Abstract
A new immobilisate of alcohol dehydrogenase (ADH) is described in which all components for the reaction, i.e. enzyme, the coenzyme NADP+, the buffer and other cofactors (trace elements), are immobilized together. It is an all-inclusive catalyst. The support is a cheap, commercially-available, superabsorbent polymer. The immobilisation is easy to achieve. The superabsorbed ADH is, even when dried, a stable and storable catalyst for at least five weeks at −18°C. Asymmetric reductions of the prochiral ketones, acetophenone, 4-acetylpyridine and ethyl acetoacetate, with a superabsorbed ADH from Lactobacillus brevis (ADH 002) and a superabsorbed ADH from Thermoanaerobicum sp. (ADH 005) in 2-propanol as both the organic solvent and the cofactor-regenerating substrate are given. Yields of chiral (R) and (S)-alcohols from 97–100% were achieved within 18 to 48 h with enantiomeric excesses of >99%. The superabsorbed ADH was easily separated by filtration and could be reused at least four times.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol dehydrogenases (ADH) play an increasingly important role in organic synthesis especially for the production of chiral alcohols, which are useful intermediates for chiral pharmaceuticals (Hussain et al. 2008; Tschaen et al. 1995), and other biologically active substances. Several ADHs, such those from Lactobacillus kefir (Metrangolo-Ruiz De Temiño et al. 2005), L. brevis (Hummel and Riebel 1997) or Thermoanaerobicum sp., are used in organic synthesis. They are produced using genetic engineering and thus are available in large quantities. Examples of enzymatically-prepared substances include, alkyl (R)-or (S)-3-hydroxybutyrate, (R)-or (S)-1-acetoxy-2-propanol (Pfaller and Stohrer 2007), aliphatic s-alcohols such as (S)-2-butanol (Pfaller and Schneider 2007) or (S)-2-pentanol (Peschko and Stohrer 2005). Usually the reactions take place in a two-phase medium. The enzyme and coenzyme are dissolved in an aqueous buffer solution, while the (in the main) less water-soluble substrates are dissolved in an organic solvent, such as ethyl acetate, methyl t-butyl ether (MTBE) or hexane. Cofactor regeneration takes place by using a second substrate, such as 2-propanol, which can also be used as a solvent to improve the solubility of the substrates. Several synthetic procedures have been described (Jeromin and Bertau 2005). To reduce costs, intensive research is being undertaken in order to make synthesis with alcohol dehydrogenases more effective. Several attempts have been made to facilitate the work-up procedure (Miethe et al. 1989) and to reuse the enzyme and coenzyme. This can be achieved by immobilizing the enzyme on a support. An efficient industrial process is described (Pfaller and Stohrer 2007), where the alcohol dehydrogenase from Lactobacillus brevis and Thermoanaerobicum sp. are immobilized on Celite in aqueous solution. After filtering the Celite from the reaction medium, most of the enzyme remains absorbed and can be reused in the next cycle. Only 15% of fresh enzyme activity and the coenzyme NADP+ needs to be added. Other attempts were made to immobilize the enzyme covalently on epoxy resins (Hildebrand and Lütz 2006) but without the cofactor. Enzyme and cofactor together have been immobilized in polyvinyl alcohol cryogels (Hischer et al. 2006), in polyacrylamide preparations (Amotz 1970) and in polyurethane polymers (Hartdegen and Swann 1980). Presented here are the results of a new and easy to produce immobilisate of an alcohol dehydrogenase (Jeromin 2008). The ADH and its coenzyme NADP+ are completely absorbed together with the buffer and trace elements on a commercial superabsobent polymer (SAP). Superabsorbent polymers are widely used in medicine and hygiene as strongly absorbent water materials. They consist basically of poly acrylic acids. Several superabsorbent polymers are commercially available. In this study, we used the superabsorbent polymer Favor from Evonik Stockhausen GmbH.
Materials and methods
Chemicals
Superabsorbent polymer (www.superabsorber.com), Favor (absorbancy under pressure at 4.8 kPa ~22 g/g, particle size distribution: >850 μm max. 1%, >600 μm ~35%, >300 μm ~44%, >150 μm ~16%, >45 μm ~3%, <45 μm max. 1%) was a free sample from Evonik Stockhausen GmbH, D-47805 Krefeld, Germany. The chemicals used were: acetophenone (>99% p.a. Fluka), 4-acetylpyridine (purum >98%, Fluka), ethyl acetoacetate (>98%, Merck) and 2-propanol (>99%, Merck).
Biocatalysts
The enzymes, ADH 002 and ADH 005, are commercial, crude alcohol dehydrogenases (E.C. 1.1.1.2). Specifications are for the ADH 002: yellow solution, activity >500 U/ml and for ADH 005: green brownish liquid, activity >100 U/ml. ADH 002 is from Lactobacillus brevis (activity 4,100 U/ml), ADH 005 is from Thermoanaerobicum sp. (activity 331 U/ml). NADP+, disodium salt, had a chemical purity of 98%. Julich Chiral Solutions GmbH, a Codexis Company, kindly provided both enzymes and the coenzyme.
Assay for ADH activities
The activity of superabsorbed alcohol dehydrogenase was determined spectrophoto-metrically by using acetophenone as a substrate and measuring the oxidation of NADPH at 340 nm after 1 min at 30°C. Activity was calculated in enzyme units (U, i.e. μmol/min). The standard assay reaction mixture for ADH 002 contains in a total volume of 1 ml, 970 μl of 11 mM acetophenone and 1 mM MgCl2 in 50 mM KH2PO4/K2HPO4 buffer, pH 7.0, 20 μl 10 mM NADPH (in buffer as given above), and 10 μl of 20 mg superabsorbed ADH 002 in 1 ml buffer as given above. The standard assay reaction mixture for ADH 005 contains in 1 ml, 970 μl 10 mM acetophenone, 10 μM ZnSO4 in 100 mM phophate buffer, pH 7.0, 20 μl 10 mM NADPH (in buffer as given above) and 10 μl of 9.1 mg superabsorbed ADH 005 in 1 ml buffer as given above.
Analytical methods
The yields of the products were analysed by GC using either a FS-FFAP-CB column (0.25 mm × 25 m) or a FS Innopeg PEG column (0.32 mm × 30 m) with N2 as carrier. The enantiomeric excess (ee) was determined with a RT BDEXcst-TM column (ß-cyclodextrin, 0.25 mm × 30 m), with He as carrier gas. TLC were performed by silica gel 60 F254, mobile phase hexane/ethyl acetate (2:1,v/v).
Synthesis procedure
Preparation of superabsorbed ADH 002
To a mixture of 35 g phophate buffer (pH 6.5, 1.5 M), 50 mg MgCl2·6H2O (25 mmol), 79 mg (0.1 mmol) NADP+ were added 2.4 ml ADH 002 (4,100 U/ml, 9,840 U) followed by 2.5 ml 2-propanol. The mixture was stirred for 10 min at the end of which 12.3 g superabsorbent polymer (Favor, Evonik Stockhausen) were added and stirred with a glass rod. After about 10 min, the mixture solidified. Next, 100 ml 2-propanol were added, stirred for 10 min with a glass rod and afterwards the immobilisate was sucked off in the vacuum via a frit. The residue was washed with 50 ml 2-propanol. This wet superabsorbed ADH 002 was used. For long term storage, it was dried in a vacuum desiccator (3 kPa over anhydrous CaCl2) for 5–6 days at room temperature the immobilisate became solid; the final yield was 22 g. The resulting product was ground in a mortar to give fine particles (particle size distribution: >2 mm ~3%, >710 μm ~66%, >355 μm 28%, <355 μm ~3%). The dried superabsorbed ADH 002 had a weight loss of 14.6% (24 h, 107°C). The superabsorbent polymer Favor had a weight loss of 3.5% (24 h,107°C). The superabsorbed ADH 002 was stored at –18°C. Enzymatic activity was measured at 249 U/g. This dry catalyst was used in 2-propanol/water (90:10, v/v) for the reduction of prochiral ketones.
Preparation of superabsorbed ADH 005
Preparation as for ADH 002 but 50 mg (0.06 mmol) NADP+, 30 ml ADH 005 (331 U/ml, 9930 U) and 20 g Favor were used. After drying for 8 d the yield was 36 g (beige). Size distribution of the ground particles: >2 mm ~7.5%, >710 μm ~81%, >355 μm ~11.4%, <355 μm ~0.1%). The dried superabsorbed ADH 005 had a weight loss of 14% (24 h, 107°C). Enzymatic activity was measured at 130 U/g.
Reduction procedure
500 U dry (about 14% water content) or wet (approximately 50% water content) superabsorbed ADH 002 or ADH 005 were suspended in 20 ml 2-propanol (10% water content) or 20 ml pure 2-propanol and stirred for 10 min. Next, 2 mmol prochiral ketone were added and the mixture magnetically stirred with a Fish-Clip stirrer at room temperature. The Fish-Clip stirrer reliably prevents the destruction of the superabsorbed enzyme particles and allows the stirring of the reaction mixture at any rpm. The reaction was monitored by TLC. After its completion in 18–48 h, the superabsorbed ADH was sucked off in the vacuum via a frit and washed with 50 ml 2-propanol. The superabsorbed ADH was than reused for the next cycle or dried in the vacuum desiccator. The resultant mixture was analysed by GC.
Results and discussion
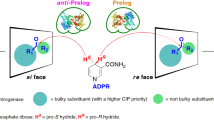
The superabsorbed alcohol dehydrogenases from Lactobacillus brevis (ADH 002) and from Thermoanaerobacter sp. (ADH 005) were tested with three prochiral ketones 1–3 (Fig. 1). Superabsorbed ADH 002 yielded the (R)-enantiomeres 1a, 2a and 3a, while the superabsorbed ADH 005 reduced the prochiral ketones 1–3 to the (S)-enantiomeres 1b, 2b and 3b. In each case, all the substrates were converted into the expected chiral alcohols with high ee’s.
Reduction of prochiral ketones 1–3 with superabsorbed ADH. For more details see Table 1
The superabsorbed ADH are ready to use after preparation when they have a water content of about 50% (w/w). Pure 2-propanol can immediately be used as a solvent. Dried superabsorbed ADH with a water content of about 14% (w/w) did not provide good results in pure 2-propanol as more water is needed for its activation. For best reduction results using the “dry” form of superabsorbed ADH, a mixture of 10% water in 2-propanol was used. The results indicated in Tables 1 and 2 were gained by this method.
We were able to use the superabsorbed ADH 002 and ADH 005 repeatedly. No further enzyme or coenzyme was added. During four cycles of reduction of ethyl acetoacetate, we found no change in the activities of ADH 002 and ADH 005. The results are listed in Table 2.
Conclusions
The new superabsorbed alcohol dehydrogenases show several advantages for the enantioselective reductions of prochiral ketones: they are simple and cheap to prepare, remain stable over at least five weeks, reductions take place under simple reaction conditions (stirring at room temperature), they can be used in organic solvents so that poorly water-soluble substrates can easily be reduced, the products and the superabsorbed ADH can be separated by filtration, the superabsorbed enzyme can be reused and the products can be isolated without the use of extraction techniques. As the conditions for the immobilization and reduction are not yet optimised, this aspect of the study is still under investigation and will be reported on in due course.
References
Amotz S (1970) Unlösliches enzymatisches reaktives material. DE Patent 1959169, 4 June 1970
Hartdegen FJ, Swann WE (1980) Immobilization of biological materials with polyurethane polymers. US Patent 4,237,229, 2 Dec 1980
Hildebrand F, Lütz S (2006) Immobilization of alcohol dehydrogenase from Lactobacillus brevis and its application in a plug-flow reactor. Tetrahedron Asymmetry 17:3219–3225
Hischer T, Steinsiek S, Ansorge-Schumacher MB (2006) Use of polyvinyl alcohol cryogels for the compartmentation of biocatalyzed reactions in non-aqueous media. Biocatal Biotransformation 24(6):437–442
Hummel W, Riebel B (1997) Alkohol-dehydrogenase und deren Verwendung zur enzymatischen Herstellung chiraler Hydroxyverbindungen. DE Patent 19610984, 25 Sep 1997
Hussain W, Pollard DJ, Truppo M, Lye GJ (2008) Enzymatic ketone reductions with co-factor recycling: Improved reactions with ionic liquid co-solvents. J Mol Catal B: Enzym 55:19–29
Jeromin GE (2008) Immobilisierung von Alkoholdehydrogenasen und deren Coenzyme sowie Verwendung des Immobilisats. DE Patent 102008038 326.0, 19 Aug 2008
Jeromin GE, Bertau M (2005) Bioorganikum, Praktikum der Biokatalyse. Wiley-VCH, Weinheim
Metrangolo-Ruiz De Temiño D, Hartmeier W, Ansorge-Schumacher MB (2005) Entrapment of the alcohol dehydrogenase from Lactobacillus kefir in polyvinyl alcohol for the synthesis of chiral hydrophobic alcohols in organic solvents. Enzyme Microb Technol 36:3–9
Miethe P,Voss H, Gruber R, Poetzsch H (1989) Verfahren zur biokatalytischen Herstellung schlecht wasserlöslicher Substanzen mit integrierter extraktiver Aufarbeitung. EP Patent 0347873, 27 Dec 1989
Peschko C, Stohrer J (2005) Enzymatic method for the enantioselective reduction of keto compounds. EP Patent 1568780, 10 Feb 2005
Pfaller R, Schneider C (2007) Production of (S)-2-Butanol by oxidative racemate resolution. US Patent 0207529 A1, 23 Feb 2007
Pfaller R, Stohrer J (2007) Method for the enzymatic production of chiral alcohols. US Patent Application 0212766 A1, 13 Sep 2007
Tschaen DM, Abramson L, Cai D, Desmond R, Dolling U-H, Frey L, Karady S, Shi Y-J, Verhoeven TR (1995) Asymmetric synthesis of MK-0499. J Org Chem 60:4324–4330
Acknowledgments
This work was supported by the Kompetenzplattform Bioengineering Jülich (KOPF), financed by the Ministry for Innovation, Science, Research and Technology of North-Rhine Westphalia (Ministerium für Innovation, Wissenschaft, Forschung und Technologie des Landes Nordrhein-Westfalen (MIWFT- NRW)), GC-analytics were made by Dr. Andrea Butzen from Julich Chiral Solutions GmbH and enzyme activities were produced by Robert Kuss of the Aachen University of Applied Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeromin, G.E. Superabsorbed alcohol dehydrogenase—a new catalyst for asymmetric reductions. Biotechnol Lett 31, 1717–1721 (2009). https://doi.org/10.1007/s10529-009-0062-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-009-0062-x