Abstract
Cell suspension cultures of Linum album were developed from internode portions of in vitro germinated plant in Gamborg’s B5 medium supplemented with 0.4 mg naphthalene acetic acid/l. The highest biomass was 8.5 g/l with podophyllotoxin and 6-methoxypodophyllotoxin at 29 and 1.9 mg/l, respectively after 12 d cultivation. Co-cultures of L. album cells with axenically cultivable arbuscular mycorrhiza-like fungi, Piriformospora indica and Sebacina vermifera, were established for the first time. These enhanced podophyllotoxin and 6-methoxypodophyllotoxin production by about four- and eight-fold, respectively, along with a 20% increase in biomass compared to the control cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants produce more than 100,000 secondary metabolites (Dixon 2001) many of which are commercially important. The low content of these compounds in whole plants, the endangered status of many plants due to their overexploitation, commercially unfeasible chemical synthesis, and geographical and genotypic variations have resulted in development of alternate biotechnological means to produce these compounds. An extensive array of secondary metabolites has been produced by plant cell culture technology as alternative strategy but, to date, limited commercial success is a major concern in this area. Application of various strategies, such as the addition of precursors, elicitation by biotic and abiotic means, renewal of medium and a combination of these, to enhance the yield of these compounds in cell cultures have been reported. Plant–fungi interaction can be used to enhance accumulation of these phytochemicals as most of these are produced due to activation of defense related biosynthetic pathways. These interactions are very complex and may be very specific to a given combination of the plant and the fungus, as there are about 250,000 species of higher plants and as many as 1.5 million species of fungi (Grayer and Kokubun 2001). The most common and prevalent symbiotic association of plants is with arbuscular mycorrhizal fungi. About 80% of the known plant species form arbuscular mycorrhizal associations (Smith and Read 1997). These fungi, represented by order Glomerales (Morton and Benny 1990), include all species capable of living symbiotically with plants. Arbuscular mycorrhizal fungi are obligate symbiont with limited saprobic ability and are dependent on the plant for their carbon nutrition (Harley and Smith 1983). But these fungi are not able to grow in pure cultures without living host roots. With the advent of axenically cultivable arbuscular mycorrhiza-like fungi, Piriformospora indica (Varma et al. 1998) and Sebacina vermifera (Warcup and Talbot 1967), a new vista has opened to study plant–fungi interaction in plant cell cultures for production of commercially important secondary metabolites. In the present report, co-cultures of these two fungi with plant cells were established to exploit their phytopromotional and elicitation potential on plant biomass and production of secondary metabolites of interest, respectively.
Podophyllotoxin (PT), widely used for synthesis of anticancer drugs like etoposide, teniposide and etopophos, has been obtained by solvent extraction from the rhizomes of Podophyllum peltatum and P. hexandrum (Berberidaceae) plants. There is an urgent need to search for alternate ways to produce these lignans by cell cultures due to threatened status of its natural source and economically unfeasible chemical synthesis. Cell cultures of Linum album are known to produce these lignans with highest productivity (Baldi et al. 2007). So L. album cell cultures capable of producing anticancer lignans namely PT and 6-methoxypodophyllotoxin (6-MPT) were used to establish co-cultures with living cells of P. indica and S. vermifera in suspension culture. This is the first report on co-culture of live plant and fungal cells for enhanced production of anticancer compounds in suspension cultures.
Materials and methods
Plant material
Seeds of Linum album were washed in 1% Savlon, surface-sterilized using 70% (v/v) ethanol HgCl2 for 1 min and rinsed thrice with sterile double-distilled water (SDDW). This was followed by treatment with 0.01% HgCl2 for 2 min and rinsing with SDDW 4–5 times. For germination, the sterilized seeds were then placed on Murashige and Skoog (MS) medium with 3% (w/v) sucrose and 1% (w/v) agar at 25 ± 1°C in complete darkness.
Initiation of L. album suspension cultures
Internode sections from 20–25 d old in vitro germinated plants were used as explants for culture initiation studies. Callus cultures were initiated by placing explants on solidified MS medium supplemented with 0.4 mg NAA/l as growth regulator.
The suspension cultures from this cell line were initiated by transferring friable fraction of callus equivalent to 2 g/l on dry cell weight (DCW) basis, into 250 ml Erlenmeyer flask containing 50 ml liquid Gamborg’s B5 media supplemented with 0.4 mg NAA/l. For establishment of growth and production kinetics, the cultures were incubated on a gyratory shaker at 125 rpm and 25°C under 16/8 h light/dark regime. The flasks, in triplicate, were harvested at an interval of 2 d till 16th d and analyzed for DCW, lignan (PT and 6-MPT) contents and residual sucrose concentration.
Establishment of co-cultures
Linum album cell suspensions were initiated in 50 ml Gamborg’s B5 media supplemented with 0.4 mg NAA/l by 2 g/l inoculum at 27 ± 1°C under 16/8 h light/dark conditions on the gyratory shaker at 125 rpm. The live, 5 d old, fungal cells of P. indica and S. vermifera, in their mid-growth phase on Kaefer medium (Kaefer et al. 1977) shaken at 200 rpm and 30°C, were aseptically added separately at different concentrations (0.5, 1, 2.5, 5 and 7.5 g/l on DCW basis) into the suspension cultures of L. album. Samples were taken at intervals and, finally, the flasks, in triplicate, were harvested on 12th d and analyzed for growth, lignan production and phenylalanine ammonia lyase (PAL) enzyme activity.
Analysis
Measurement of DCW and residual sugar
To determine DCW, the cells were collected, blotted on a filter paper and dried at 25 ± 2°C until constant weight was achieved. Residual sucrose was estimated in spent medium by phenol/sulphuric acid method (Dubois et al. 1956).
Extraction and quantitative analysis of podophyllotoxins
For extraction, dried and powdered cells (100 mg) were sonicated with 5 ml methanol for 15 min at 4–6°C and then held at 4–6°C for 24 h for complete extraction. The supernatant was removed after centrifugation and evaporated to dryness. The extract was redissolved in HPLC-grade methanol, filtered through 0.22 μm filter and analyzed by HPLC. Quantitative estimation was done by using Nova Pak RP-C18 column (Waters, USA) (250 × 4.6 mm2) with elution by 0.01% v/v phosphoric acid in water/acetonitrile (72:28 v/v) at 0.8 ml/min. The column was maintained at 30°C. PT and 6-MPT were detected at 290 nm at residence times of 3 and 4 min, respectively.
Determination of fungal and plant biomass
Total biomass (plant biomass + fungal biomass) as g/l on DCW basis was determined for each set of co-culture experiment in triplicate. These dried biomass samples (1 g) were subjected to acid hydrolysis and subsequent colorimetric analysis, to estimate the concentration of fungal biomass on the basis of their chitin content. Plant biomass produced in co-culture experiments was then determined by subtracting the fungal biomass from the total biomass.
Acid hydrolysis for chitin estimation
Chitin is present in fungal cell walls but not in plant cells so its estimation was used to determine the growth of the fungal cells in co-culture.
Air-dried biomass from each of the fungal cultures of P. indica and S. vermifera or from total biomass from co-culture experiments were washed with 50 ml 5 M H2SO4 under agitation at 100 rpm for 15 min, centrifuged at 4,000g for 40 min and rinsed with distilled water twice and incubated with 10 ml 10 M HCl at 25°C for 16 h. The volume was made up to 100 ml with double-distilled water and the samples were then autoclaved for 2 h at 121°C. Finally, it was neutralized to pH 7 using NaOH.
Colorimetric assay for chitin estimation
Estimation of chitin using 3-methyl-2-benzothiazolone hydrazone hydrochloride (MBTH) was performed with slight modification in method described previously (Smith and Gilkerson 1979). To 1 ml acid-hydrolyzed fungal/total (fungal + plant) biomass sample, 2 ml 2.5% (w/v) NaNO2 was added. Then 500 μl 0.5 M HCl was added and the reaction mixture was vortexed and kept at 25°C for 15 min. To this, 1 ml 12.5% (w/v) ammonium sulphamate was added, vortexed again and kept at 25°C for 5 min. The excess NaNO2 was liberated in the form of HNO2 by vortexing. Then 1 ml 0.25% (w/v) MBTH was added and again vortexed. The reaction mixture was then held at 65°C for 60 min. To this, 1 ml freshly prepared 0.5% (w/v) FeCl3 was added and the absorbance at 650 nm measured after 15 min.
The fungal biomass was correlated with A650 by plotting standard curves for the fungi in range of 0.15–1.5 g/l of respective fungal biomass on DCW basis.
PAL enzyme assay
Fresh weight of plant cells (1 g) was frozen and homogenized using liquid N2 in a precooled mortar and pestle at 4°C. To powdered cells, 3 ml borate buffer (0.1 M, pH 8) containing 5% (v/v) glycerol and 50 mM β-mercaptoethanol was added. The samples were ultrasonicated for 1 min. The homogenate was centrifuged at 8,000g for 30 min at 4°C and supernatant was collected. For activity assay, 1 ml 50 mM phenylalanine was added to 0.5 ml supernatant and held at 40°C for 60 min. The reaction was stopped by addition of 0.2 ml 6 M HCl. The reaction mixture was extracted with 4 ml toluene by vortexing for 15 s. This mixture was then centrifuged to separate the phases. Toluene phase was then analyzed for the trans-cinnamic acid recovered against a toluene blank at 290 nm. PAL enzyme activity was expressed as μkat (μmol cinnamic acid formed per s) per kg protein. Total protein estimation was based on the principle of protein–dye binding using bovine serum albumin as standard.
Results
Establishment of growth and production kinetics
The growth, lignan production and substrate consumption profiles of L. album cells in suspension culture were established and are given in Fig. 1. Maximum biomass (8.5 g/l), PT (29 mg/l) and 6-MPT (1.9 mg/l) were obtained at 12th d of cultivation. These growth and production levels are comparable with earlier reports on L. album cell suspension cultures (Smollny et al. 1998; Siedel et al. 2002, van Furden et al. 2005, Federolf et al. 2007). Nearly complete consumption of sugar was observed on 12th d when maximum growth and lignan production were achieved. Lignan production, therefore, was primarily a growth associated process. Lignans were produced intracellularly and the highest production occurred during the late log phase of L. album cells. This phenomenon had also been observed in lignan production by cell suspension cultures of P. hexandrum (Chattopadhyay et al. 2001) and L. album (Siedel et al. 2002).
Effect of co-culture on growth of plant cells
The co-cultures of both arbuscular mycorrhiza-like fungi, P. indica and S. vermifera, were established with L. album by inoculating different levels of the fungi (as inoculum) in a growing culture of L. album (at 9th, 10th and 11th d of its cultivation). Both fungi showed phytopromotional effect on plant cells in suspension cultures. Plant cell growth was increased by 21% (10.2 g/l) in comparison to control cultures (8.4 g/l), when P. indica was inoculated at a concentration of 1 g/l on 11th d and co-cultivated for 24 h. Similarly, 17.6% enhancement in plant biomass was obtained when S. vermifera was co-cultured for 24 h by inoculating it at a concentration of 2.5 g/l on 11th d. The increase in biomass could be attributed to the fact that arbuscular mycorrhizal colonization can dramatically increase absorption of mineral nutrients by plant cells (Safir et al. 1971). The exposure time of fungal cells to plant cells greatly affected this phytopromotional effect. As the co-culture time was increased to 48 and 72 h, fungal concentration dependent decrease in plant cell biomass was observed in comparison to control cultures. This might be due to unavailability of nutrients to plant cells due to their preferential uptake by fungal cells on longer exposure.
Piriformospora indica, an endophyte isolated from the soil of Rajasthan, showed growth promotional activities with diverse medicinal and economically important plants like Bacopa moniera (Sahay and Varma 1999, 2000), Azadirachta indica, Coffea arabica (Singh et al. 2002, 2003), Withania somnifera, Spilanthes calva (Rai et al. 2001), Tridex procumbans, Abrus procatorius (Kumari et al. 2004), Adhatoda vasica (Rai and Varma 2005) and Chlorophytum borivilianum (Chauhan et al. 2006). On the other hand, Sebacina sp. interfered with defense signaling by ethylene inhibition but allowed Nicotiana attenuata plants to increase growth rates at the expense of herbivore resistance mediated by triose phosphate isomerases (Barazani et al. 2005). All of these studies had been carried out on tissue culture raised or pot/field grown plants. Studies on organ (root) culture with arbuscular mycorrhizal fungi with the objective of studying plant–fungal symbiotic interaction have also been reported (Tiwari and Adholeya 2002). But the present investigation is the first report on phytopromotional capability of these fungi in plant cell suspension cultures.
Effect of co-culture on production of podophyllotoxins
The effect of co-cultivation of live fungal cells with L. album cells on lignan accumulation is shown in Figs. 2–5. The maximum increments of 3.6 times (102 mg/l) and 7.4 times (13.7 mg/l) in PT and 6-MPT accumulation, respectively, were obtained with P. indica when it was inoculated at 1 g/l on 11th d of L. album cultivation. Sebacina vermifera improved PT and 6-MPT production by 3.9 times (112 mg/l) and 7.6 times (14 mg/l) in co-cultivation experiments when it was inoculated at 2.5 g/l on 11th d of cultivation of L. album. With both fungi, the production of lignan decreased with increased period of co-cultivation (48 and 72 h) apparently because of decreased plant cell biomass. In most of the studies done till date about enhancement of commercially important plant derived compounds by addition of fungal elicitors to cell culture, pathogenic fungi were tested either in the form of culture broth or dead cell extract. But this report presents successful enhancement of anticancer lignans in cell suspension cultures by interaction of live fungal and plant cells.
The elicitation is the basic mechanism responsible for the increase in the lignan content in plant cells because the fungi mainly induce hypersensitive response in the plant cells; this results in activation of plant defense pathways and, therefore, increased phytoalexin production. This was further proved by significant increase in phenylalanine ammonia lyase (PAL) enzyme activity during the co-culture studies.
Production of podophyllotoxins and PAL activity
PAL catalyzes the first step of phenylpropanoid pathway i.e. conversion of phenylalanine to cinnamic acid by deamination. This is the bridge reaction between primary metabolism and natural product biosynthesis and also known as the rate limiting step of lignan biosynthesis (Nicholas et al. 1994). The effect of interaction of fungal and plant cells during co-cultivation on PAL activity is given as Table 1.
Lignan accumulation and PAL enzyme activity directly correlated with maximum lignan accumulation and coincided with the maximum increase of PAL enzyme activity. Enhancement of 2.7 times (216 μkat/kg protein) and 2.4 times (208 μkat/kg protein) in PAL activities was observed in co-cultivation with optimum concentrations of P. indica (1 g/l) and S. vermifera (2.5 g/l), respectively. The exposure of fungal cells for longer duration (72 h) resulted in decrease in PAL enzyme activity with corresponding decrease in lignan production.
Effect of addition of dead fungal cells
In order to study the effect of dead cells of the two fungi on production of podophyllotoxins by elicitation, the dead fungal cells were added to L. album cells in suspension culture. This also enhanced production of podophyllotoxins but to a lesser extent in comparison to live cells. Addition of 1 g/l dead P. indica cells on 10th d resulted in accumulation of 36 mg PT/l and 2.3 mg 6-MPT/l, which were 1.25- and 1.5-times higher in comparison to control cultures. The maximum enhancement of 1.4-fold (40.7 mg/l) and 1.8-fold (3.4 mg/l) in PT and 6-MPT production, respectively, was achieved by addition of 1 g/l dead S. vermifera cells on 10th d. PAL activity also increased by 1.2-fold (96.5 μkat/kg protein) and 1.4-fold (109 μkat/kg protein) on addition of dead cells of P. indica and S. vermifera, respectively, in comparison to control cultures (80 μkat/kg protein). These values were significantly lower than PAL activity in co-culture experiments.
Discussion
Co-cultures of L. album cells with live arbuscular mycorrhiza-like fungi, P. indica and S. vermifera, were successfully established. The present study is the first successful approach to mimic the induction of symbiotic and elicitation responses by plant cells due to fungal interaction for enhanced production of secondary metabolites. Interaction of live plant and fungal cells resulted in higher accumulation of podophyllotoxins in comparison to that with dead cells. This was probably due to activation of defense pathway for a longer period of time (24 h of co-cultivation) in live co-cultures which resulted in continuous release of signaling molecules responsible for production of defense compounds like podophyllotoxins. This fact was further supported by higher PAL activity observed in co-culture experiments. This methodology also avoided the problems associated with cultivation of arbuscular mycorrhizal fungi in pure cultures and may provide a valuable tool to study symbiotic mycorrhizal association in plants at cellular level. Further research for identification of compounds responsible for phytopromotional and elicitation effects in plant cell cultures is in progress.
References
Baldi A, Bisaria VS, Srivastava AK (2007) Biotechnological approaches for production of some plant based chemotherapeutics. In: Kayser O, Quax WJ (eds) Medicinal plant biotechnology—from basic research to industrial applications. Wiley-VCH Verlag, Weinheim, Germany, pp 117–156
Barazani O, Benderoth M, Groten K, Kuhlemeier C, Baldwin IT (2005) Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuate. Oecologia 146:234–243
Chattopadhyay S, Srivastava AK, Bhojwani SS, Bisaria VS (2001) Development of suspension culture of Podophyllum hexandrum for the production of podophyllotoxin. Biotechnol Lett 23:2063–2066
Chauhan AK, Das A, Kharkwal AC, Varma A (2006) Impact of micro-organisms on environment and health. In: Chauhan AK, Varma A (eds) Microbiology series—microbes: health and environment, I.K. International Publishing House Pvt. Ltd, New Delhi, pp 1–12
Dixon RA (2001) Natural products and plant disease resistance. Nature 411:843–847
Dubois M, Gilles KA, Hamilton JK, Roberts PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Federolf K, Alfermann AW, Fuss E (2007) Aryltetralin–lignan formation in two different cell suspension cultures of Linum album: deoxypodophyllotoxin 6-hydroxylase, a key enzyme for the formation of 6-methoxypodophyllotoxin. Phytochem 68:1397–1406
Grayer RJ, Kokubun T (2001) Plant–fungal interactions: the search of phytoalexins and other antifungal compounds from higher plants. Phytochem 56:253–263
Harley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic Press, New York, p 483
Kaefer E (1977) Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet 19:33–131
Kumari R, Pham GH, Sachdev M, Garg AP, Varma A (2004) Symbiotic fungi for eco-friendly environment: a perspective. Nat Prod Rad 3:396–400
Morton JB, Benny GL (1990) Revised classification of arbuscular mycorrhizal fungi (Zygomycetes); A new order, Glomales, two new suborders, Glomineae and Gigasporineae, with an emendation of Glomaceae. Mycotaxon 37:471–491
Nicholas JB, John OW, Meromit A, Hassar TN (1994) Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Plant Biol 91:7608–7612
Rai MK, Singh An, Arya D, Varma A (2001) Positive growth responses of the medicinal plants Withania somnifera and Spilanthes calva to inoculation by Piriformospora indica in a field trial. Mycorrhizae 11:123–128
Rai MK, Varma A (2005) Arbuscular mycorrhizae—like biotechnological potential of Piriformospora indica, which promotes the growth of Adhatoda vasica. Electron J Biotechnol ISSN 8:107–112
Safir GR, Boyer JS, Gerdemann JW (1971) Mycorrhizal enhancement of water transports in soybean. Science 172:581–583
Sahay NS, Varma A (1999) Piriformospora indica: a new biological hardening tool for micropropogated plants. FEMS Microbiol Lett 181:297–302
Sahay NS, Varma A (2000) Biological approach towards increasing the survival rates of micropropogated plants. Curr Sci 78:126–129
Singh AN, Singh AR, Kumari M, Rai MK, Varma A (2003) Biotechnology importance of Piriformospora indica—a novel symbiotic mycorrhiza-like fungus: an overview. Indian J Biotechnol 2:65–75
Singh AN, Singh AR, Varma A (2002) Piriformospora indica—in vitro raised leguminous plants: a new dimension in establishment and phyto-promotion. Indian J Biotechnol 1:372–376
Smith RL, Gilkerson E (1979) Quantitation of glycosaminoglycan hexosamine using 3-methyl-2-benzothiazolone hydrazone hydrochloride. Anal Biochem 98:478–480
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, New York
Smollny T, Wichers H, Kalenberg S, Shahsavari A, Petersen M, Alfermann AW (1998) Accumulation of podophyllotoxin and related lignans in cell suspension cultures of Linum album. Phytochem 48:575–579
Tiwari P, Adholeya A (2002) In-vitro coculture of two AMF isolates—Gigaspora margarita and Glomus intraredices on Ri-T DNA transformed roots. FEMS Microbiol Lett 206:39–43
van Furden B, Hamburg A, Fuss E (2005) Influence of methyl jasmonate on podophyllotoxin and 6-methoxypodophyllotoxin accumulation in Linum album cell suspension cultures. Plant Cell Rep 24:312–317
Verma S, Varma A, Rexer KH, Kost G, Sarbhoy A, Bisen P, Butehorn B, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 95:896–903
Warcup JH, Talbot PHB (1967) Perfect states of Rhizoctonias associated with orchids. New Phytologists 66:631–641
Acknowledgment
The authors are thankful to Prof. Ajit Varma, Amity Institute of Herbal and Microbial Studies, Noida, India for providing fungal cultures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Intellectual property rights of the work is covered under Patent application no. 1266/DEL/2007.
Rights and permissions
About this article
Cite this article
Baldi, A., Jain, A., Gupta, N. et al. Co-culture of arbuscular mycorrhiza-like fungi (Piriformospora indica and Sebacina vermifera) with plant cells of Linum album for enhanced production of podophyllotoxins: a first report. Biotechnol Lett 30, 1671–1677 (2008). https://doi.org/10.1007/s10529-008-9736-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-008-9736-z



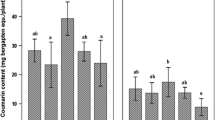



 , 72 h; and
, 72 h; and
 , control)
, control)

