyme. Biomaterials 28:5215–5224
Jurgensen K, Aeschlimann D, Cavin V, Genge M, Hunziker EB (1997) A new biological glue for cartilage–cartilage interfaces: tissue transglutaminase. J Bone Joint Surg Am 79:185–193
Kleman JP, Aeschlimann D, Paulsson M, van der Rest M (1995) Transglutaminase-catalyzed cross-linking of fibrils of collagen V/XI in A204 rhabdomyosarcoma cells. Biochemistry 34:13768–13775
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4:140–156
McHale MK, Setton LA, Chilkoti A (2005) Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng 11:1768–1779
Nurminskaya MV, Belkin AM (2012) Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol 294:1–97
Nurminsky D, Shanmugasundaram S, Deasey S, Michaud C, Allen S, Hendig D, Dastjerdi A, Francis-West P, Nurminskaya M (2011) Transglutaminase 2 regulates early chondrogenesis and glycosaminoglycan synthesis. Mech Dev 128:234–245
Orban JM, Wilson LB, Kofroth JA, El-Kurdi MS, Maul TM, Vorp DA (2004) Crosslinking of collagen gels by transglutaminase. J Biomed Mater Res A 68:756–762
Rosenthal AK, Derfus BA, Henry LA (1997) Transglutaminase activity in aging articular chondrocytes and articular cartilage vesicles. Arthritis Rheum 40:966–970
Shanmugasundaram S, Logan-Mauney S, Burgos K, Nurminskaya M (2012a) Tissue transglutaminase regulates chondrogenesis in mesenchymal stem cells on collagen type XI matrices. Amino Acids 42:1045–1053
Shanmugasundaram S, Logan-Mauney S, Burgos K, Nurminskaya M (2012b) Tissue transglutaminase regulates chondrogenesis in mesenchymal stem cells on collagen type XI matrices. Amino Acids 42:1045–1053
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Spurlin TA, Bhadriraju K, Chung KH, Tona A, Plant AL (2009) The treatment of collagen fibrils by tissue transglutaminase to promote vascular smooth muscle cell contractile signaling. Biomaterials 30:5486–5496
Summey BT Jr, Graff RD, Lai TS, Greenberg CS, Lee GM (2002) Tissue transglutaminase localization and activity regulation in the extracellular matrix of articular cartilage. J Orthop Res 20:76–82
Williams R, Khan IM, Richardson K, Nelson L, McCarthy HE, Analbelsi T, Singhrao SK, Dowthwaite GP, Jones RE, Baird DM, Lewis H, Roberts S, Shaw HM, Dudhia J, Fairclough J, Briggs T, Archer CW (2010) Identification and clonal characterisation of a progenitor cell sub-population in normal human articular cartilage. PLoS ONE 5:e13246
Acknowledgments
This study was supported by Grants from Maryland Stem Cell Research Foundation (MSCRFE-0156) and the National Institute of Health (AR057126).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niger, C., Beazley, K.E. & Nurminskaya, M. Induction of chondrogenic differentiation in mesenchymal stem cells by TGF-beta cross-linked to collagen-PLLA [poly(l-lactic acid)] scaffold by transglutaminase 2. Biotechnol Lett 35, 2193–2199 (2013). https://doi.org/10.1007/s10529-013-1301-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1301-8