Abstract
Phlebiopsis gigantea fungus used in biological control of root rot is currently cultivated commercially in disposable, sterilizable plastic bags. A novel packed bed bioreactor was designed for cultivating P. gigantea and compared to the plastic bag method and to a tray bioreactor. The spore viability of 5.4 × 106 c.f.u./g obtained with the packed bed bioreactor was of the same order of magnitude as the viabilities obtained with the other cultivation methods. Furthermore, the packed bed bioreactor was less time and space consuming and easier to operate than the tray bioreactor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In solid state cultivation the microorganisms are grown within a porous carrier. This cultivation method is mainly used for growing yeasts and fungi because they tolerate low water activity. Many of the solids used as cultivation media are of agricultural origin and the support itself acts as a carbon source (Pandey et al. 2000). Solid state cultivation has been exploited for example in the production of enzymes (Pandey et al. 1999), various secondary metabolites (Barrios-González and Tosmani 1996; Medeiros et al. 2001; Barrios-González and Mejía 1996) and fungal spores (Larroche 1996; Deshpande 1999). Common bioreactor types used in solid state applications are tray, static packed bed, mixed packed bed and rotating drum bioreactors (Mitchell et al. 1999).
The advantages of solid state cultivations over submerged cultivations are lower energy consumption, less wastewater production and lower capital investment. In addition, solid state cultivations can be used for the utilization of otherwise unusable raw materials (Pandey 2003; Fasidi et al. 1996). However, solid state cultivation is much less studied than submerged cultivation mostly due to the lack of suitable large scale process equipment (Mitchell et al. 1999). Solid state cultivation is, however, the only possible option for many biotechnical production processes, owing for example to the physiology of the microbe used. Also better yields and higher end-concentrations of products are in some cases gained by solid state than by submerged cultivation (Hölker et al. 2004). For instance, the fungus Phlebiopsis gigantea produces more spores in solid state cultivation than in submerged state (Seiskari et al. 1992).
Phlebiopsis gigantea is a white rot fungus that can be used to control root and butt rot after cutting down pines and spruces. It is a natural antagonist of the fungus, Heterobasidion annosum, which causes yearly losses of over € 790 million for the European forest industry (Woodward et al. 1998). Colonization by H. annosum can be prevented by spraying a solution containing spores of P. gigantea onto fresh stumps of cut trees (Pratt et al. 1999). The commercial P. gigantea product is currently manufactured by solid state cultivation in sterilizable plastic bags by the Finnish biotech company Verdera Oy. Demand for the product is increasing due to the growing awareness of the severity of economical losses caused by H. annosum and the rapid spreading of the pathogen to healthy forests.
The aim of this study was to develop an economical cultivation process for P. gigantea. Cultivation of P. gigantea in larger bioreactors would reduce manual work and improve the efficiency of the production process. A novel static bioreactor was developed for this purpose and compared to the plastic bag method and to a tray bioreactor.
Materials and methods
The organism and the culture medium
The inoculum of P. gigantea 1835 (Verdera, Finland) was cultivated in 1 l shake flasks containing 0.5 l malt extract (Biokar) solution (20 g/l) at 150 rpm and at 28°C for 5 days. The inoculum was homogenized with ultra-turrax (Ika labortechnik, T25 basic) to chop the mycelium pellets before inoculation of the bioreactors.
The culture medium used in the plastic bag, tray and packed bed cultivations contained 55.6% (w/v) water, 16.2% (w/v) condensed starch mash (Altia Corp.), 26.9% (w/v) silica (Sipernat, Degussa) and 1.3% (w/v) dolomite lime (Nordkalk Oyj). In the novel packed bed bioreactor, a cultivation at elevated moisture content (61.2% (w/v) water) was also performed. The culture medium used in the tray bioreactor, and in the plastic bags was sterilized at 121°C for 40 min. The medium used in the novel packed bed bioreactor was sterilized for 1 h.
Sterilizable plastic bags
Sterilizable plastic bags were made of high-density polyethylene (Amerplast). The bags were 450 × 700 mm holding 2 kg culture medium. The bag was closed with a polycarbonate collar and a cotton wool plug through which a silicone tube was inserted. The inocula (5% w/v) were pumped into the plastic bags through the silicone tubes. The culture media were mixed by shaking the bags manually. The cells were cultivated at 28°C for 10 days and filtered (Millex vent filter units, Millipore) air was blown into the bags through the silicone tube at 0.3 l/(min kg) during the cultivation.
Tray bioreactor
The tray bioreactor was encased with a polycarbonate box with the dimensions: height 300 mm, width 340 mm and depth 420 mm. The substrate layer (30–70 mm thick) was placed on 320 × 400 × 50 mm trays with perforated bases. The bioreactor held 7.5 kg culture medium. The reactor was loaded and unloaded from the end cap. The inoculum (5% w/v) was sprayed on the surface of the culture media with a syringe. The fungus was cultivated at 28°C for 10 days with aeration at 0.3 l/(min kg).
The novel packed bed bioreactor
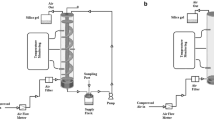
The capacity of the pilot bioreactor was 8.5 kg culture medium. The material of the vessel was stainless steel (AISI 316). The reactor parts presented in Fig. 1 are: vibrator (1) for achieving homogenous inoculation; pipe with a fog nozzle (2) to spray the inoculum to the cultivation medium; lower compartment (3) where the cultivation takes place; upper compartment (4) where the culture medium is placed during the sterilization; screen (5) to provide uniform transition of the cultivation medium from the upper compartment to the lower compartment during the inoculation; air inlet (6); and air outlet in the lid (7) for forced aeration.
The bioreactor was loaded with the culture medium up to the screen and the lid was closed. Before autoclaving sterile filters (Millex vent filter units, Millipore) were mounted on the air inlets and the inoculation pipe was shut. After sterilisation the vessel was turned lid down for inoculation and attached to the vibrator (Pneumatic roller vibrator, Netter Vibrationstechnik, NCR10). A hose pump (Masterflex, model 7549-44) was connected to the inoculation pipe. The fog nozzle (BETE WT42) was situated in the middle of the bioreactor and it was directed towards the lid. The reactor was vibrated during the aseptic spraying of the inoculum (5% w/v) into the bioreactor through the fog nozzle. The nozzle was a hollow cone nozzle with a spraying angle of 140° and a capacity of 0.5 l/min at 0.3 bar. The screen, made of acid-proof steel, had apertures of the size of 37 × 15 mm. Vibration caused the solid medium to fall gradually through the screen which resulted in homogenous inoculation. The oscillating frequency of the vibrator motor depended on the size of the bioreactor and on the screen aperture size. The speed of the feed pump (300 ml/min) was adjusted with the falling rate of the culture medium.
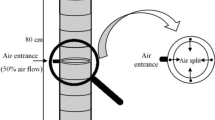
The fungus was cultivated at an ambient temperature of 24°C for 10 days using an airflow of 0.1 l/(min kg) from top to bottom. The temperature of the culture medium was measured from the center of the vessel and recorded using a data logging device (MadgeTech 2.00). After the cultivation the vessel was turned again, the lid opened and the culture poured out.
Viability determination
After the cultivation the culture medium containing P. gigantea spores was air-dried and the viability of the spores determined. Known amount of dried medium powder was mixed with water using an ultra-turrax homogenizer. From this homogenized mixture aliquots were plated on potato/dextrose/agar (Difco). The plates were incubated at 28°C for 24 h and the germinated spores were counted by examination of at least 15 microscopic fields. The diameter of a microscopic field was measured and the amount of viable spores was calculated accordingly.
Results
Cultivation
The cultivations were performed in a temperature controlled room. At the production scale the temperature control of the packed bed bioreactor by cooling jackets would however be more practical. Temperature rises did not pose a problem in the plastic bags or in the tray bioreactor. In the novel packed bed bioreactor the generation of metabolic heat caused the temperature of the culture medium to rise significantly, although the ambient temperature was 4°C lower than with the tray bioreactor and with the plastic bags.
A cultivation with increased moisture content (by 10%) was performed in the novel packed-bed bioreactor and compared to the cultivation at the same moisture content as was used in the plastic bags and in the tray bioreactor cultivations. The air velocities were kept constant in both cases. The temperature rise during the cultivations is presented in Fig. 2. Results from preliminary experiments indicated that P. gigantea did not grow above 32°C (data not shown). In the cultivation with no additional water the temperature in the center of the substrate bed rose from 24 to 35.1°C, which was too high for P. gigantea growth. In the experiment with additional water the temperature rose to a maximum temperature of 31.6°C.
In the packed bed cultivations air had to be blown from top to bottom in order to avoid formation of air channels in the culture medium. According to Auria et al. (1993) the pressure drop across an aerated bioreactor may be a problem in packed bed cultivations. However, no significant pressure drop could be observed during the cultivation in the novel packed bed bioreactor.
Harvesting and spore viability
The bioreactors could not be opened during the cultivation because of the contamination risk and thus no samples were taken. Culture media were compact, in one piece and fully colonized by P. gigantea at the end of all cultivations. The sporulation of P. gigantea was more vigorous on the surface area than in the center of the medium. The contents of the packed bed bioreactor and the plastic bags could be poured out easily. In the tray bioreactor the growth medium adhered to the perforated base of the trays and the medium had to be shoveled out.
Figure 3 shows the comparison of different cultivation strategies in terms of viability of P. gigantea spores. The viabilities obtained in the bioreactor cultivations were somewhat lower than, but of same magnitude as in the plastic bags. The viability in the tray bioreactor was on average 8.0 × 106 c.f.u./g and in the novel packed bed bioreactor 4.2 × 106 c.f.u./g and 5.4 × 106 c.f.u./g with elevated moisture content in the latter. The results indicate that P. gigantea can be cultivated successfully both in tray type and in packed bed type bioreactors, but that somewhat higher viability is obtained in tray type bioreactor.
Discussion
Solid state cultivation of P. gigantea in plastic bags and in two types of bioreactors was studied in this work. Only static solid-state cultivation methods were used because agitation is known to disturb the growth of P. gigantea (Seiskari et al. 1992) and because the silica based culture medium does not tolerate mixing.
The demand for P. gigantea spores is growing. Scale-up of the conventional plastic bag method would only be possible by increasing the number of cultivation bags. However, it would be a labor intensive, uneconomical and space consuming solution. The aim of the present work was therefore to develop a novel, practical bioreactor for the production of a biofungicide based on P. gigantea spores.
The novel packed bed bioreactor compares favorably with the tray bioreactor tested. The operation of the tray bioreactor required a lot of manual labor, because each tray had to be loaded, emptied and cleaned individually. By contrast, the packed bed bioreactor was easy to fill and empty by simply pouring the culture medium in and out. Dead space between the trays was needed for adequate air circulation in the tray bioreactor. Although tray bioreactors are generally very simple in design, only a thin layer of solid substrate can be used in order to avoid overheating problems (Hardin and Mitchell 1998; Fasidi et al. 1996). The bioreactor size per product was consequently smaller in the packed bed bioreactor than in the tray bioreactor.
According to the literature the major disadvantage of packed bed bioreactors is poor heat transfer, which is due to the thick substrate layer (Muller dos Santos et al. 2004; Hardin and Mitchell 1998; Ashley et al. 1999). Temperature gradients in the culture medium may lead to large variation in microbial growth and product formation (Ghildyal et al. 1994). Heat can be removed from the vessel for example by using a cooling jacket to enhance the radial heat conduction, by increasing the air velocity or by air reversal strategy (Ashley et al. 1999). Also moisture can be added to the culture medium to make the evaporative cooling more effective (Sangsurasak and Mitchell 1995). The constant flow of air through the culture medium may cause moisture to escape from the medium. This problem was avoided by the use of silica as the carrier in the culture medium. Silica has a high moisture retention capacity and it helps in keeping the moisture content of the medium constant. The results obtained in the present work show that by increasing the moisture content of the culture medium the temperature of the bed can be more effectively controlled.
The spore viability appeared to be somewhat higher in the tray bioreactor than in the packed bed bioreactor. This was possibly due to the poorer heat transfer in the packed bed cultivation. The differences are, however, relatively small and presumably insignificant in terms of industrial production. Furthermore, higher viability can most likely be obtained by the use of suitable instrumentation and by more efficient control of the cultivation conditions. The performance of the bioreactor could be improved for example by on-line moisture and temperature controls and by sampling. Practical devices for these purposes are yet to be designed. This work is currently underway in our laboratories. Further studies are also needed to evaluate whether the novel bioreactor can be used for solid state cultivation of other micro-organisms and whether this reactor type can be scaled up to industrial scale.
References
Ashley V, Mitchell D, Howes T (1999) Evaluating strategies for overcoming overheating problems during solid-state fermentation in packed-bed bioreactors. Biochem Eng J 3:141–150
Auria R, Morales M, Villegas E, Revah S (1993) Influence of mold growth on the pressure drop in aerated solid state fermentors. Biotechnol Bioeng 41:1007–1013
Barrios-Gonzalez J, Mejía A (1996) Production of secondary metabolites by solid-state fermentation. Biotechnol Ann Rev 2:85–121
Barrios-González J, Tosmani A (1996) Production of aflatoxins in solid state fermentation. J Sci Ind Res 55:424–430
Deshpande MV (1999) Mycopesticide production by fermentation: potential and challenges. Crit Rev Microbiol 25:229–234
Fasidi I, Isikhuemhen O, Zadrazil F (1996) Bioreactors for solid-state fermentation of lignocellulosics. J Sci Ind Res 55:450–456
Ghildyal NP, Gowthaman MK, Raghava Rao KSMS, Karenth NG (1994) Interaction between transport resistances with biochemical reaction in packed-bed solid state fermentors: effect of temperature gradients. Enzyme Microb Technol 16:253–257
Hardin M, Mitchell D (1998) Recent developments in the design, operation and modelling of bioreactors for solid state fermentation. Recent Res Dev Ferment Bioeng 1:205–223
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Larroche C (1996) Microbial growth and sporulation behaviour in solid state fermentation. J Sci Ind Res 55:408–423
Medeiros ABP, Pandey A, Christen P, Fontoura PSG, Freitas RJS, Soccol CR (2001) Aroma compounds produced by Kluyveromyces marxianus in solid state fermentation on a packed bed column bioreactor. World J Microbiol Biotechnol 17:767–771
Mitchell DA, Pandey A, Sangsurasak P, Krieger N (1999) Scale-up strategies for packed-bed bioreactors for solid-state fermentation. Process Biochem 35:167–178
Muller dos Santos M, Souza da Rosa A, Dal’Boit S, Mitchell DA, Krieger N (2004) Thermal denaturation: is solid-state fermentation really a good technology for the production of enzymes? Bioresour Technol 93:261–268
Pandey A (2003) Solid-state fermentation. Biochem Eng J 13:81–84
Pandey A, Soccol CR, Mitchell D (2000) New developments in solid state fermentation: I-bioprocesses and products. Process Biochem 35:1153–1169
Pandey A, Selvakumar P, Soccol C, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77:149–162
Pratt JE, Gibbs JN, Webber JF (1999) Registration of Phlebiopsis gigantea as a forest biocontrol agent in the UK: recent experience. Biocontrol Sci Tech 9:113–118
Sangsurasak P, Mitchell DA (1995) Incorporation of death kinetics into a 2-D dynamic heat transfer model for solid state fermentation. J Chem Technol Biotechnol 64:253–260
Seiskari P, Pulkkanen H, Vapaaoksa P (1992) Solid support medium for microbe preparations and a method for cultivation of microbes. WO 92/18623, Oct 1992
Woodward S, Stenlid J, Karjalainen R, Hütterman A (1998) Heterobasidion annosum. Biology, ecology, impact and control. CAB International, Wallingford, UK
Acknowledgments
The authors thank Seppo Jääskeläinen for valuable technical support. The research was funded by Verdera Oy and by the Finnish Funding Agency for Technology and Innovation (TEKES).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Virtanen, V., Nyyssölä, A., Vuolanto, A. et al. Bioreactor for solid-state cultivation of Phlebiopsis gigantea . Biotechnol Lett 30, 253–258 (2008). https://doi.org/10.1007/s10529-007-9538-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9538-8