Abstract
Chemical and surface characteristics of sulfite-pretreated royal palm sheath (RPS) fibers and parenchyma cells were investigated in order to study cell-type-dependent biomass hydrolysis by cellulase. Size, chemical composition, cellulose crystallinity and the exposure of cellulose microfibrils in pretreated RPS biomass affected the enzymatic accessibility and digestibility of different cell-type substrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Palm residues are widely available in tropical and subtropical areas as an abundant byproduct of the agriculture and food industries (Daud and Law 2011). They are a primary source of lignocellulosic biomass for producing second-generation biofuels. Enzymatic conversion of cellulose from palm residues biomass to yeast-fermentable sugars is one of the most promising approaches (Jørgensen et al. 2007). However, the natural recalcitrance of lignocellulose to cellulase hydrolysis significantly hinders the enzymatic conversion efficiency (Saritha et al. 2012). Therefore, various sophisticated pretreatment techniques have been developed in order to increase the accessibility and digestibility of cellulose by enzymes (Zhu and Pan 2010). However, the detailed mechanisms involved remain unclear even though the pretreatment methods dramatically promote lignocellulose conversion. Pretreatment efficiency greatly depends on removing lignin and hemicellulose, increasing substrate porosity, reducing particle size and/or cellulose crystallinity (Sun and Cheng 2002). The types of tissues in plant materials may determine biomass recalcitrance and therefore affect the pretreatment efficiency; for example, stalk piths were more readily hydrolyzed than leaves or rinds (Zeng et al. 2011). However, how the cellular types in plant biomass affect pretreatment and the subsequent enzymatic hydrolysis remains unsolved and warrants further studies.
Our group aims to maximize the utilization of palm residues for pulp and biofuels production. In this study, we investigated how chemical and surface characteristics of sulfite-pretreated palm fibers and parenchyma cells affected the enzymatic accessibility and digestibility. Our findings may help design novel biorefining strategies for efficient conversion of parenchyma-abundant biomass.
Materials and methods
Sulfite pretreatment
Royal palm sheath (RPS) chips (15–30 mm × 3–10 mm) were treated with sulfite compounds supplemented with H2SO4 or Na2CO3 at 175 °C for 30 min. Disintegrated solids were washed and then subjected to a Bauer-McNett Classifier, in which, parenchyma fines were separated from fiber bundles. Fines represent particles that passed through a 200 mesh screen but were retained on a 400 mesh. The bundles were further defibrated in a KRK disc refiner to yield homogeneous substrates.
Enzymatic hydrolysis
The pretreated material was hydrolyzed with 15 FPU/gsubstrate Celluclast 1.5L and 34 CBU/gsubstrate of Novozym 188 at 50 °C, pH 4.8 and 150 rpm for 72 h. Cellulose enzymatic digestibility was determined as the percentage of glucan hydrolyzed to glucose based on the initial glucan content in the pretreated biomass.
Analytical methods
Light microscopy was carried out by using a Model BX51 microscope following ISO 9184-3-1990. Atomic force microscopy (AFM) of RPS biomass samples was performed in tapping mode using a Veeco Multimode IIIa AFM with a J-scanner and a TESP tip (290–346 kHz). Crystallinity of RPS biomass was determined by a D8 Advance X-ray diffraction analyzer. Biomass crystallinity index was calculated using Segal et al. described method (1959). Cellulose crystallinity index was calculated using O’Dwyer’s prediction equation (Zhu et al. 2010).
Results and discussion
Cell diversity and size change in RPS biomass
RPS biomass mainly consists of vascular fibers and parenchyma cells (Fig. 1a). Fibers are narrow and end-pointed with a width of ca. 17 μm and a length ranged from 0.5 to 2 mm. Thin-walled parenchyma cells varying up to 250 μm in length and 150 μm in diameter are abundant. Sulfite pretreatment caused deformation and even fragmentation of parenchyma cells without significant defibrillization of fibers (Fig. 1b, c). Sulfite RPS under acid and alkali conditions appeared no different under microscopy observation (Fig. 1). Stained fibers appeared much yellower than parenchyma cells, most likely lignin prevented the color reaction between hemicellulose and stains. The content of fines in solids was retained around 32 % although undergone treatments (Supplementary Table 1). Thus, rare additional fines were separated from fibers and parenchyma cells were still the predominate constitution in fines.
AFM phase composition
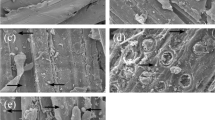
The morphology and phase composition of sulfite RPS biomass were investigated by AFM. The difference was marginal between acid and alkali sulfite pretreatments (Fig. 2; Supplementary Fig. 1). Granule-like structures (20–150 nm) were predominant throughout the surface of RPS fibers. Fibril-like structures fully but uniformly covered the surface of parenchyma. The fibrils with a width of 20–50 nm were likely to be cellulose microfibrils. Some of those granules might be lignin or hemicellulose because neither lignin nor hemicellulose has been reported to be fibril-like form (Koljonen et al. 2004). To verify the assumption, further chlorite delignification was performed to completely remove residual lignin (Chunilall et al. 2012) from sulfite RPS samples. Few granules were retained on fibers after delignification (Fig. 2c; Supplementary Fig. 1c). Instead, cellulose microfibrils appeared, occupying most of the scanning area. Some smaller residual granules (15–50 nm) were observed, probably accounting for hemicellulose not removable by chlorite. No granular structure was found in parenchyma cells after delignification, indicating the granules present in Fig. 2b and Supplementary Fig. 1b were lignin.
AFM observation demonstrated the surface of sulfite RPS fibers was covered by layers of lignin together with hemicellulose. Thus, lignin and/or hemicellulose possibly form physical barriers, preventing access of enzyme to cellulose surface. Lignin and/or hemicellulose may also cause nonproductive binding inhibition to cellulase, which consequently decreases the enzymatic hydrolysis efficiency of cellulose. Furthermore, AFM showed cellulose in sulfite RPS parenchyma formed microfibril-like structure. The exposure of cellulose on parenchyma surface is critically important for efficient enzymatic hydrolysis because the available surface area of cellulose exposed to enzyme attack is a key factor limiting the hydrolysis rate, especially at the initial stage (Sun and Cheng 2002).
Chemical composition and biomass crystallinity change
The chemical compositions of RPS biomass samples are summarized in Table 1. The content of glucan is lower than in wood but close to residues of date palm and oil palm (Daud and Law 2011; Khiari et al. 2010). The content of lignin was relatively low but ash was at the same level if compared to that in wheat straw and banana pseudo-stems (Khiari et al. 2010).
Sulfite pretreatment led to glucan-enriched solids by partially removing lignin, hemicellulose, extractives and inorganic substances (Table 1). Acid and alkali sulfite pretreatments increased the glucan contents in RPS biomass at least 100 and 85 % respectively. Acid sulfite pretreatment removed 82 % hemicellulose and 22.5 % lignin. Alkali sulfite pretreatment removed 55.5 % hemicellulose and 36 % lignin. Acid treatment led to more dissolution or degradation of polysaccharides than alkali treatment, and both fibers and parenchyma cells were more severely destructed as evidenced by Fig. 1. For both sulfite pretreatments, the glucan content was higher in parenchyma fines than that in fibers. This agreed with the AFM observations. Acid sulfite RPS fines were particularly rich of glucan up to 66.5 % of the solids, which is 17 % higher than that in the alkali sulfite fines. A lesser amount of lignin and hemicellulose in RPS fines than in fibers allows a higher accessibility of cellulose to cellulase (Shuai et al. 2010).
Cellulose crystallinity is a crucial factor that affects enzymatic hydrolysis (Sun and Cheng 2002). Cellulose crystallinity index (CrIc) in RPS solids yielded by acid sulfite pretreatment was higher than that obtained by alkali sulfite pretreatment (52 and 48 % respectively, Supplementary Fig. 2). This result indicated acid sulfite pretreatment removed more amorphous cellulose. Under the same pretreatment conditions (e.g. acid condition), parenchyma cells had a lower CrIc (49 %) than fibers (54.5 %). AFM results shown in Fig. 1 provide a probable explanation. Cellulose microfibrils exposed on parenchyma surface can be more severely damaged by pretreatment chemicals than those embedded by lignin-hemicellulose matrix in fibers. The lower cellulose crystallinity means a higher accessibility of the glycosidic bonds to cellulase (Sun and Cheng 2002).
Cellulose enzymatic digestibility
Cellulose enzymatic digestibilities (CED) of sulfite pretreated RPS biomass samples were studied (Fig. 3). Parenchyma fines exhibited the fastest initial hydrolysis rate and the highest CED, indicating that they were most susceptible fractions to cellulase. The time-dependent CED plots for acid and alkali sulfite RPS fines are almost overlapped, suggesting enzymatic cellulose hydrolysis was more affected by other factors such as hemicellulose content despite the difference in cellulose crystallinity. For acid sulfite fines, 83 % glucan was rapidly converted to glucose in the initial 12 h, while 91.5 % was completely hydrolyzed after 72 h. The CED pattern of alkali sulfite fines was similar to that of acid sulfite fines. Fibers were more recalcitrant to cellulase than fines. Only 38 and 44 % cellulose were hydrolyzed to glucose for acid and alkali sulfite fibers after incubated with cellulase for 12 h, respectively.
In conclusion, the main causes of the poorer digestibility of RPS fibers are: larger size dimension in RPS fibers (Fig. 1), more non-cellulose components (lignin and hemicellulose, Table 1), higher crystallinity of cellulose (Supplementary Fig. 2), together with nearly full surface coverage by lignin (Fig. 2). The hydrolysis of biomass by cellulose thus relies on the diversity and distribution of lignocellulosic cells. Sulfite RPS fibers have an average length of 1 mm (data not shown) and mechanical properties comparable to bamboo fibers (Rao and Rao 2007). Thus, we can propose a new integrated biorefining strategy for efficient utilization of palm residues or other parenchyma-rich biomass by separating fibers and parenchyma for respective purpose of pulping and biorefining.
References
Chunilall V, Bush T and Erasmus RM (2012) Investigating the lignocellulosic composition during delignification using confocal raman spectroscopy, crosspolarization magic angle spinning carbon 13-nuclear magnetic resonance (CP/MAS 13C-NMR) spectroscopy and atomic force microscopy. Cellulose Chem Technol 46: 269–267
Daud WRW, Law KN (2011) Oil palm fibers as papermaking material: potentials and challenges. Bioresources 6:901–917
Jørgensen H, Kristensen JB, Felby C (2007) Enzymatic conversion of lignocellulose into fermentable sugars: challenges and opportunities. Biofuel Bioprod Bior 1:119–134
Khiari R, Mhenni MF, Belgacem MN, Mauret E (2010) Chemical composition and pulping of date palm rachis and Posidonia oceanica—a comparison with other wood and non-wood fibre sources. Bioresource Technol 101:775–780
Koljonen K, Österberg M, Kleen M, Fuhrmann A, Stenius P (2004) Precipitation of lignin and extractives on kraft pulp: effect on surface chemistry, surface morphology and paper strength. Cellulose 11:209–224
Rao KMM, Rao KM (2007) Extraction and tensile properties of natural fibers: vakka, date and bamboo. Compos Struct 77:288–295
Saritha M, Arora A, Nain L (2012) Pretreatment of paddy straw with Trametes hisuta for improved enzymatic saccharification. Bioresource Technol 104:459–465
Segal L, Creely JJ, Martin AE (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-Ray diffractometer. Text Res J 29:786–794
Shuai L, Yang Q, Zhu JY (2010) Comparative study of SPORL and dilute-acid pretreatments of spruce for cellulosic ethanol production. Bioresource Technol 101:3106–3114
Sun Y, Cheng JY (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Zeng MJ, Ximenes E, Ladisch MR, Mosier NS, Vermerris W, Huang CP, Sherman DM (2011) Tissue-specific biomass recalcitrance in corn stover pretreated with liquid hot-water: enzymatic hydrolysis (Part 1). Biotechnol Bioeng 109:390–397
Zhu JY, Pan XJ (2010) Woody biomass for cellulosic ethanol production: technology and energy consumption evaluation. Bioresour Technol 101:4992–5002
Zhu L, O’Dwyer JP, Chang VS, Granda CB, Holtzappled MT (2010) Multiple linear regression model for predicting biomass digestibility from structural features. Bioresource Technol 101:4971–4979
Acknowledgments
We thank the support of Fundamental Research Funds for the Central Universities (No. 2012ZB0020) and National Natural Science Foundation of China (No. 31170549).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10529_2012_1079_MOESM1_ESM.tif
Supplementary Figure 1: AFM phase images of alkali sulfite RPS fibers (a), alkali sulfite RPS fines (b), delignified alkali sulfite fibers (c)and delignified alkali sulfite fines (d), recorded at a scan rate of 1 Hz and a scan size of 2 × 2 μm2 (TIFF 4137 kb)
10529_2012_1079_MOESM2_ESM.tif
Supplementary Figure 2: X-ray diffraction patterns and the crystallinity indices of RPS biomass.Note: Biomass crystallinity index (CI) was calculated from the height ratio between the intensity of the crystalline peak(I002 − Iam) and total intensity (I002) (1959). I002 refers to the peak intensity for the crystalline portion of biomass at about2θ = 22.5° and Iam represents the intensity for the amorphous portion at about 18°. Cellulose crystallinity index (CrIc)11 was predicted using O’Dwyer’s empirical equation, CrIc = 1.097 (CrIb) + 0.939 (Xylan content) − 11.433 (TIFF 577 kb)
Rights and permissions
About this article
Cite this article
Li, N., Liu, H., Fu, S. et al. Cell-type-dependent enzymatic hydrolysis of palm residues: chemical and surface characterization of fibers and parenchyma cells. Biotechnol Lett 35, 213–218 (2013). https://doi.org/10.1007/s10529-012-1079-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1079-0