Abstract
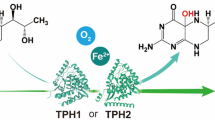
Hyoscyamine 6β-hydroxylase (H6H; EC 1.14.11.11) converts hyoscyamine to scopolamine in the last step of scopolamine biosynthetic pathway. The gene encoding H6H in Anisodus acutangulus was cloned and expressed in Escherichia coli and the recombinant proteins fused with His-tag or GST-tag at its N-terminal were purified and then confirmed by Western bolt analysis. The biofunctional assay revealed that the His-AaH6H and GST-AaH6H converted hyoscyamine (40 mg/l) to scopolamine at 32 and 31 mg/l, respectively. This is the first report on AaH6H expression, purification and functional characterization facilitates further genetic improvement of scopolamine yield in A. acutangulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropane alkaloids (such as hyoscyamine and scopolamine) are used medicinally as anticholinergic agents that act on the parasympathetic nervous system (Suzuki et al. 1999; Kai et al. 2009a, 2009b). Compared to hyoscyamine, scopolamine exhibits higher pharmaceutical activity and less side-effects, therefore, it is more valuable with ten-fold higher commercial demand in the clinical market (Yun et al. 1992; Zhang et al. 2004; Kai et al. 2007). However, because of the relatively low production of scopolamine in resource plants, it is of great significance and importance to obtain enough scopolamine to meet the expansion of clinical need by biotechnology.
Hyoscyamine 6β-hydroxylase (H6H, EC 1.14.11.11) considered as the rate-limiting enzyme involves in two consecutive steps (hyoscyamine hydroxylation and epoxide formation) in tropane alkaloid pathway producing scopolamine (Hashimoto et al. 1993) (Fig. 1). H6H genes have been found in Solanaceae plants such as Hyoscyamus niger (Matsuda et al. 1991), Atropa belladonna (Suzuki et al. 1999) and Anisodus tanguticus (Liu et al. 2005), and the functional characterization for the conversion of hyoscyamine to scopolamine has been described in H. niger and A. tanguticus (Matsuda et al. 1991; Liu et al. 2005). In addition, H6H genes have been used to improve scopolamine production via genetic engineering in hairy root cultures of A. belladonna, H. muticus and A. baetica (Yun et al. 1992; Jouhikainen et al. 1999; Zarate et al. 2006). We previously reported an additional H6H gene (AaH6H) isolated from native Chinese Solanaceous plant A. acutangulus (Kai et al. 2007). In this report, we expressed AaH6H in E. coli BL21 (DE3), via IPTG induction, and confirmed the purified protein by western blot analysis. We furthermore characterized the biofunction of AaH6H by whole-cell biotransformation in vitro, and the data suggested that the recombinant AaH6H can effectively convert hyoscyamine to the end product, scopolamine. This work provides an important clue for further genetic improvement of scopolamine by metabolic engineering and the genetically-engineered strains obtained in this work can be potentially utilized as bioconversion system for drug production.
Materials and methods
DNA constructs
The coding region of AaH6H was cloned into BamHI and SalI sites of bacterial expression vectors (pET30a and pGEX4T-1) under the control of T7 promoter. The resulting plasmids, pET30a-AaH6H and pGEX4T-1-AaH6H, were introduced into BL21 (DE3) and used for fusion protein expression.
Expression and purification
E. coli BL21 (DE3) cells transformed with pET30a-AaH6H were grown at 37°C in sterile Luria–Bertani (LB) medium supplemented with 50 μg kanamycin/ml. When the OD600 reached ~0.6, IPTG was added at 0.5 mM and the culture was grown for 2 days at 20°C. The cells were harvested by centrifugation at 5,000×g for 10 min at 4°C and resuspended in a lysis buffer (50 mM Tris/HCl, 200 mM NaCl, pH 8.0) containing 20 mM imidazole. The resuspended cells were sonicated for 10 min and the debris was removed by centrifugation at 14,000×g for 20 min at 4°C. The recombinant H6H (His-AaH6H) was purified according to the method described by Novagen (pET System Manual) with needed modifications. The target proteins were respectively eluted by two column volumes (CV) of lysis buffer (50 mM Tris/HCl, 200 mM NaCl, pH 8.0) containing 50 mM (elute 1), 70 mM (elute 2), 100 mM (elute 3) and 140 mM (elute 4) imidazole. For purification of GST-AaH6H protein, the target proteins were eluted with lysis buffer containing 7 mM glutathione. Protein concentrations were determined by Bradford assay using bovine serum albumin (fraction V, Sigma) as standard (Hashimoto and Yamada 1987).
Whole-cell biotransformation, alkaloids extraction and gas chromatography-mass spectroscopy analysis
Hyoscyamine (40 mg/l) and IPTG (0.5 mM) were simultaneously added to the culture of BL21 (DE3) cells harboring pET30a-AaH6H or empty pET30a, pGEX4T-1-AaH6H or empty pGEX4T-1 after the OD600 reached ~0.6. After 2-day induction, alkaloids in cell culture media were extracted with ethyl acetate by vortexing for three times. The organic phases were separated and evaporated with rotary evaporators. The extracts were dissolved in methanol/water (1:1, v/v) and analyzed by GC–MS. Tropane alkaloids were quantified as previously described (Liu et al. 2005). GC–MS was performed with Agilent 6850 Series GC-System using a 30 m × 250 × 0.25 μm HP-5 column. The splitless injection volume was 1 μl. Helium was used as the carrier gas at 1 ml/min. The inlet temperature was 280°C. The temperature program was: initial 50°C, 3 min isothermally, 5°C/min up to 200°C, 2 min isothermally, 3°C/min up to 280°C, 10 min isothermally. A mass detector (Agilent 5975) was used for detection.
Results and discussion
Protein purification and western blot analysis
The different fractions from protein Ni–NTA affinity purification were fractionated by SDS–PAGE gel. A major band corresponding to the putative target protein (His-AaH6H)) of 43 kDa was present in supernatant, precipitant and flow-through fractions as well as the eluted fractions but was absent in the wash fraction (Fig. 2a), indicting His-AaH6H was being expressed in bacteria. His-AaH6H was further confirmed by western blot analysis using anti-His tag antibody, which revealed a strong blotting band corresponding to the expected size (43 kDa) (Fig. 2b).
Expression, purification and Western blot analyses of recombinant H6H. The different fractions from AaH6H protein purifications were resolved in 12% SDS–PAGE gel. The gels were stained with Coomassie brilliant blue R-250 and were subsequently transferred onto the membranes for Western blots using rabbit polyclonal antibody against His or GST tags. Arrows indicate the band corresponding to recombinant AaH6H proteins. a SDS–PAGE analysis of His recombinant H6H. b Western blot analysis of His recombinant H6H protein from the supernatant. c SDS–PAGE analysis of GST-tag recombinant H6H. d Western blot analysis of GST- recombinant H6H protein from the supernatant
These results implied that His-AaH6H was abundantly expressed in bacterial cells. Similarly, a major band at 64 kDa, corresponding to the expected size of GST-AaH6H, was detected by Coomassie Brilliant Blue R-250 staining and western blot analysis using anti-GST antibody (Fig. 2c and d) suggesting that GST-AaH6H was also expressed in the cells. Finally, the yields of the purified recombinant His- and GST-AaH6H proteins were 0.6 and 0.9 mg/ml, respectively.
Bioconversion of hyoscyamine to scopolamine in E. coli
The biofunction of AaH6H were further investigated by whole-cell biotransformation in vitro. Both scopolamine and 6β-hydroxyhyoscyamine were detected in the media of cells carrying pET30a-AaH6H or pGEX4T-1-AaH6H not the empty vectors (pET30a and pGEX4T-1) (Table 1 and Fig. S1 in Supplementary material). Peaks corresponding to hyoscyamine, 6β-hydroxyhyoscyamine and scopolamine were identified by MS; the MS of scopolamine and 6β-hydroxyhyoscyamine from the bacterial media were identical to the standard alkaloids (Fig. S2 in Supplementary material). The yields of scopolamine extracted from the media of the cells carrying pET30a-AaH6H or pGEX4T-1-AaH6H were 32 and 31 mg/l, respectively. Both of ratios of 6β-hydroxyhyoscyamine to scopolamine were about 1:10 (Table 1).
Consistent with our findings, a similar phenomenon was previously reported in H. niger (Hashimoto et al. 1993). Recently, a H6H gene from Brugmansia candida was functionally expressed in yeast indicating a good potential for use as a biocatalyst (Cardillo et al. 2008). Because the complex structures of alkaloids make chemical synthesis complicated and expensive, our genetically engineered bacterial strains which can express bioactive product provide an alternative promising bioreactor to produce scopolamine as a new biocatalyst.
References
Cardillo AB, Talou JR, Giulietti AM (2008) Expression of Brugmansia candida Hyoscyamine 6beta-Hydroxylase gene in Saccharomyces cerevisiae and its potential use as biocatalyst. Microb Cell Fact 7:17
Hashimoto T, Yamada Y (1987) Purification and characterization of hyoscyamine 6β-hydroxylase from root cultures of Hyoscyamus niger. Eur J Biochem 164(2):277–285
Hashimoto T, Matsuda J, Yamada Y (1993) Two-step epoxidation of hyoscyamine to scopolamine is catalyzed by bifunctional hyoscyamine 6β-hydroxylase. FEBS Lett 329(1–2):35–39
Jouhikainen K, Lindgren L, Jokelainen T, Hiltunen R, Teeri T, Oksman-Caldentey KM (1999) Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 208:545–551
Kai GY, Chen JF, Li L, Zhou GY, Zhou LM, Zhang L, Chen YH, Zhao LX (2007) Molecular cloning and characterization of a new cDNA encoding hyoscyamine 6β-hydroxylase from root of Anisodus acutangulus. J Biochem Mol Biol 40(5):715–722
Kai GY, Zhang Y, Chen JF, Li L, Yan XM, Zhang R, Liao P, Lu X, Wang W, Zhou GY (2009a) Molecular characterization and expression analysis of two distinct putrescine N-methyltransferases from roots of Anisodus acutangulus. Physiol Plant 135(2):121–129
Kai GY, Li L, Jiang YX, Yan XM, Zhang Y, Lu X, Liao P, Chen JB (2009b) Molecular cloning, characterization of two tropinone reductases in Anisodus acutangulus and enhancement of tropane alkaloids production AaTRI transformed hairy roots. Biotechnol Appl Biochem 54(3):177–186
Liu T, Zhu P, Cheng KD, Meng C, He HX (2005) Molecular cloning, expression and characterization of hyoscyamine 6beta-hydroxylase from hairy roots of Anisodus tanguticus. Planta Med 71(3):249–253
Matsuda J, Okabe S, Hashimoto T, Yamada Y (1991) Molecular cloning of hyoscyamine 6β-hydroxylase, a 2-oxoglutarate-dependent dioxygenase, from cultured roots of Hyoscyamus niger. J Biol Chem 266(15):9460–9464
Suzuki K, Yun DJ, Chen XY, Yamada Y, Hashimoto T (1999) An Atropa belladonna hyoscyamine 6-hydroxylase gene is differentially expressed in the root pericycle and anthers. Plant Mol Biol 40:141–152
Yun DJ, Hashimoto T, Yamada Y (1992) Metabolic engineering of medicinal plants: Transgenic Atropa belladonna with an improved alkaloid composition. Proc Natl Acad Sci USA 89(24):11799–11803
Zarate R, El Jaber-Vazdekis N, Medina B, Ravelo AG (2006) Tailoring tropane alkaloid accumulation in transgenic hairy roots of Atropa baetica by over-expressing the gene encoding hyoscyamine 6β-hydroxylase. Biotechnol Lett 28(16):1271–1277
Zhang L, Ding RX, Chai YR, Bonfill M, Piñol MT, Xu TF, Pi Y, Wang ZN, Zhang HM, Kai GY, Liao ZH, Sun XF, Tang K (2004) Engineering tropane biosynthetic pathway in Hyoscyamus niger hairy root cultures. Proc Natl Acad Sci USA 101(17):6786–6791
Acknowledgments
This work was supported by National Natural Science Fund (30900110), National Transgenic Organism New Variety Culture Key Project (2009ZX08012-002B), Project from Ministry of Science and Technology of China (NC2010AE0075, NC2010AE0372), Shanghai Science and Technology Committee Project (10JC1412000, 09QH1401900, 06QA14038, 08391911800, 073158202, 075405117, 065458022, 05ZR14093), Kunshan Municipal Science & Technology Project (KS0914), Zhejiang Provincial Natural Science Fund (Y2080621), Program from Shanghai Municipal Education Commission (06DZ015, 09ZZ138), Fujian Science and Technology Committee Key Special Project (2008NZ0001-4), Leading Academic Discipline Project of Shanghai Municipal Education Commission (J50401) and Project from Shanghai Normal University.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kai, G., Liu, Y., Wang, X. et al. Functional identification of hyoscyamine 6β-hydroxylase from Anisodus acutangulus and overproduction of scopolamine in genetically-engineered Escherichia coli . Biotechnol Lett 33, 1361–1365 (2011). https://doi.org/10.1007/s10529-011-0575-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0575-y